Abstract
This notes examines the mode of cell death of HL-60 cells exposed to 70 kHz and 1.3 W/cm2 in the presence of 1% Pluronic P105 and 1.67 μg/ml doxorubicin (Dox). The cells were ultrasonicated for 30, 60 and 120 minutes. They were then lysed, electrophorised, stained using propidium iodide, and their DNA profile captured using a fluorescent microscope. The gradual DNA damage observed and the comet tails captured after 1 and 2 hours of insonation suggest that the mode of cell killing is apoptosis.
Keywords: Micellar drug delivery, Doxorubicin, Comet Assay, Ultrasound, Apoptosis
1. Introduction
Our research group is investigating the use of Pluronic P105 micelles to deliver anthracycline agents or other drugs to cancerous tissues. Our previous publication has shown that no DNA damage was observed when cells were treated with 10 wt% P105 with and without exposure to ultrasound at a frequency and power density of 70 kHz and 1.3 W/cm2, respectively, for up to 3 hours [1]. In another set of experiments, DNA damage increased with incubation time when cell suspensions were incubated in the presence of 10 μg/ml free Dox. When cells were exposed to 10 μg/ml Dox in the presence of 10% P105 no significant DNA damage was observed for up to 9 hours of incubation. However, extensive DNA damage was observed within 1 h of exposure to ultrasound in the presence of Pluronic-encapsulated Dox. The damage increased as the insonation time increased up to 2 hours [1].
We have also used a custom ultrasonic exposure chamber with real-time fluorescence detection to measure the release of Dox and Ruboxyl (Rb, a paramagnetic analog of Dox) from Pluronic P105 micelles using low frequency ultrasound [2]. The amount of drug release increased as the ultrasonic power density increased and the frequency decreased. The observations reported by our group earlier suggest that Dox can be loaded in Pluronic P105 micelles and released when exposed to ultrasound. Based on these findings, a targeted drug delivery system aimed at reducing chemotherapy side effects by exposing cancerous tissues to ultrasonic power can be envisioned [3].
The single cell electrophoresis assay (comet assay) has been used extensively to detect DNA damage induced by hyperthermia and radiation [4-9]. It has been also used to measure DNA repair, supercoiling and replication [10, 11]. The principle behind this assay is that the negatively-charged broken DNA molecules are free to migrate in an electric field towards the anode, with the shorter fragments moving faster. The pattern of migration produces a profile resembling the shape of a comet. Two main principles determine comet formation patterns: the size of DNA fragments and the number of fragments. In this paper, the comet assay was used to examine the mode of cell death for the ultrasonic delivery of Dox to HL-60 cells from P105 micelles.
2. Materials and Methods
Cells:
HL-60 cells were cultured in RPMI medium supplemented with 10% fetal calf serum, 6 mM L-glutamine and 7.5% sodium bicarbonate. The culture was maintained in 75-ml plastic tissue flasks at 37°C in humidified 5% CO2 and was passaged every 3 to 4 days. For each experiment, 160 ml of cells were cultured for three days until they reached a density of about 106 cells/ml. The cells were concentrated by centrifugation (to a final concentration of 1x107 cells/ml) and were then resuspended in 10 ml of supplemented RPMI medium before treatment.
Drugs:
Doxorubicin in HCl (Sigma, St Louis, MO) was dissolved in phosphate buffered saline (PBS) and sterilized by filtration through a 0.2 μm filter.
Drug incorporation inside Pluronic micelles:
Pluronic P105 was obtained from BASF (New Jersey). To prepare a stock solution, P105 was dissolved in a phosphate buffered saline (PBS) solution to a final concentration of 20-wt% (20 grams P105 in 80 ml of PBS). The solution was then sterilized by filtration through a 0.2 μm filter. Dox was introduced into Pluronic P105 micellar solutions by mixing stock solutions at room temperature. A previous study has shown that Dox will accumulate in the hydrophobic core when mixed with Pluronic P105 [12]. Final Dox concentration was 1.67 μg/ml.
Ultrasonication:
Ultrasonic power was generated by a Sonicor SC-100 ultrasonicating bath (Sonicor Instr., Copaique, N. Y.) operating at 70 kHz. The insonation intensity was controlled by adjusting the input voltage using a variable A.C. transformer (variac) [13]. The insonation intensity as a function of applied voltage was determined using a calibrated hydrophone (Bruel and Kjaer model 8103, Decatur, GA). For subsequent experiments, the variac voltage was adjusted to produce an intensity of 1.3 W/cm2. Since we were not studying the effect of hyperthermia induced by insonation, the temperature of the bath was maintained at 37°C using a recirculating thermostatic bath. In this report, HL-60 cells were insonated (70 kHz, 1.3 W/cm2) in the presence of 1.67 μg/ml of Dox and 1% Pluronic P105 in PBS solution.
Ultrasonic intensities used in our experiments were in agreement with the optimum power densities suggested by Rapoport et al. [14-16]. Our experiments were conducted within the window of opportunity (what they refer to as the window of power densities). In their article [27], the group provides a range of power densities were Dox can be released from the core of unstabilized Pluronic P105 micelles without causing permanent cell damage as evidenced by cell lysis. At 70 kHz, the therapeutic range is reported to be between 1.2 W/cm2 and 2.3 W/cm2. Our experiments were conducted at a power density of 1.3 W/cm2, well within the suggested range.
Comet Assay:
The comet assay, performed as described by Fairbairn et al.[4], was used to determine the mode of cell death of ultrasonically delivered Dox to HL-60 cells from Pluronic P105 micelles [7]. A summary of the comet assay is given below.
Two hundred microliters of 106 cells/ml suspension were mixed with 600 μl of 1% low melting point agarose at 37°C (0.75% final concentration) and immediately layered on custom frosted slides which feature a clear centered window [17]. The slides were placed on a chilled plate to solidify the agarose. The slides were then bathed in freshly prepared lysing solution (2.5 M NaCl, 10 mM Tris-base, 0.1% sodium sarcosinate, pH = 12.3) in the dark for one hour at room temperature. After lysing, the slides were placed in alkaline electrophoresis buffer (0.3 N NaOH, 1 mM EDTA, pH = 12.3) for 30 minutes, allowing for salt equilibration and further DNA unwinding. Electrophoresis was performed at 20 V and 400 mA for 10 minutes. The slides were then immersed in a bath of distilled water (at room temperature) for five minutes in order to anneal the DNA. Then they were stained with propidium iodide (2.5 mg/ml) for 20 minutes and covered with a glass cover slip before analysis.
The image of the electrophoresed DNA appears like a comet, with undamaged DNA in the head, and fragmented DNA migrating to form a tail. Comet images were captured on an Axioscope fluorescent microscope equipped with a standard Photonics Camera.
3. Results and Discussion:
Figures 1, 2, 3, and 4 are comet figures obtained as a result of exposing HL-60 cells to ultrasound (70 kHz and 1.3 W/cm2) in the presence of 1% P105 and 1.67 μg/ml of Dox. Figure 1 is the control (DNA of untreated cells); no DNA breaks are apparent and the profile representing the nuclear DNA is round. Figure 2 is the DNA of a cell that has been ultrasonicated in the presence of encapsulated Dox for 30 minutes. The DNA damage is not substantial but a few broken fragments that migrated in the electric field are observed. More damaged is observed in Figure 3 where HL-60 cells were acoustically treated for 1 hour in the presence of encapsulated Dox. Figure 4 shows the nuclear DNA of a cell that has been ultrasonicated for 2 hours in the presence of encapsulated Dox. The DNA is completely fragmented and the profile of nuclear DNA is barely recognizable as evidenced by the low florescence observed in the comet head region.
Figure 1.

DNA of a live cell. No DNA damage is observed.
Figure 2.

After 30 minutes of sonication in the presence of encapsulated DOX, most of the nuclear DNA is intact but small pieces have migrated.
Figure 3.
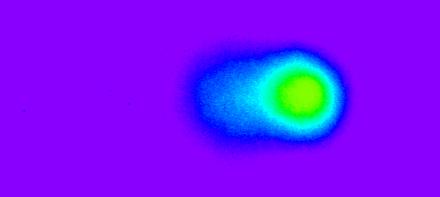
More DNA damage but the cells are still alive (1 hr of sonication in the presence of encapsulated DOX).
Figure 4.

The cell clearly dead by apoptosis. The DNA in the cell is fragmented and the nuclear DNA can barely be recognized (2 hours of sonication in the presence of encapsulated DOX).
There are two distinct pathways for the cell death process: apoptosis and necrosis. Apoptosis is a mechanism by which organisms maintain a balance between cell proliferation and cell death. It is defined as the programmed death of aging cells following a gene-directed mechanism characterized by “the biochemical activity of endogenous endonucleases acting at internucleosomal sites, producing a DNA ladder upon electrophoresis” [7].
The other form of cell death is necrosis, which is caused by subjecting cells to severe environmental stress. Necrotic cells usually swell until their cell membranes rupture [6, 7]. DNA profiles shown in Figures 2, 3, and 4 provide strong evidence that the DNA damage is caused by apoptosis [5, 7]. Figure 4 shows that the majority of the DNA content migrated towards the tail. In necrosis, the majority of the damage remains in the comet head, although smaller DNA fragments can migrate the same distance as that encountered in apoptosis DNA profiles shown in Figures 2, 3, and 4 provide strong evidence that the DNA damage is caused by apoptosis [5, 7].
In this article, our conclusions are based on the shape of the comet in addition to the amount of DNA fragmentation. Our previous publication focused on the amount of DNA damage induced as a result of exposing HL-60 cells to a combination of ultrasound, doxorubicin and pluronic micelles [1]. Here, we focus on the shape of the comet to deduce the mode of cell death. Several articles have been published showing that tail moments and comet shapes can be used to differentiate between an apoptotic population and a necrotic one [5,7].
Dox is also known to induce apoptosis in cancer cells and therefore plays an important role in the mechanism of cell death [18, 19]. The anti-neoplastic agent is among the most common chemotherapy drugs that belong to the anthracycline family [20]. Anthracycline drugs are topoisomerase I and II inhibitors that intercalate between paired bases of the DNA, affecting many of the cell functions including DNA replication and RNA transcription [21]. We have reported earlier that incubation and ultrasonication with free Dox causes substantial DNA damage to HL-60 cells [1].
With respect to the mechanism of the enhanced cell killing by ultrasound, we have reported that ultrasonic power releases Dox from P105 micelles [2, 22, 23]. This release causes an increase in the amount of Dox that penetrates the cells. We have already reported a decrease in cell viability (trypan blue exclusion) and an increase in DNA damage (comet assay) when incubated and insonated cells were assayed after exposure to 10 μg/ml of free and P105 encapsulated Dox [1]. The rate of DNA damage was higher for the insonated than for incubated cells. Several reports in literature have shown that ultrasound forms holes or pores in cells by at least two mechanisms: microconvection currents and shock waves. Microconvection currents are created as a result of gas bubbles oscillating in liquid media. This phenomenon is called cavitation. The gas bubbles originate as dissolved nucleates on impurities in the liquid [24]. As a result of the low- and high-pressure phases of ultrasound, the bubbles oscillate by growing and shrinking in size. At greater ultrasonic power densities, these bubbles collapse completely during the shrinking cycle; this phenomenon is known as transient cavitation. As these bubbles implode the local temperature increases by thousands of degrees (due to adiabatic compression), resulting in the formation of free radicals and the generation of a shock wave [25,26]. Shock waves are created as a result of bubbles collapsing near the cell membrane. The collapse causes a high velocity ‘micro-jet’ of liquid to be directed towards the cell surface [27].
Thus, microconvention currents and shock waves are the primary mechanisms involved in the formation of membrane pores as a result of the application of ultrasound, a phenomenon known as sonoporation. These pores are caused by disturbances in the phospholipid bilayer of the cell membrane. By causing temporary pores to form in the cell membrane, the rate of encapsulated and free DOX diffusion into the cell is enhanced, which leads to more drug available to interact with the nuclear contents, causing more DNA breaks. Saito et al. reported that exposure to ultrasound induces an increase in plasma membrane permeability of corneal endothelium cells [28]. This increase in permeability is reversible as the cells regained their membrane integrity after several minutes. In another study by Tachibana [29], HL-60 cells exposed to ultrasonic waves (255 kHz and 0.4 W/cm2 for 30 seconds in the presence of a photosensitive drug (15 μg/ml of merocyanine 540) showed “multiple surface pores” and “dimple like craters” when examined using a scanning electron microscope. In some cells, the cytoplasm seemed to have extruded through pores formed in the cell membrane as a result of sonoporation [29]. When exposed to ultrasound alone, the cells showed minor disruptions in the cell membrane. Neither ultrasound nor merocyanine alone showed any cytotoxic effect on cell viabilities (95% and 96% respectively). The cell viability decreased to 47% when exposed to both ultrasonic irradiation and merocyanine. These studies suggest that the higher rate of DNA damage in HL-60 cells observed in sonicating free and encapsulated Dox is due to a rapid sonoporation process during ultrasonic exposure in the presence of the chemotherapeutic agent.
In summary, the figures presented above suggest that the mode of cell death in ultrasonically triggered micellar drug delivery is apoptosis, rather than necrosis.
Abbreviations
- (Dox)
Doxorubicin
- (Rb)
Ruboxyl
- (PBS)
Phosphate buffered saline
- (HL-60)
Human Leukemia cell line
References
- 1.Husseini GA, et al. DNA damage induced by micellar-delivered doxorubicin and ultrasound: comet assay study. Cancer Lett. 2000;154:211–216. doi: 10.1016/s0304-3835(00)00399-2. [DOI] [PubMed] [Google Scholar]
- 2.Husseini GA, et al. Factors Affecting Acoustically-Triggered Release of Drugs from Polymeric Micelles. J. Contr. Release. 2000;69:43–52. doi: 10.1016/s0168-3659(00)00278-9. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JL, et al. Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Res. 2002;62:7280–7283. [PubMed] [Google Scholar]
- 4.Fairbairn DW, Olive PL, O'Neill KL. The comet assay: a comprehensive review. Mutation Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- 5.Fairbairn DW, O'Neill KL. Letter to the Editor: Necrotic DNA Degradation Mimics Apoptotic Nucleosomal Fragmentation Comet Tail Length. In Vitro Cell. Dev. Biol. 1995;31:171–173. doi: 10.1007/BF02639429. [DOI] [PubMed] [Google Scholar]
- 6.Fairbairn DW, Neill KLO. The neutral comet assay is sufficient to identify an apoptotic 'window' by visual inspection. Apoptosis. 1996;1:91–94. [Google Scholar]
- 7.Fairbairn DW, et al. Key Morphologic Changes and DNA Strand Breaks in Human Lymphoid Cells: Discriminating Apoptosis from Necrosis. Scanning. 1996;18:407–416. doi: 10.1002/sca.1996.4950180603. [DOI] [PubMed] [Google Scholar]
- 8.Vijayalaxmi, Tice RR, Strauss GHS. Assessment of radiation-induced DNA damage in Human blood lymphocytes using single-cell electrophoresis technique. Mutation Res. 1992;271:243–252. doi: 10.1016/0165-1161(92)90019-i. [DOI] [PubMed] [Google Scholar]
- 9.Vijayalaxmi, Strauss GHS, Tice RR. An analysis of γ- ray induced DNA damage in human blood leukocytes, lymphocytes and granulocytes. Mutation Res. 1993;292:123–128. doi: 10.1016/0165-1161(93)90139-q. [DOI] [PubMed] [Google Scholar]
- 10.Vogelstein B, P.D. M, Coffey DS. Supercoiled Loops and Eucaryotic DNA Replication. Cell. 1980;22:79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh NP, et al. A microgel electrophoresis technique for the direct quantitation of DNA damage and repair in individual fibroblasts cultured on microscope slides. Mutation Res. 1991;252:289–296. doi: 10.1016/0165-1161(91)90008-v. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport N, Pitina L. Intracellular distribution and intracellular dynamics of a spin-labeled analog of Doxorubicin. J. Pharm. Sci. 1998;87(3):321–325. doi: 10.1021/js970271g. [DOI] [PubMed] [Google Scholar]
- 13.Qian Z, Sagers RD, Pitt WG. The role of insonation intensity in acoustic-enhanced antibiotic treatment of bacterial biofilms. Colloids and Surfaces B: Biointerfaces. 1997;9:239–245. [Google Scholar]
- 14.Rapoport N, Marin A, Christensen DA. Ultrasound-Activated Drug Delivery. Drug Delivery Systems and Sciences. 2002;2:37–46. [Google Scholar]
- 15.Marin A, Muniruzzaman M, Rapoport N. Mechanism of the ultrasound activation of micellar drug delivery. J. Control. Rel. 2001;75:69–81. doi: 10.1016/s0168-3659(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport N. Factors Affecting Ultrasound Interactions with Polymeric Micelles and Viable Cells. in: Carrier-Based Drug Delivery. In: Swenson S, editor. ACS Symposium Series. Vol. 879. Washington, DC: 2004. pp. 161–173. [Google Scholar]
- 17.Smith MJ, O'Neill KL. Microscope slides for enhanced analysis of DNA damage using comet assay. Biotechniques. 1998;25:49–50. doi: 10.2144/98251bm10. [DOI] [PubMed] [Google Scholar]
- 18.Grassilli E, et al. Loss of MYC Confers Resistance to Doxorubicin-induced Apoptosis by Preventing the Activation of Multiple Serine Protease- and Caspase-mediated Pathways. The journal of Biological Chemistry. 2004;279(20):21318–21326. doi: 10.1074/jbc.M313532200. [DOI] [PubMed] [Google Scholar]
- 19.Yeh YY, et al. Phosphorylation of p53 on Thr55 by ERK2 is necessary for doxorubicin-induced p53 activation and cell death. Oncogene. 2004;23:3580–3588. doi: 10.1038/sj.onc.1207426. [DOI] [PubMed] [Google Scholar]
- 20.Gilman AG, et al. 9th McGraw-Hill; New York: 1996. Goodman and Gilman's the Pharmacological Basis of Therapeutics. [Google Scholar]
- 21.Nip J, et al. E2F-1 cooperates with topoisomerase II inhibition and DNA damage to selectively augment p53-independent apoptosis. Mol. Cell Biol. 1997;17(3):1049–1056. doi: 10.1128/mcb.17.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husseini GA, et al. Kinetics of ultrasonic release of doxorubin from pluronic P105 micelles. Coll Surf B: Biointerfaces. 2002;24:253–264. [Google Scholar]
- 23.Husseini GA, et al. Ultrasonic release of doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J. Controlled Rel. 2002;83(2):302–304. doi: 10.1016/s0168-3659(02)00203-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Lewis TN, Prausnitz MR. Non-Invasive Assessment and Control of Ultrasound-Mediated Membrane Permeabilization. Pharm. Res. 1998;15(6):918–924. doi: 10.1023/a:1011984817567. [DOI] [PubMed] [Google Scholar]
- 25.Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423(6936):153–156. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- 26.Tomita Y, Shima A. High-speed photographic observations of laser-induced cavitation bubbles in water. Acustica. 1990;71(3):161–171. [Google Scholar]
- 27.Rooney JA. Hemolysis Near an Ultrasonically Pulsating Gas Bubble. Science. 1970;169:869–871. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- 28.Saito K, et al. Plasma Membrane Disruption Underlies Injury of the Corneal Endothelium by Ultrasound. Exp. Eye Res. 1999;68:421–427. doi: 10.1006/exer.1998.0626. [DOI] [PubMed] [Google Scholar]
- 29.Tachibana K, et al. Enhanced cytotoxic effect of Ara-C by low intensity ultrasound to HL-60 cells. Cancer Lett. 2000;149:189–194. doi: 10.1016/s0304-3835(99)00358-4. [DOI] [PubMed] [Google Scholar]


