Abstract
Micelle-like nanoparticles that could be used as drug delivery carriers were developed. The unique feature of these nanoparticles was that the core of poly(ethylene oxide)-b-poly(N-isopropylacrylamide) (PEO-b-PNIPAAm) micelle was lightly crosslinked with a biodegradable crosslinker N,N-bis(acryloyl)cystamine (BAC). The nanoparticles were characterized by dynamic light scattering and fluorescence measurements. When the BAC was from 0.75 wt% to 0.2 wt% of the mass of NIPAAm, the diameters of the nanoparticles were less than 150 nm. The anti-cancer drug doxorubicin (Dox) and 1,6-diphenyl-1,3,5-hexatriene (DPH) were used as fluorescent probes to study the hydrophobicity of the cores of the nanoparticles; the results showed that the cores of the nanoparticles were hydrophobic enough to sequester Dox and DPH. The nanoparticles with 0.5 wt% BAC stored at room temperature were stable up to two weeks, even at dilute concentrations. The degradation of BAC by reducing agent β-mercaptoethanol was investigated, and the nanoparticles were not detectable 14 days after adding β-mercaptoethanol.
Keywords: Nanoparticle, poly(ethylene oxide)-b-poly(N-isopropylacrylamide), N, N-bis(acryloyl)cystamine, crosslink, doxorubicin
Introduction
Amphiphilic copolymers with poly(ethylene oxide) (PEO) as hydrophilic segments have attracted much interest over the recent years because they often self–assemble into core–shell structures in aqueous solutions and thus form polymer micelles [1–5]. PEO has unique biocompatible properties, such as low protein adsorption and cell adhesion. It has also been shown that proteins and drugs coupled to PEO have enhanced lifetime in the bloodstream [6,7]. Such studies indicate that PEO is an excellent material for use in polymer drug carrier systems.
During the past decade, block copolymers of PEO and poly(N-isopropylacrylamide) (PNIPAAm) have been synthesized and studied [3,8,9]. PNIPAAm, one of the most studied thermosensitive polymers, has a lower critical solution temperature (LCST) of about 32°C in aqueous solution [10,11]. The polymer changes from hydrophilic to hydrophobic when the solution temperature increases through the LCST. In addition, the LCST of PNIPAAm in PEO-b-PNIPAAm copolymer decreases 2–3°C due to the hydrophilic PEO segment [8]. Because of this thermal transition of PNIPAAm, PEO-b-PNIPAAm copolymers are soluble in water at room temperature but form polymer micelles at temperatures above the LCST of the PNIPAAm. These polymer micelles have potential as drug carriers, but they would eventually dissolve upon dilution in blood, even above the LCST.
In previous work in our lab, Pruitt et al. synthesized a stabilized drug carrier called Plurogel® [5,12], and it was successfully used with ultrasound to treat tumors in rats [13]. In this study, the cores of the PEO-b-PNIPAAm micelles were lightly crosslinked with a biodegradable crosslinker N, N-bis(acryloyl)cystamine to form micelle-like nanoparticles that are stable upon dilution. We anticipate that these nanoparticles have the appropriate size and temporal stability, and thus could also be employed as an ultrasonically activated drug carrier.
Experiment
Materials
Methoxypoly(ethylene glycol), N-isopropylacrylamide, ammonium cerium nitrate and N,N-bis(acryloyl)cystamine were obtained from Sigma-Aldrich and used without further purification. Doxorubicin hydrochloride was obtained from Pharmacia & Upjohn Company, Kalamazoo MI in dosage form. 1,6-diphenyl-1,3,5-hexatriene was obtained from Molecular Probes (Fugene, Oregon). Pluronic® P105 was kindly provided by BASF Corp, NJ.
Particle Synthesis
Polymerization was done in a round bottom flask connected to a water condenser and a nitrogen purge. 20 mL of double-distilled water was poured into the flask, stirred magnetically, and purged with nitrogen for two hours at 60°C to remove oxygen. Subsequently, 1 g methoxypoly(ethylene glycol), 0.476 g N-isopropylacrylamide (NIPAAm) and measured amounts of N,N-bis(acryloyl)cystamine (BAC) were added. After 5 minutes purging, 0.376 g ammonium cerium nitrate in 4 mL of 1 M nitric acid was added. After 4 hours of reaction, the solution was cooled to room temperature and 1 M NaOH solution was added to precipitate out the cerium salts [8]. The supernate of the reaction (containing copolymer) was centrifuged 0.5 hour (10,000 rpm, Eppendorf 5415C) at 40°C to separate the unreacted PEO. The precipitated copolymer products were dissolved with double-distilled water to form a solution with a concentration of 2.0±0.1 mg/mL. Some solutions were stored at room temperature, some were stored at 37°C, and others were frozen.
The BAC concentration was varied in several experiments from 20 wt% of that of the NIPAAm monomer to 2.0, 1.5, 1.0, 0.75, 0.5, 0.2 and 0.1wt%.
Measurements
The LCSTs of the copolymer systems at 2.0 mg/mL and 0.2 mg/mL solutions were quantified by measuring their absorbance of 600 nm light at various temperatures with a Beckman Coulter DU 640 spectrophotometer.
Sizes of the nanoparticles were measured by dynamic light scattering (DLS) with a Brookhaven 90Plus submicron particle size analyzer. Measurements were performed at scattering angle 90º. The CONTIN algorithm that gives the intensity distribution was used to analyze data. For each sample, 3–6 measurements were taken and each measurement took 2 minutes. The average count rate of the background was 15 kcps and that of each measurement was between 200~ 500 kcps. The temperature was controlled at 40°C or room temperature (about 20°C).
Fluorescence measurements were carried out at 40°C to study the nanoparticle stabilities and microenvironments of the nanoparticle cores. A Perkin-Elmer LS50B luminescence spectrometer was used with 1,6-diphenyl-1,3,5-hexatriene (DPH) or an anti-cancer drug doxorubicin (Dox) as the fluorescent probe. A stock solution of DPH or Dox in tetrahydrofuran (THF) was added to an empty glass vial, and the THF was evaporated. The copolymer solution with different nanoparticle concentration was then added to the vial. The vial was kept in a water bath at 40°C for 0.5 hour to thoroughly dissolve the fluorescent probe. The concentration of DPH or Dox in each sample was 0.1 or 40 μg/mL respectively. Fluorescence intensity was measured with λexcitation = 360 nm and λemission= 430 nm for DPH, and λexcitation = 488 nm and λemission = 590 nm for Dox.
Results
During PEO-b-PNIPAAm copolymerization, micelles commenced formation by 3–5 minutes following the addition of the ammonium cerium nitrate, as indicated by a color change from orange to white, which is consistent with the report of Topp et al. [8]. Because the biodegradable crosslinker BAC is hydrophobic, we assume it was sequestrated into micelle cores and was copolymerized with the NIPAAm, thus crosslinking the cores of the micelles.
Particle Size
After resuspending the PEO-b-PNIPAAm-BAC nanoparticles made with 20 wt% or 2 wt% BAC, a white flocculate was always apparent and settled after several days. Thus no particle size analysis was done for these higher concentrations. For the nanoparticles with less than 2.0 wt% BAC, the stock solutions (in which the nanoparticle concentrations were 2.0 mg/mL) were subsequently diluted to 0.2 mg/mL and 0.02 mg/mL. Figure 1 shows the diameters of the particles at all three dilutions measured by DLS. The nanoparticles with 1.5 wt% BAC or 1.0 wt% BAC had diameters over 200 nm at all three dilutions, indicating that large particles had formed. The diameters of the nanoparticles with BAC amounts from 0.75 wt% to 0.2 wt% were less than 150 nm and were detectable at all three dilutions. The nanoparticles with 0.1 wt% BAC were not detectable at concentrations lower than 0.2 mg/mL, indicating that not enough crosslinking occurred to prevent particles from dissolving upon dilution. Topp et al. reported that the diameters of PEO-b-PNIPAAm micelles varied from 61 nm to 117 nm at 37°C and depended on the molar ratio of EO and NIPAAm repeat units in the copolymer [8]. Thus the nanoparticles stabilized with 0.75% to 0.20% BAC are comparable in size to the micelles reported by Topp.
Figure 1.
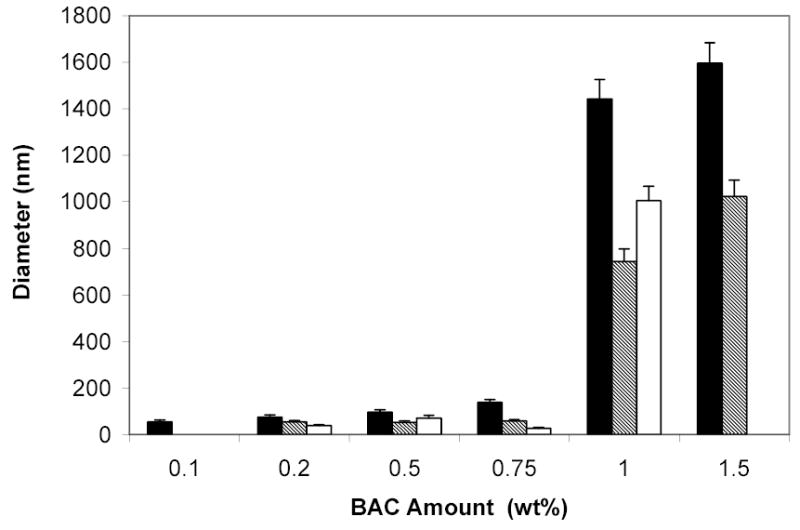
Diameters of PEO-b-PNIPAAm-BAC particles with different amounts of BAC upon dilution at 40°C. The black bar represents data at 2 mg/mL solution, the hatched bar represents data at 0.2 mg/mL dilution, and the white bar represents 0.02 mg/mL dilution. Repeatable results were not obtained for the particles with 1.5 wt% BAC in 0.02 mg/mL solution and thus are not shown. Error bars represent the standard deviations (n=3).
As mentioned earlier, PEO-b-PNIPAAm copolymers are soluble in water at room temperature and form polymer micelles at temperatures above the LCST of the PNIPAAm. Quantified from measuring their absorbance of 600 nm light at various temperatures, the LCSTs of the PEO-b-PNIPAAm-0.5 wt% BAC nanoparticles at 2.0 mg/mL and 0.2 mg/mL solutions were 29 ± 1°C. It is postulated that PEO-b-PNIPAAm-BAC particles also form a micelle-like structure at temperatures above the LCST of the PNIPAAm (such as during polymerization and during subsequent experiments at 40°C), but would not totally dissolve at room temperature due to the crosslinked cores. However, cooling below the LCST would cause the PNIPAAm core to expand and the observed hydrodynamic diameters would increase. This hypothesis is supported by the data presented in Figure 2. At room temperature, PEO-b-PNIPAAm-0.5 wt% BAC particles were detectable at concentrations as low as 0.002 mg/mL, and their diameters increased 37–102% compared to the particles at same concentration 40°C. The particles in 0.002 mg/mL solution showed the largest increase in diameter, perhaps because the driving force to disassociate the crosslinked particle caused a large uptake of water.
Figure 2.

Diameters of PEO-b-PNIPAAm-0.5 wt% BAC particles upon dilution at 20 and 40°C. The white bar represents data at 40°C and the black bar represents data at 20°C. Error bars represent the standard deviations (n=3).
Figure 3 shows the changes in the nanoparticle diameters over time. The experiments were carried out over one month and the samples were stored at room temperature, so the PNIPAAm core was expanded to provide driving force for dissolution. In general, the nanoparticle diameters decreased with time, although the diameters of particles at 0.2 mg/mL dilution are not significantly different at 3 and 4 weeks (p>0.05). The nanoparticles in 0.002 or 0.02 mg/mL solutions became undetectable at three or four weeks respectively, indicating they degraded within these time periods. Nanoparticles that were stored at 37°C or frozen showed no change in diameter during 4 weeks of storage (data not shown).
Figure 3.
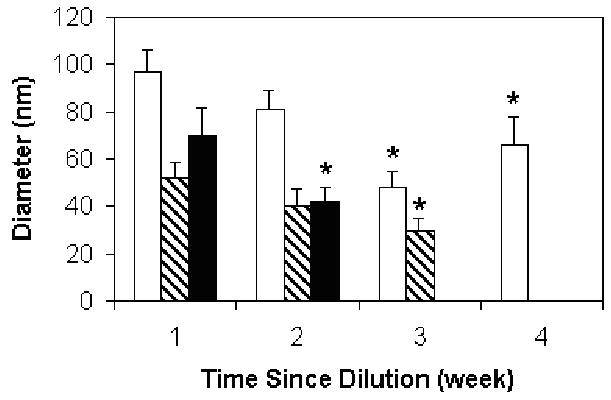
Diameters of PEO-b-PNIPAAm-0.5 wt% BAC nanoparticles as a function of time. The data was measured at 40°C. The white bar indicates 0.2 mg/mL dilution, the hatched bar indicates 0.02 mg/mL dilution and the black bar indicates 0.002 mg/mL dilution. Error bars represent the standard deviations (n=3). * indicates samples with diameters statistically less than the value at 1 week.
Fluorescence Measurement
DPH and Dox are hydrophobic compounds and have fluorescent emissions at 430 nm and 590 nm respectively. Their emission intensities depend strongly on the hydrophobicity of the local environment. DPH has almost no fluorescence in an aqueous solution while it is highly fluorescent in a hydrophobic environment. Dox has less fluorescence in water than in a hydrophobic environment. When the probes are mixed with micelle solutions, they are trapped in the hydrophobic cores of the micelles, and their emission intensities will report the hydrophobic nature of the cores. Once the micelle loses its stability and dissolves, Dox or DPH will be released to the aqueous environment and the emission intensity will decrease or go to zero, respectively. Thus we can tell how fast the micelles are dissolving.
The DPH emission intensities of PEO-b-PNIPAAm-BAC nanoparticles with different amount of BAC at 40°C are shown in Figure 4. The BAC concentration was varied from 0 wt% to 0.2, 0.5, 0.75, 1.0 and 1.5 wt%. The data show that the fluorescent intensity increased as the amount of crosslinker BAC increased. Such data supports the hypothesis that as the ratio of BAC to NIPAAm increased, the distance between crosslink points decreased, and the crosslinked network became tighter. We speculate that less water was able to penetrate into the core, and thus there was less quenching of the fluorescence. Pruitt et al. reported that the DPH emission intensities of the stabilized Pluronic® P105 micelles (called Plurogel®), in 1 mg/mL and 0.1 mg/mL solutions with same DPH concentration (0.1μg/mL) were 20 and 3 respectively [5]. Compared with Pruitt’s results, the PEO-b-PNIPAAm-BAC nanoparticles with more than 1 wt% BAC provides similar hydrophobic core environments as Plurogel®.
Figure 4.

DPH emission intensities of water and PEO-b-PNIPAAm-BAC nanoparticles with different amounts of BAC at 40°C. The diamond represents 2 mg/mL solution, the square represents 0.2 mg/mL solution, and the horizontal line, just above zero, represents the emission intensity of DPH in water.
Figure 5 shows the DPH emission intensities of PEO-b-PNIPAAm-0.5 wt% BAC particles at different concentrations at 40°C. The DPH emission intensities decreased almost linearly upon dilution, indicating that no significant micelle degradation occurred upon dilution. In contrast to Plurogel®, whose DPH emission intensities in 0.1 and 0.01 mg/mL solutions with same DPH concentration were both greater than 1, the DPH emission intensities of PEO-b-PNIPAAm-0.5 wt% BAC particles at the same concentrations were less than 1 [5]. These suggested that the cores of PEO-b-PNIPAAm-0.5 wt% BAC particles are less hydrophobic than the cores of Plurogel® at a similar dilute concentration. This might occur because the hydrophobic part of PEO-b-PNIPAAm is PNIPAAm while the hydrophobic part of P105 is poly(propylene oxide) (PPO), and PNIPAAm is less hydrophobic than PPO.
Figure 5.

DPH emission intensities of water and PEO-b-PNIPAAm-0.5 wt% BAC nanoparticles at 40°C. The horizontal dotted line is the emission intensity of 0.1 μg/mL DPH in water.
When the solution concentration was diluted to 0.001 mg/mL, the emission intensity of the particles was only slightly above the emission intensity observed for a saturated solution of DPH in distilled water.
The Dox emission intensities at 40°C of PEO-b-PNIPAAm-0.5 wt% BAC particles and unstabilized Pluronic® P105 micelles at different concentrations are shown in Figure 6. For unstabilized P105, there was a sharp drop of the emission intensity when the concentration was lower than 1 mg/mL. This is because the critical micelle concentration (CMC) of unstabilized P105 at 40°C is around 1 mg/mL [5]. The Dox was released from the micelles into aqueous solution and was quenched. For PEO-b-PNIPAAm-0.5 wt% BAC nanoparticles, the emission intensities decreased upon dilution gradually. At concentrations below 0.2 mg/mL, they have higher emission intensities than unstabilized Pluronic® P105 micelles. These observations indicate the stabilized particles did not dissolve at low concentrations and are consisting with the data shown in Figure 5.
Figure 6.

Emission intensities of 40 μg/mL Dox in water (dotted line), unstabilized Pluronic® P105 (dashed line) and PEO-b-PNIPAAm-0.5 wt% BAC nanoparticles (solid line) at 40°C.
One of the reasons BAC was chosen as the crosslinker is that it contains a disulfide bond that can be broken by reducing agents such as glutathione possessed by cells at physiological conditions. To test the degradability of BAC, a reducing agent β-mercaptoethanol was added to 2 and 0.2 mg/mL PEO-b-PNIPAAm-0.5 wt% BAC solution. The molar ratio of β-mercaptoethanol to BAC was 10:1. The solutions were stored at 37°C. Fluorescence tests were carried out immediately following addition, then 7 and 14 days after adding β-mercaptoethanol. The results are shown in Figure 7. After β-mercaptoethanol was added, DPH emission intensities of the particles with 0.5 wt% BAC in both solution concentrations decreased with time gradually and reached the values of the particles without BAC at corresponding concentrations after 14 days. Without β-mercaptoethanol in the solutions, DPH emission intensities of the nanoparticles with 0.5 wt% BAC decreased only slightly after 14 days in both concentrations. These data indicate that BAC can be degraded by β-mercaptoethanol and the particle appears to be totally degraded after 14 days.
Figure 7.
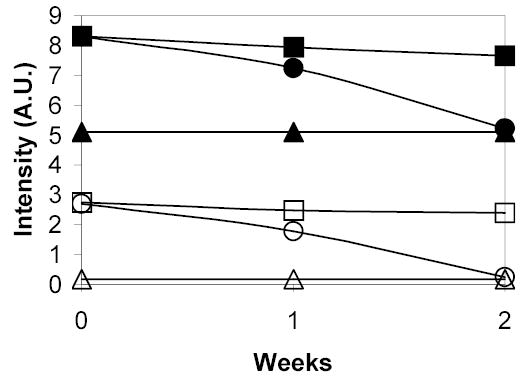
Emission intensities of 0.1 μg/mL DPH in PEO-b-PNIPAAm-0.5 wt% BAC nanoparticles at 40°C with or without reducing agent β-mercaptoethanol as a function of time. The solid symbols indicate 2 mg/mL solution and the open symbols indicate 0.2 mg/mL dilution. The squares represent data with no β-mercaptoethanol, the circles represent data with 10:1 molar ratio β-mercaptoethanol and the triangles represent data for non-stabilized PEO-b-PNIPAAm.
Discussion
Micelle-like nanoparticles PEO-b-PNIPAAm-BAC have been synthesized as potential drug delivery carriers. Synthesis was achieved by copolymerization of NIPAAm with the crosslinker BAC onto PEO polymers. The ratio of BAC to NIPAAm in polymerizations was varied over a wide range.
The diameters of PEO-b-PNIPAAm-BAC nanoparticles measured by DLS varied with the amount of BAC (Figure 1). The physical properties of the nanoparticles could be categorized into four groups. Nanoparticles polymerized with 2 wt% or greater BAC never dissolved in water even below the LCST of PNIPAAm. We speculate that the high amount of BAC rendered the core sufficiently hydrophobic that the resulting micelles were hydrophobic at room temperature and they coagulated. It is also possible that at these high concentrations of BAC, some crosslinking occurred outside of the micelle core, and could have linked the nanoparticles together during polymerization.
Nanoparticles with BAC amount from 1.5 wt% to 1.0 wt% had diameters larger than 200 nm. These larger sizes may also be due to aggregation of particles or interparticle crosslinking happening during copolymerization, but perhaps at a lesser extent than above 2 wt% BAC.
In the range of 0.75 wt% and 0.2 wt% BAC, the nanoparticle sizes were less than 150 nm. This was about the same size as non-crosslinked micelles and as Plurogel® particles [5]. This BAC concentration range appears to be ideal for producing small stable micelles. Below this range the micelles were not stable upon dilution. Stable micelles of less than 100 nm would be suitable for IV administration of drug carries [14]. In addition, these nanoparticles are biocompatible because they would eventually degrade.
Particle size analysis and fluorescence measurement showed that with a suitable amount of the crosslinker BAC, temporally stable PEO-b-PNIPAAm-BAC nanoparticles could be obtained. The nanoparticles appear to be stable up to two weeks even at dilute concentrations (Figure 3). This is much longer than Plurogel® which has a half-life in dilute aqueous solution of only about 18 hours [5]. During degradation, the diameters of the nanoparticles at different solution concentrations decreased with time. This slow degradation (in the absence of β-mercaptoethanol) of large nanoparticles might have occurred because some PEO-b-PNIPAAm copolymers forming the micelles during polymerization might not have been crosslinked, but only been entangled with other copolymers within the micelles. When the solutions were stored at room temperature, the non-crosslinked-copolymer dissolved into aqueous solutions gradually.
Because the diameters of diluted nanoparticles decreased over time (Figure 3), it appears that some copolymer diffused out and did not remain with the nanoparticles even at 40°C, above the LCST. However, in the absence of diffusional driving forces, such as when storing undiluted samples at 37°C (above the LCST) or below the freezing point, the diameters did not decrease in size during 4 weeks of storage.
As mentioned earlier, Dox is not only a fluorescent probe but also an anti-cancer drug. The fluorescence measurement showed that PEO-b-PNIPAAm-BAC nanoparticles sequester Dox (Figure 6). Future experiments will show whether Dox can be released from these nanoparticles using ultrasound [15,16]. Furthermore, the particles are stable for up to two weeks even when stored at room temperature and are eventually biodegradable so that they do not build up in the body (Figure 7). All these properties make PEO-b-PNIPAAm-BAC nanoparticles a potential candidate for anti-cancer drug delivery.
Acknowledgments
Financial support from Chemical Engineering Department of Brigham Young University, NIH (CA 76562) and CIBA Vision are gratefully acknowledged. The authors also wish to thank Dr. D. Woodbury from Department of Physiology & Developmental Biology of Brigham Young University for his assistance with dynamic light scattering.
References
- 1.Zhu PW, Napper DH. Aggregation of block copolymer microgels of poly(N-isopropylacrylamide) and poly(ethylene glycol) Macromolecules. 1999;32:2068–2070. [Google Scholar]
- 2.Virtanen J, Holappa S, Lemmetyine H, Tenhu H. Aggregation in aqueous poly(n-isopropylacrylamide)-block -poly(ethylene oxide) solutions studied by fluorescence spectroscopy and light scattering. Macromolecules. 2002;35:4763–4769. [Google Scholar]
- 3.Maeda Y, Taniguchi N, Ikeda I. Changes in the hydration state of a block copolymer of poly(N-isopropylacrylamide) and Poly(ethylene oxide) on thermosensitive micellization in water. Macromol Rapid Commun. 2001;22:1390–1393. [Google Scholar]
- 4.Hagan SA, Coombes AGA, Garnett MC, Dunn SE, Davies MC, Illum L, Davis SS. Polylactide-poly(ethylene glycol) copolymers as drug delivery stystems. 1. characterization of water dispersible micelle-forming systems. Langmuir. 1996;12:2153–2161. [Google Scholar]
- 5.Pruitt JD, Husseini G, Rapoport N, Pitt WG. Stabilization of Pluronic P-105 Micelles with an Interpenetrating Network of N,N-Diethylacrylamide. Macromolecules. 2000;33:9306–9309. [Google Scholar]
- 6.Dubruel P, Schacht E. Effect of polyethylene oxide blocks or grafts on the physicochemical properties of poly(2-N-(dimethylaminoethyl) methacrylate) DNA complexes. Journal of Bioactive and Compatible Polymers. 2000;15:279–296. [Google Scholar]
- 7.Veronese FM, Caliceti P, Schiavon O. Branched and Linear Poly(Ethylene Glycol): Influence of the Polymer Structure on Enzymological, Pharmacokinetic, and Immunological Properties of Protein Conjugates. Journal of Bioactive and Compatible Polymers. 1997;12:196–207. [Google Scholar]
- 8.Topp MDC, Dijkstra PJ, Talsma H, Feijen J. Thermosensitive micelle-forming block copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) Macromolecules. 1997;30:8518–8520. [Google Scholar]
- 9.Topp MDC, Leunen IH, Dijkstra PJ, Tauer K, Schellenberg C, F J. Quasi-living polymerization of N-isopropylacrylamide onto poly(ethylene glycol) Macromolecules. 2000;33:4986–4988. [Google Scholar]
- 10.Yoshioka H, Mikami M, Mori Y, Tsuchida E. Preparation of poly(N-isopropylacrylamide)-b-poly(ethylene glycol) and calorimetric analysis of its aqueous solution. Pure Appl Chem. 1994;A31:109–112. [Google Scholar]
- 11.Virtanen J, Tenhu H. Thermal Properties of Poly(N-isopropylacrylamide)-g-poly(ethylene oxide) in Aqueous Solutions: Influence of the Number and Distribution of the Grafts. Macromolecules. 2000;33:5970–5975. [Google Scholar]
- 12.Rapoport N. Stabilization and activation of Pluronic micelles for tumor-targeted drug delivery. Colloids and Surfaces B: Biointerfaces. 1999;16:93–111. [Google Scholar]
- 13.J. L. Nelson. In Chemical Engineering; Brigham Young University: Provo, UT, 2002; p 79.
- 14.Ha TS, Kim D. Drug releasing characteristics of PDLLA-MPEG DI- and MPEG-PDLLA-MPEG triblock copolymer micelles. JMS-Pure Appl Chem. 1999;A36:1031–1044. [Google Scholar]
- 15.Husseini GA, Christensen DA, Rapoport NY, Pitt WG. Ultrasonic release of doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J Controlled Rel. 2002;83:302–304. doi: 10.1016/s0168-3659(02)00203-1. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Res. 2002;62:7280–7283. [PubMed] [Google Scholar]


