Abstract
Involuntary post-contraction muscle activity may occur after performing a strong long-lasting (about 30 s) isometric muscle contraction (Kohnstamm phenomenon). Here we examined how this putative excitatory state may interact with a locomotor movement. The subjects stood upright and were asked to oppose a rotational force applied to the pelvis for about 30 s either in the clockwise or in the counterclockwise direction. After that, they were asked to perform various motor tasks with the eyes closed. During quiet standing, we observed an involuntary post-contraction torsion of the trunk. During walking, the post-contraction facilitatory effect of body torsion was not overridden by the voluntary activity, but instead significantly influenced the forward locomotor program such that subjects walked along a curved trajectory in the direction of the preceding torsion. In contrast, we did not observe any rotational component when subjects were asked to step in place. We conclude that the post-contraction rotational aftereffect does not transfer to just any motor task but apparently manifests itself in those movements that incorporate the activated axial muscle synergy or rotational component. We argue that central excitability changes following the voluntary effort may contribute to the phenomenon and highlight the role of tonic influences in fine-tuning of the spinal cord.
Introduction
At the beginning of the last century, Kohnstamm (1915) reported a very intriguing phenomenon related to involuntary tonic activity. The classic description of one of its variants is the following. An individual standing with the arms at his side was asked to exert force with the back of one of his hand against a wall for about 30–40 s. The cessation of the effort resulted in an automatic upward arm movement accompanied by a particular sensation of ‘lightness’, and the individuals performing this task often report that the arms seem to lift themselves. The ‘Kohnstamm phenomenon’ comprises an extensive literature (see Craske and Craske 1986 for a review). Muscular after-contraction can be observed in flexors and extensors of the wrist, shoulder, ankle, knee, hip, and also neck muscles. In healthy humans, these post-contraction aftereffects emerge most clearly in proximal rather than in distal joint muscles (Gurfinkel et al. 1989). They are commonly thought to result from a long-lasting change in the excitatory state of the neuromuscular system; however, the exact mechanisms have not yet been identified. The current hypotheses about the origin of the Kohnstamm phenomenon consider both a prolonged excitation of central structures and an increased afferent inflow—post-contractory sensory discharge (see Duclos et al. 2004 for a review). The appearance of involuntary tonic activity is accompanied by a facilitation of selected motor reactions (e.g., motor-evoked potentials; Mathis et al. 1996). Also, post-activation phenomena can be used as a tool to study tonic influences (Craske and Craske 1986; Gurfinkel et al. 1999).
Tonic influences may play a regulating role both in sustaining and shaping the locomotor pattern (Grillner 1981). To elicit locomotor movements, the descending inputs may fine-tune the ‘tonic’ locomotor state of the spinal cord (Shik 1983; Hultborn 2001). This tuning apparently necessitates a certain period of time since locomotor movements do not start/stop immediately after the onset/cessation of electrical stimulation of locomotor regions in the brain. In addition, the enhancement of postural tonus precedes initiation of locomotion (Mori et al. 1982). Finally, tonic facilitations may cause locomotor circuits to transition from an inhibited to active state (Grillner 1981; Shik 1983). For example, by means of the Kohnstamm phenomenon (e.g., following a long-lasting isometric flexion of the knee joint), it is possible to evoke an involuntary air-stepping of the suspended leg in humans (Gurfinkel et al. 1999). Here we report a new manifestation of the Kohnstamm phenomenon in the standing position in humans, the torsional postural aftereffect, and its interaction with locomotor movements. We hypothesized that an asymmetrical change of the tonic state of the neuromuscular system (by the post-contraction phenomenon) may result in a rotational movement component compliant with the role of the neuronal circuits being activated.
Methods
Twenty-one healthy subjects (15 males and 6 females, age=34±15) participated in this study. It is known that some individuals do not show prominent behavioral effects of muscle pre-contraction (see Craske and Craske 1986). For this reason, in a preliminary session, we tested the standard Kohnstamm phenomenon (i.e., shoulder abduction) during quiet standing with eyes closed. Out of 28 healthy subjects tested, 21 were sensitive to pre-contraction from the very first application, and these subjects subsequently participated in the laboratory experiments (14 of them participated also in the walking-in-open-space experiments, see below). None of the subjects had any history of neurological disease or vestibular impairment. The studies conformed to the Declaration of Helsinki, and informed consent was obtained from all participants.
To evoke rotational post-contraction aftereffects, the subjects, standing upright with eyes closed and wearing headphones, were first asked to oppose an approximately constant rotational force applied by an experimenter to the pelvis (40±5 Nm across all subjects, as estimated from the force plate measurements, Kistler 9287B, Switzerland) (Fig. 1a). These torsional contractions were made either in the clockwise (CWT) or in the counterclockwise (CCT) direction (i.e., as viewed from above; the direction refers to the torque generated by the subject, not by the experimenter) for ≈30 s. The distance between the heels was about 12 cm. Numerous muscles around the hip may generate this torque (Dostal et al. 1986), as well as asymmetrical bilateral leg muscle activity may considerably contribute to the net torsional effort due to the ‘closed’ kinematic chain when standing on both legs. After cessation of the voluntary pelvis-torque the subjects were asked to perform various motor tasks with eyes closed: quiet standing (for ≈1 min), forward walking, and stepping in place. The same motor tasks were also performed prior to the applied-torque experiments (control). For each motor task, three trials were usually performed by each subject: control, after CWT, and after CCT.
Fig. 1.
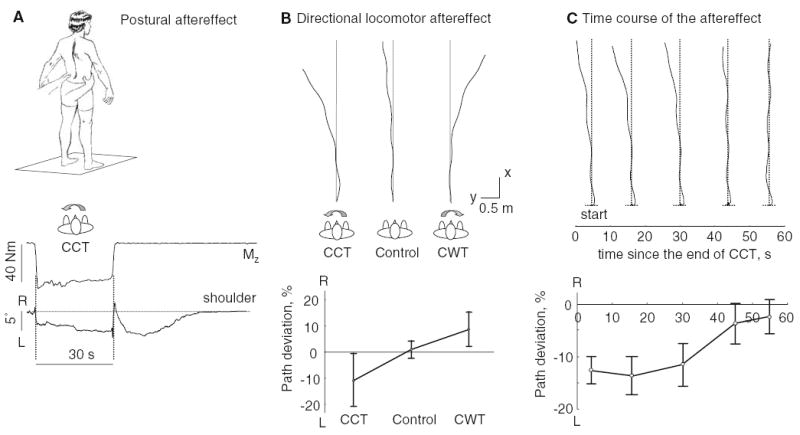
Aftereffect of long-lasting (30 s) externally applied torsion on standing and walking. a An example of the postural aftereffect in one subject. From up to down: rotational torque (Mz) measured by the force plate and shoulder rotation (R right, L left). b Directional aftereffect of body torsion on walking trajectories. Path traces of a representative subject in three test conditions (CWT, CCT, and Control) and average path deviation (±SD, n=21, computed as a medio-lateral deviation of the trajectory during two strides divided by the path traveled) are shown. c Time course of the post-contraction effect on walking trajectories. Walking trajectories performed at different delays after the end of the CCT in a representative subject and average deviation of the trajectory as a function of time since the end of the CCT in six subjects are shown. Note a decay of the rotational aftereffect with time
Walking was tested in two different experimental settings: (1) short-distance walking in the laboratory and (2) walking in a large flat open space. Unless stated otherwise, the walking task was initiated 3–4 s following the cessation of externally applied torsion. The subjects were asked to walk at their preferred speed with the arms swinging naturally. In the first walking setting, the subjects walked along a 6-m path across the room. In addition, six of these subjects who showed a prominent post-contraction aftereffect during walking were asked to initiate walking at different delays (3, 15, 30, 45, and 55 s) after the cessation of the active resistance to externally applied torsion. In the second walking setting, the subjects (n=14) walked in a large horizontal open space (30×50 m). To avoid providing subjects with feedback about their locomotor trajectories, they were guided to a new unknown position after each trial with eyes closed.
Between trials, the subjects sat quietly in a chair for more than 5 min, since this period seemed sufficient to attenuate any significant aftereffect of the preceding contraction, as verified in a few subjects. The total duration of the experimental session was approximately 2 h.
Data recording and analysis
In the laboratory, kinematic data were acquired using a Motion Analysis system (Santa Rosa, CA, USA; sampling at 60 Hz) or a Vicon-612 system (Oxford, UK; sampling at 100 Hz). The spatial error of either system is ≤ 1 mm (RMS). Each subject was outfitted with infrared reflective markers attached to the skin of both sides of the subject overlying the gleno-humeral joint, the midpoint between the anterior and the posterior superior iliac spine (ilium), the greater trochanter, the lateral femur epicondyle, the lateral malleolus, and the fifth metatarso-phalangeal joint. Gait cycle was determined using a relative amplitude criterion for the vertical displacement of the foot markers (Ivanenko et al. 2005). Locomotor trajectories were based on the path of the mid-position of the two ilium markers. During standing and stepping in place, shoulder position was used to compute changes in the body orientation.
During walking in a large open space, locomotion trajectories were approximated. An experimenter walked behind the subject and marked the estimated vertical projection of the trunk on the ground every 1–1.5 m of the path. The walking area was marked ahead of time with a grid (1×1 m) so that, after the trial, the approximate x, y positions of markers relative to this grid could be measured visually (±15 cm). These measurements were then entered manually into a computer to generate smoothed locomotor trajectories and for further quantitative analysis. Walking speed was estimated as the total distance traveled divided by the total duration, as measured with a stopwatch. The smoothed locomotor trajectories were reconstructed by re-sampling the measured coordinates in the space domain by means of polynomial interpolation of the x, y time series (0.1 m steps).
Statistical analyses (ANOVA) were used where appropriate. Results were considered significant at a probability level of P<0.05.
Results
Post-contraction postural rotational aftereffect
In agreement with the original demonstration of the Kohnstamm phenomenon on the deltoid muscle, we found that, notwithstanding individual variations (see also Craske and Craske 1986), the aftereffect of prolonged voluntary contraction at the pelvis is a steady rise to a plateau angle of shoulder rotation followed a gradual return over a period of ≈20 s (Fig. 1a). In some instances, the effect lasted as briefly as 7 s or as long as 40 s. The mean peak angle of shoulder rotation was 4±4° across subjects, relative to the mean shoulder orientation prior to the voluntary exertion. The force was applied by an experimenter to the pelvis and therefore the shoulder axis could rotate slightly during the isometric contraction in the direction of the effort (by a few degrees, Fig. 1a). It typically returned to the initial (prior to the effort) shoulder yaw orientation, although sometimes it remained in a deviated position (<5°).
Short distance walking
In the control trials (i.e., no preceding contraction), the subjects produced nearly straight walking trajectories. The principal observation in subjects who walked with eyes closed following a prolonged torsional contraction was a curved trajectory to the walking path in the direction of the preceding trunk torsion (Fig. 1b). To estimate the amount of turning, we computed the medio-lateral deviation of the walking trajectory during the first two strides (normalized to the total path length). This curved trajectory was significantly different from the path produced without a preceding torsional contraction (F2,20=13.3, P<0.0001). These deviations from a straight path were on average 10% of the total path length, while in the control condition path errors of 1% were typical (Fig. 1b).
Time course of the post-contraction effects
The time course of the ‘Kohnstamm phenomenon’ on walking trajectory deviation was studied in single trials performed at different intervals after cessation of the long-lasting voluntary CCT (Fig. 1c). Measured in this way, the deviation from a straight-line path decayed with a time-constant of a few tens of seconds, comparable to the postural aftereffect shown in Fig. 1a. Despite some inter-subject variability, the amplitude of trajectory deviations decreased with time (F4,5=9.6, P<0.0002) (Fig. 1c).
Walking in a large open space
To analyze longer paths, we performed experiments in a large horizontal open space (see methods). As with shorter walking trajectories, trajectories in the larger space tended to deviate in the direction of the preceding postural torsion (Fig. 2a). The change in walking direction after a 20-s period of movement was defined, in this case, as the difference between the initial and final headings (derived from the smoothed walking trajectories, Fig. 2a). In the control trials, the mean veering was only 2±7° (mean±SD). In contrast, the change in the heading direction after 20-s stepping was on average −117±86° in the CCT condition and 132±81° in the CWT condition, thus showing a prominent direction-specific effect (Fig. 2c).
Fig. 2.
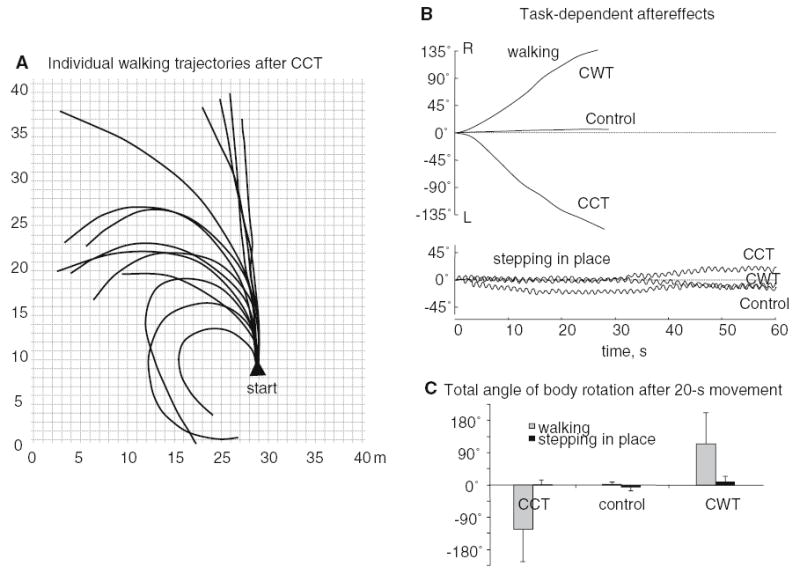
Aftereffect of long-lasting externally applied torsion on walking in a large open area and on stepping in place. a Individual walking trajectories. The actual starting points of trials could be in different locations of the walking area but for the purpose of illustration they are superimposed. b An example of changes in body orientation during walking (4.1 km h−1) and stepping in place in one subject. Changes in body orientation during walking (upper panel) were limited to ≈30-s recordings since walking trajectories were terminated at this time by approach to some physical obstruction. c Average total angle of body rotation (mean ± SD) after 20 s movements
Subjects either always experienced a straight walking trajectory (i.e., they were unaware of turning, yet, the walking trajectory was curvilinear) or reported that ‘something was slightly pushing them laterally’. The individual angular path deviations during walking correlated with the maximal shoulder rotation during postural aftereffect (r=0.71±0.23), suggesting a correspondence between the subject’s sensitivities to pre-contraction and the effects on postural and walking tasks.
The curvature and angular velocity of walking tended to be roughly constant during the walking task (Fig. 2a). The range of maximum angular velocities differed considerably across subjects (1–20°/s), although maximum angular velocity calculations assumed that forward-progression walking speed was constant during the test. Individual trajectories were usually terminated after about 25–40 s by approach to some physical obstruction. Therefore, two subjects who showed a prominent aftereffect were also tested on a separate day in a very large horizontal open area (about 100×100 m) in order to verify whether the post-contraction path deviation persisted over a longer period. In both subjects, the angular deviation of the walking trajectory waned considerably after 1 min of walking: the change in the heading direction during the final 20-m portion of the path was only 2° and 7° in these two subjects (while during the initial 20-m portion of the path it was much larger, 160° and 85°).
Stepping in place
Since many of the same muscles are activated during stepping in place and walking, we looked for post-torsion rotational effects during stepping in place at a comfortable cadence (0.5–1 Hz). We examined whether body orientation progressively shifted in the direction of the preceding axial torsion, as was the case for walking. Surprisingly, we found no rotational post-effect during stepping movements as illustrated in Fig. 2b. On occasion, we observed small spontaneous changes in the body orientation. However, on average, the total absolute change in the trunk orientation after 20 s movements was only 9±8° (CCT and CWT combined) and did not depend on the direction of the preceding rotational effort (F2,20=1.26, P=0.29) (Fig. 2c).
Discussion
The paper describes the aftereffect of voluntary torsional contraction while standing. We examined interactions of this ‘asymmetrical’ post-contraction excitatory state of the neuromuscular system with whole body motor tasks and found that it resulted in a prominent deviation of the locomotor trajectory, the direction of deviation being dependent on the direction of pre-contraction.
The post-contraction time course of the trajectory deviation (decay after 20–40 s, Fig. 1c) resembled a typical time course of the involuntary muscle activity following sustained voluntary effort of moderate intensity against a static load (Kohnstamm phenomenon). The neurophysiological substrates underlying such behavior are largely unknown. However, it is unlikely that purely peripheral factors such as post-contractory sensory discharge and thixotropic properties of muscle spindles can account for the observed phenomenon (Gregory et al. 1988; Ribot-Ciscar et al. 1991; Gilhodes et al. 1992; Hagbarth and Nordin 1998) since the aftereffect during walking would be expected to disappear after 1–2 steps. Indeed, any muscle thixotropy decreases considerably following muscle shortening/ lengthening (Gurfinkel et al. 1990; Proske et al. 1993). In contrast, the post-contraction locomotor aftereffect lasts for about 20–40 steps (Fig. 2a). In addition, the motor aftereffect may depend on postural, sensory and cognitive contexts (see Duclos et al. 2004), and on the duration of the pre-contraction (Craske and Craske 1986). Therefore, we believe that it has central rather than peripheral origin.
Task-dependent aftereffects
The act of walking along a curved path can exploit the basic mechanisms of the spinal locomotor generator with only little additional tuning of the inner and outer leg muscle activity (Courtine and Schieppati 2003). Moreover, curvilinear deviations of the locomotor trajectory may possibly involve unidirectional activation of a specific pelvis-stabilizing rotational component of the motor pattern that is already incorporated in normal forward walking (Saunders et al. 1953).
The preparedness of lower-level structures for pending suprasegmental motor commands is an essential aspect of the vertebrate’s ability to select an appropriate response from a vast motor repertoire and to avoid undesirable movements triggered by external or internal sources. Assuming that involuntary post-contraction activity is a sign of a long-lasting excitatory state of specific neuronal networks associated with a preceding motor activity, this excitatory state should involve numerous brain structures in addition to motoneurons. Indeed, α- and γ-motoneurons constitute only a very small part of spinal (inter-) neurons participating in any motor activity (Jankowska and Edgley 1993; Hultborn 2001; Poppele and Bosco 2003). For example, neuronal networks involved in ‘readiness’ in the control of voluntary movements (Bernstein 1967), including both diffuse and specific tuning effects (see also Evarts et al. 1984; Prochazka 1989; Gurfinkel et al. 1999), may influence the direction of gait following prolonged axial torsion. Changes in tuning at this level may activate specific ‘‘functional units’’ (Hultborn 2001) or muscle synergies (Bizzi et al. 2002) in the spinal cord. In addition, the specific properties of proprioceptive reflexes are context-dependent (Stuart 2002) and can potentially be influenced by the Kohnstamm phenomenon (Ribot-Ciscar et al. 1991). Selecting the appropriate muscle pattern for a particular motor task may be achieved by employing a modular architecture of neuronal networks (Hultborn 2001; Bizzi et al. 2002) and motor programs (Ivanenko et al. 2005). We believe that our findings on task-dependent aftereffects are consistent with this hypothesis. The activated rotational motor component might be appropriate for curving the walking trajectory but not appropriate for stepping in place movements since the two tasks may engage somewhat different muscle synergies for stepping and turning.
Acknowledgments
We thank Drs. W. Miller and G. Bosco for comments on the earlier version of the manuscript and V. Sabia for the subject’s drawing (Fig. 1a).
References
- Bernstein N (1967) The co-ordination and regulation of movements. Pergamon Press, Oxford
- Bizzi E, D’Avella A, Saltiel P, Tresch M. Modular organization of spinal motor systems. Neuroscientist. 2002;8(5):437–442. doi: 10.1177/107385802236969. [DOI] [PubMed] [Google Scholar]
- Courtine G, Schieppati M. Human walking along a curved path. II Gait features and EMG patterns. Eur J Neurosci. 2003;18(1):191–205. doi: 10.1046/j.1460-9568.2003.02737.x. [DOI] [PubMed] [Google Scholar]
- Craske B, Craske JD. Oscillator mechanisms in the human motor system: investigating their properties using the after-contraction effect. J Mot Behav. 1986;18(2):117–145. doi: 10.1080/00222895.1986.10735374. [DOI] [PubMed] [Google Scholar]
- Dostal WF, Soderberg GL, Andrews JG. Actions of hip muscles. Phys Ther. 1986;66(3):351–361. doi: 10.1093/ptj/66.3.351. [DOI] [PubMed] [Google Scholar]
- Duclos C, Roll R, Kavounoudias A, Roll JP. Long-lasting body leanings following neck muscle isometric contractions. Exp Brain Res. 2004;158(1):58–66. doi: 10.1007/s00221-004-1871-8. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Shinoda Y, Wise SP (1984) Neurophysiological approaches to higher brain functions. Wiley, New York
- Gilhodes JC, Gurfinkel VS, Roll JP. Role of Ia muscle spindle afferents in post-contraction and post-vibration motor effect genesis. Neurosci Lett. 1992;135(2):247–251. doi: 10.1016/0304-3940(92)90447-f. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. After-effects in the responses of cat muscle spindles and errors of limbs position sense in man. J Neurophysiol. 1988;59:1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Handbook of physiology. The nervous system. Motor control, sect 1, vol 2, part 1. Am Physiol Soc, MD, Bethesda, pp 1179–1236
- Gurfinkel VS, Levik YS, Lebedev MA. Immediate and remote postactivation effects in the human motor system. Neurofiziologia. 1989;21:247–253. [PubMed] [Google Scholar]
- Gurfinkel VS, Ivanenko YP, Levik YS (1990) Unusual mechanical behavior of skeletal muscles during a slow change in its length. In: Cherniy GG, Regirer SA (eds) Contemporary problems of biomechanics. Mir Publishers, CRC Press, Boston, pp 236–256
- Gurfinkel VS, Ivanenko YP, Levik YS, Kazennikov OV, Selionov VA (1999) The neural control of posture and locomotion: a lock with two keys. In: Gantchev GN, Mori S, Massion J (eds) Motor control today and tomorrow. Academic Publishing House, Sofia, pp 113–121
- Hagbarth KE, Nordin M. Postural after-contractions in man attributed to muscle spindle thixotropy. J Physiol. 1998;506:875–883. doi: 10.1111/j.1469-7793.1998.875bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. State-dependent modulation of sensory feedback. J Physiol. 2001;533:5–13. doi: 10.1111/j.1469-7793.2001.0005b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Cappellini G, Domimici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci. 2005;25(31):7238–7253. doi: 10.1523/JNEUROSCI.1327-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley S. Interactions between pathways controlling posture and gait at the level of spinal interneurones in the cat. Prog Brain Res. 1993;97:161–171. doi: 10.1016/s0079-6123(08)62274-8. [DOI] [PubMed] [Google Scholar]
- Kohnstamm O. Demonstration einer katatonieartigen erscheinung beim gesunden (katatonusversuch) Neurol Zentral Bl. 1915;34S:290–291. [Google Scholar]
- Mathis J, Gurfinkel VS, Struppler A. Facilitation of motor evoked potentials by postcontraction response (Kohnstamm phenomenon) Electroenceph Clin Neurophysiol. 1996;101:289–297. doi: 10.1016/0924-980x(96)95599-x. [DOI] [PubMed] [Google Scholar]
- Mori S, Kawahara K, Sakamoto T, Aoki M, Tomiyama T. Setting and resetting of level of postural muscle tone in decerebrate cat by stimulation of brain stem. J Neurophysiol. 1982;48:737–748. doi: 10.1152/jn.1982.48.3.737. [DOI] [PubMed] [Google Scholar]
- Poppele R, Bosco G. Sophisticated spinal contributions to motor control. Trends Neurosci. 2003;26(5):269–276. doi: 10.1016/S0166-2236(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Progr Neurobiol. 1989;3:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41(6):705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Tardy-Gervet MF, Vedel JP, Roll JP. Post-contraction changes in human muscle spindle resting discharge and stretch sensitivity. Exp Brain Res. 1991;86(3):673–678. doi: 10.1007/BF00230541. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Inman VT, Eberhart HD. The major determinants in normal and pathological gait. J Bone Joint Surg Am. 1953;35-A(3):543–558. [PubMed] [Google Scholar]
- Shik ML (1983) Action of the brainstem locomotor region on spinal stepping generators via propriospinal pathways. In: Kao CC, Bunge RP, Reier RJ (eds) Spinal cord reconstruction. Raven Press, New York, pp 421–434
- Stuart DG. Reflections on spinal reflexes. Adv Exp Med Biol. 2002;508:249–257. doi: 10.1007/978-1-4615-0713-0_30. [DOI] [PubMed] [Google Scholar]


