Abstract
Herpes simplex virus (HSV) establishes productive (lytic) infections in nonneuronal cells and nonproductive (latent) infections in neurons. It has been proposed that HSV establishes latency because quiescent neurons lack cellular factors required for productive infection. It has been further proposed that these putative factors are induced following neuronal stress, as a requirement for HSV reactivation. To date, the identity of these putative cellular factors remains unknown. We have demonstrated that cyclin-dependent kinase (cdk) 1, 2, or 7 is required for HSV replication in nonneuronal cells. Interestingly, cdks 1 and 2 are not expressed in quiescent neurons but can be induced in stressed neurons. Thus, cdks may be among the cellular proteins required for HSV reactivation whose neuronal expression is differentially regulated during stress. Herein, we determined that neuronal expression of nuclear cdk2, cdk4, and cyclins E and D2 (which activate cdks 2 and 4, respectively) was induced following explant cultivation, a stressful stimulus that induces HSV reactivation. In contrast, neuronal expression of cdk7 and cytoplasmic cdk4 decreased during explant cultivation, whereas cdk3 was detected in the same small percentage of neurons before and after explant cultivation and cdks 1, 5, and 6 were not detected in neuronal cell bodies. HSV-1 reactivated specifically in neurons expressing nuclear cdk2 and cdk4, and an inhibitor specific for cdk2 inhibited HSV-1 reactivation. We conclude that neuronal levels of cdk2 are among the factors that determine the outcome of HSV infections of neurons.
Herpes simplex virus type 1 (HSV-1) establishes both productive (usually lytic) and nonproductive (latent) infections. Infections of nonneuronal cells are productive, and infections of neurons may be either latent or, more rarely, productive (reviewed in reference 51). One of the models proposed to explain the distinct outcomes of HSV-1 infections of neurons and nonneuronal cells postulates that nonneuronal cells constitutively express all the cellular factors that are required for HSV-1 replication, whereas neurons express some of these factors only under certain conditions (34).
Many cellular functions are differentially expressed in neurons and nonneuronal cells. For example, neurons are terminally differentiated and consequently do not express several of the proteins that regulate progression into and through the cell cycle. Thus, many cyclin-dependent kinases (cdks), including cdks 1 and 2, are not expressed in terminally differentiated neurons, whereas they are commonly expressed in cultured nonneuronal cells (13, 45, 67). Interestingly, we have found that pharmacological inhibition of cdks 1, 2, and 7, which regulate progression into the cell cycle, inhibits replication of HSV-1 in cultured nonneuronal cells (38, 58-60). Thus, we propose that cdk 1, 2, or 7 may be among the proteins that are required for productive HSV-1 replication and differentially expressed in resting neurons versus stressed neurons and nonneuronal cells.
Although they are generally nonpermissive for HSV replication, neurons can support productive HSV infection under certain conditions. For example, physically and emotionally stressful stimuli induce a subset of latently infected neurons to become permissive for HSV reactivation (10, 11, 27). Interestingly, expression of several cdks has been shown to be induced in neurons subjected to stressful stimuli (4, 6, 13, 18, 21, 24, 29, 37, 40, 46-49, 56, 69). We therefore hypothesize that expression of cdks normally involved in cell cycle regulation is induced in neurons by the same stressful stimuli that promote HSV reactivation (and replication) in neurons and that expression of these cdks renders neurons permissive for HSV-1 replication and thus promotes HSV-1 reactivation.
The family of cdks currently includes nine proteins, most of which are required for progression through the specific phases of the cell cycle (39, 42, 70). cdks 4 and 6, activated by the D-type cyclins, allow progression into late G1. cdk3 is required for entry into late G1, but its specific function and activating cyclins are unknown. cdk2 is required for progression into and through S phase when it is activated by cyclins E and A, respectively. cdk1 is activated by the B-type cyclins and allows the cell to proceed into mitosis. cdk7, which associates with cyclin H, has both cell cycle-associated and cell cycle-independent functions in that it regulates the activities of other cdks and of transcription proteins (12, 33, 61, 62). The remaining cdks are not known to play major roles in regulating cell cycle progression. cdks 8 and 9, which associate with cyclins C (cdk8) and T and K (cdk9), phosphorylate transcription proteins (28, 50, 53), and cdk5, which associates with p25, is active only in terminally differentiated neurons, where it phosphorylates proteins of the neuroskeleton (20, 22, 31, 66, 67). The recently completed sequencing of the human genome sequence indicates that only nine other proteins have any significant homology to cdks. However, none of these nine proteins is activated by the known cyclins, and only three previously unknown cyclins were identified. Thus, it is likely that most, if not all, cdks have been identified (42).
The expression and functions of cdks in cycling (nonneuronal) cells have been the focus of numerous studies since the 1980s. In contrast, expression of cdks and cyclins in neurons has been examined only in the past few years. Briefly, the expression and activities of cdks 1 and 2 wane in neuronal progenitor cells as they withdraw from the cell cycle during terminal differentiation (19, 41, 45, 67). These two kinases are undetectable in mature, resting neurons (13). cdk5 activity is detected exclusively in terminally differentiated neurons, where cdk5 protein is excluded from the cell bodies and localizes to the axons (20, 31, 66, 67). cdks 4 and 6 have been detected in neurons in some studies (13, 26) but not in others (4, 18, 37, 40, 69, 74). Neuronal expression of cdks 1, 2, 4, and 6 and their cyclin partners has been reported to be induced during apoptosis (4, 13, 18, 21, 37, 40, 47, 52, 56, 63, 69). Inhibitors of cdks 1, 2, 4, and 6 prevent neuronal apoptosis in vitro and also in a mouse model (14, 46, 48, 49). Finally, neuronal expression of cdks 3, 7, 8, and 9 has not been examined.
Several pharmacological inhibitors specific for cdks have been developed. Of these, the most specific and best characterized are the 2,6,9-trisubstituted purine-derived drugs, such as roscovitine (38). Roscovitine inhibits cdks 1, 2, 5, and 7 with high potency and erks 1 and 2 at concentrations ≈50-fold above the cdk inhibitory concentrations. Roscovitine does not inhibit cdks 4, 6, and 8 or 36 other enzymes. All known cellular effects of roscovitine can be accounted for by the inhibition of its recognized cdk targets (15, 38). Depending on cell type, the cellular effects of roscovitine are observed at concentrations that range from 10 to 100 μM (2, 9, 23, 32, 36, 38), which is consistent with the concentrations of roscovitine required to inhibit cdk activities at physiological concentrations of ATP (unpublished data). We have shown recently that roscovitine inhibits HSV gene expression, DNA synthesis, and hence productive replication in nonneuronal cells as a consequence of inhibition of cell cycle-associated cdks (57-60). Thus, we concluded that cdks play an important role in HSV replication in nonneuronal cells. In the experiments presented in this paper, we began to investigate the potential roles of cdks in HSV replication in neuronal cells
In the experiments reported below, we used immunohistochemistry to examine expression of cdks, cyclins, and HSV antigen in neurons of trigeminal ganglia that had and had not been stressed by explant cultivation. We found that most resting neurons from mock and latently infected trigeminal ganglia express cytoplasmic cdk4 and nuclear and cytoplasmic cdk7 but no detectable cdk1, 2, or 6. Expression of cdk2 and nuclear translocation of cdk4 were induced in a significant number of neurons during explant cultivation, whereas levels of cdk7 declined significantly in most explant-cultivated neurons. Moreover, HSV reactivation from latency, as assessed by expression of viral antigens, correlated with neuronal expression of cdk2 and translocation of cdk4 to neuronal nuclei. Lastly, roscovitine, which specifically inhibits cdks 1, 2, 5, and 7 (but not cdks 4 and 6), completely inhibited HSV reactivation. Thus, we propose that the level of cdk2 (and perhaps cdk4) in neurons is a factor that determines whether neurons can support productive HSV infection and that expression and relocalization of cell cycle-associated cdks 2 and 4 can be induced in neurons following the stress associated with explant cultivation.
MATERIALS AND METHODS
Animals.
ICR mice (5 to 7 weeks old) were anesthetized, their corneas were scarified with a 22.5-gauge needle, and 2.0 μl of inoculum containing 2.5 × 105 PFU of HSV-1 strain KOS or no virus (mock infections) was applied to each cornea. At 35 days postinfection, mice were euthanized by CO2 inhalation, and trigeminal ganglia were removed immediately (in less than 7 min) and either fixed or explanted into 1.0 ml of minimal essential medium (MEM) supplemented with 10% fetal bovine serum, antibiotics, and, where indicated, 30 or 40 μM roscovitine. Explanted trigeminal ganglia were fixed at the indicated times postexplant. All animal experiments were performed under protocols approved by the local institutional animal care and use committee, which followed all applicable local and federal regulations.
Immunohistochemistry.
Trigeminal ganglia were fixed at 4°C by rocking in 10% buffered formalin, dehydrated, embedded in paraffin, and sectioned by standard procedures. Sections were deparaffined in xylenes, rehydrated, and incubated in 0.01 M citric acid (pH 6.0) at 95 to 102°C for 10 min. After cooling to room temperature, sections were blocked (Power Block; BioGenex, San Ramon, Calif.) for 10 min, incubated for 30 min with primary antibody, washed for 10 min in phosphate-buffered saline-0.05% Tween 20, and incubated for 10 min with secondary antibody (Dako Corporation, Carpenteria, Calif.). Sections were then washed, incubated for 45 min in avidin-biotin-alkaline phosphatase conjugate, washed again, and developed with Vector Blue and Vector Red (Vector Laboratories, Inc., Burlingame, Calif.), as indicated, in the presence of levamisole (BioGenex). Sections were counterstained with methyl green unless otherwise indicated. Sections from nonexplanted and explanted trigeminal ganglia from mock- and HSV-infected animals were processed in parallel, providing internal controls for all experiments. The specificity of the technique was verified further in control tests in which primary antibodies were omitted. A total of 200 to 400 neurons (in 6 to 12 sections) were counted per time point. Results are presented as the percentage of antigen-positive neurons.
The antibodies used in these studies were 17 (cdk1), C19 (cdk1), M2 (cdk2), Y-20 (cdk3), C-22 (cdk4), C-8 (cdk5), H96 (cdk6), C-19 (cdk7), C-4 (cdk7), C-19 (cyclin A), GNS1 (cyclin B1), R-124 (cyclin D1), 34B1-3 (cyclin D2), C-16 (cyclin D3), and M-20 (cyclin E), all from Santa Cruz Biotechnologies (Santa Cruz, Calif.), and B0114 (HSV), from Dako Corporation. Except for Y-20 (1:50), GNS1 (1:25), and 34B1-3 (1:25), all antibodies were diluted 1:100 in blocking solution.
Reactivation studies.
Latently and mock-infected mice were euthanized 35 days after infection. One trigeminal ganglia from each animal was explanted in the absence of roscovitine, a specific inhibitor of cdks 1, 2, 5, 7, and likely 3, and the other was explanted in the presence of 30 μM (two experiments) or 40 μM (two experiments) roscovitine. These concentrations of roscovitine are nontoxic for neurons and actually promote their survival (47). All trigeminal ganglia removed after necropsy were explanted without feeder cells to minimize the potential inhibitory effects of roscovitine on secondary replication of HSV in nonneuronal cells. As an indication of the presence of reactivated virus, culture supernatants were tested daily for the ability to produce cytopathic effects in Vero cells as an indication of the presence of reactivated virus. Inocula were removed from the indicator cells at 8 h postinfection. Importantly, roscovitine at 30 and 40 μM inhibits HSV replication in Vero cells inefficiently and in a manner that is reversible within 24 h (58).
RESULTS
A subset of cell cycle-associated cdks are constitutively or inducibly expressed in neurons of trigeminal ganglia.
To determine if terminally differentiated sensory neurons retain the capacity to express cell cycle-associated cdks, we tested whether these cdks are expressed in mock- or latently infected neurons and in neurons stressed during explant cultivation. To this end, expression of cdks 1 through 7 (all but cdk5 are involved in cell cycle regulation) was examined in neurons of nonexplanted and explanted trigeminal ganglia. Specifically, trigeminal ganglia of mock- and latently infected mice were fixed immediately after necropsy (nonexplanted neurons) or on days 1, 2, and 3 postexplant (explanted neurons). Expression of proteins rather than mRNAs was evaluated because expression of cdks and cyclins is regulated at several posttranscriptional levels. Moreover, there is often no good correlation between mRNA and protein levels in neurons (35). Thus, mRNA levels may not be the best indicators of the actual levels of cdks and cyclins in neurons.
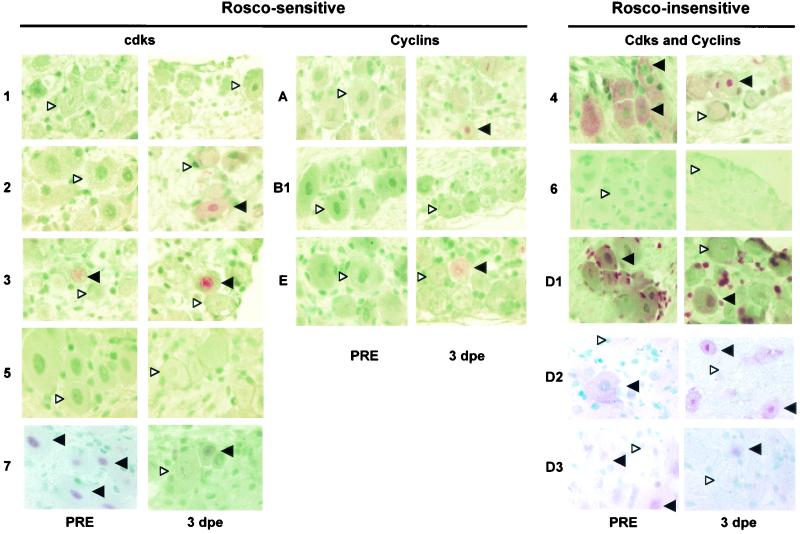
cdks 1 and 2 have not been detected in resting neurons, and cdk5 localizes only to axons (13, 67, 71). Consistent with these findings, we did not detect these cdks in the cell bodies of nonexplanted trigeminal ganglia neurons (Fig. 1, 2A, and 3). Neuronal expression of cdks 3 and 7 has not been examined to date. cdk3 immunoreactivity was detected in only ∼5% of neuronal nuclei, whereas nearly all neurons exhibited strong cdk7 immunoreactivity (Fig. 1, 2A, and 3). Notably, antibody C-4 detected primarily nuclear cdk7 (Fig. 1), whereas antibody C-19 recognized primarily cytoplasmic cdk7 (Fig. 3). These two antibodies were raised against different cdk7 epitopes. Since cdk7 forms complexes with different sets of cellular proteins, it is not surprising that different epitopes of cdk7 are accessible to antibodies in different cellular compartments.
FIG. 1.
Expression of cdks and their cyclin partners in neurons of trigeminal ganglia before and after explant. Sections of latently infected trigeminal ganglia fixed immediately after necropsy (PRE) and after 3 days in explant culture (3 dpe) were processed for immunohistochemistry and stained with antibodies specific for the indicated cdks and cyclins. Sections were developed with a red substrate and counterstained with methyl green. Solid arrowheads pointing to the left indicate representative neurons that are positive for the indicated antigen. White arrowheads pointing to the right indicate neurons that are negative for the indicated antigen. cdks and cyclins are labeled roscovitine (Rosco) sensitive and roscovitine insensitive based on the susceptibility of the respective cdk/cyclin holoenzymes to this inhibitor. Original magnification, ×1,000.
FIG. 3.
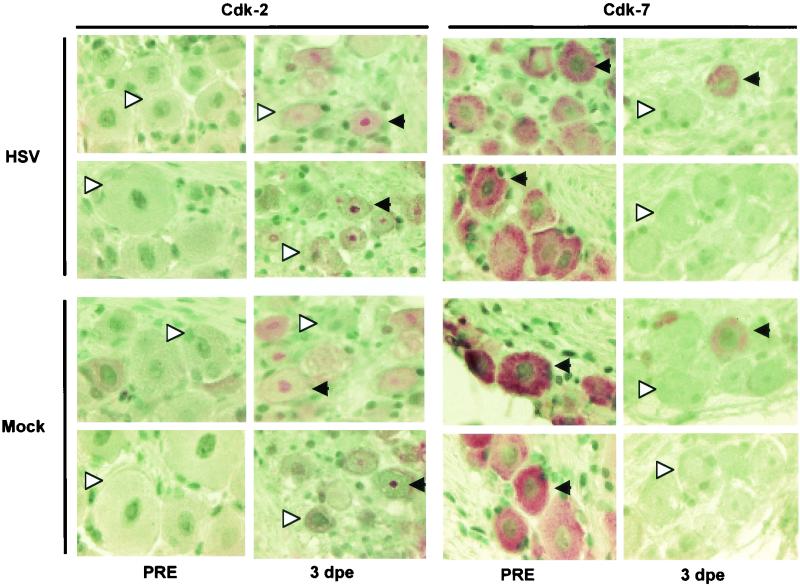
Changes in expression of cdks 2 and 7 in trigeminal ganglia neurons on day 3 postexplant are independent of HSV infection. Trigeminal ganglia from mock-infected (Mock) and latently infected (HSV) mice were fixed immediately after necropsy (PRE) and 3 days after explant (3 dpe). Black arrowheads pointing to the left indicate representative neurons that are positive for a specific cdk. White arrowheads pointing to the right indicate representative neurons that are negative for a specific cdk. Original magnification, ×1,000.
Some investigators (13, 26, 71) but not others (18, 40, 56, 69) have detected cdks 4 and 6 in resting neurons. In this study, a small percentage of resting neurons (∼5%) exhibited very weak immunoreactivity with the cdk6 antibody. This immunoreactivity was difficult to capture photographically (Fig. 1) and may well be nonspecific. In contrast, strong cdk4 immunoreactivity was detected in the cytoplasm but not in the nucleus of nearly all nonexplanted neurons (Fig. 1 and 4).
FIG. 4.
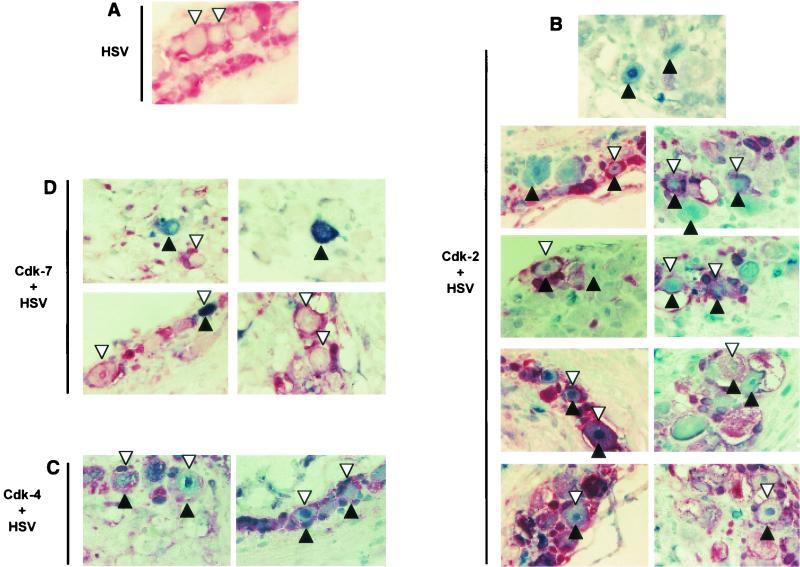
Colocalization of HSV antigen with cdk2 and nuclear cdk4 but not with cdk7 in explanted neurons. Trigeminal ganglia in which HSV antigen had been detected in screening experiments were processed with antibodies specific for a given cdk and for HSV antigen. cdks were developed in blue, and HSV antigen was developed in red. These sections were not counterstained. Black arrowheads pointing up indicate neurons that are positive for a specific cdk. White arrowheads pointing down indicate neurons that are HSV antigen positive. Double-positive neurons are designated by black and white arrowheads. Sections are from trigeminal ganglia explanted for 4 to 6 days; no differences in colocalization patterns were observed during this period. Original magnification, ×1,000. (A) A section processed only with HSV-specific antibodies (red) is presented for comparison. (B) Sections stained with antibodies specific for cdk2 (blue) and HSV (red). The top panel shows two neurons that are cdk2+/HSV− and several neurons that are cdk2−/HSV−. All other panels show neurons that are cdk2+/HSV+ and cdk2+/HSV−. As cdk2 is primarily nuclear, its expression can be determined best in neurons whose nuclei are visible. In some cases, it is difficult to determine whether HSV staining localizes to the periphery of the neuron or to adjacent satellite cells. (C) Sections stained with antibodies specific for cdk4 (blue) and HSV antigen (red). Nuclear cdk4 staining is clear in HSV+ neurons. (D) Sections stained with antibodies specific for cdk7 and HSV antigen. Note the numerous HSV+/cdk7− neurons in which the nucleus is clearly visible. Only one potentially double-positive neuron is visible in these sections.
The stress associated with explant cultivation of trigeminal ganglia consistently induces reactivation of latent HSV, although the cellular changes that convert latently infected neurons from a nonpermissive to a permissive state have not been identified. A slight increase in the level of some viral mRNAs has been observed by reverse transcription (RT)-PCR, tentatively at 2 h postexplant and more clearly after 4 to 6 h postexplant (10, 64). Viral transcripts and proteins can be detected by in situ techniques only 24 to 48 h postexplant (11, 27) (Fig. 5), and infectious (reactivated) virus is commonly detected 48 to 72 h postexplant. Given the kinetics of reactivation, we analyzed changes in the percentage of neurons expressing individual cdks and cyclins during the first 72 h postexplant.
FIG. 5.
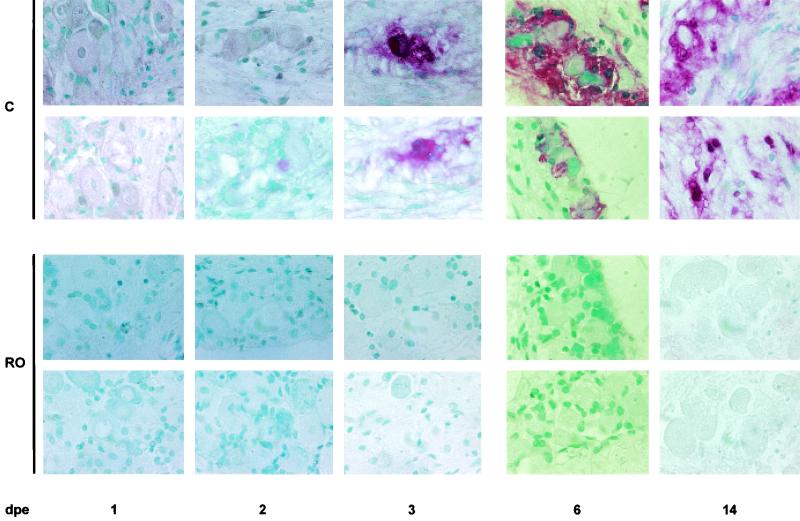
Roscovitine, which inhibits cdk2, prevents HSV antigen expression in neurons. Sections from trigeminal ganglia that had been explanted for 1, 2, 3, 6, and 14 days in the presence (RO) and absence (C) of 40 μM roscovitine were fixed and stained for HSV antigen. These sections are from three different experiments (1, 2, 3, 6, and 14 days postexplant [dpe]). In the absence of roscovitine, HSV antigen was detected at 2 days postexplant in a very few neurons. More HSV-positive neurons were visible on day 3 postexplant. Large foci of HSV-positive cells were visible in trigeminal ganglia fixed on days 6 and 14 postexplant, when infection had spread to many nonneuronal cells. No HSV-positive cells were observed in trigeminal ganglia explanted in the presence of roscovitine. Original magnification, ×1,000.
Expression of cell cycle-associated cdks was found to be differentially regulated in neurons stressed by explant cultivation. The percentage of cdk2+ neurons, for example, increased from <1% on day 1 to ∼25% on day 3 postexplant (Fig. 2A). The percentage of neurons expressing nuclear cdk4 also increased from days 1 to 3 postexplant (Fig. 1 and 2A), whereas the percentage of neurons expressing cytoplasmic cdk4 decreased from ∼95% of nonexplanted trigeminal ganglia neurons to 5 to 10% by day 3 postexplant. In contrast to cdk2 and nuclear cdk4, the percentage of cdk7+ neurons declined rapidly after explant (Fig. 1, 2A, and 3). On day 1 postexplant, cdk7 was detected in only 10 to 30% of trigeminal ganglia neurons and on day 3 postexplant, cdk7 was detected in very few neurons (Fig. 1, 2A, and 3). Similar results were obtained with antibodies C-19 (which recognizes primarily nuclear cdk7) and C-4 (which recognizes primarily cytoplasmic cdk7) (compare Fig. 1 and 3).
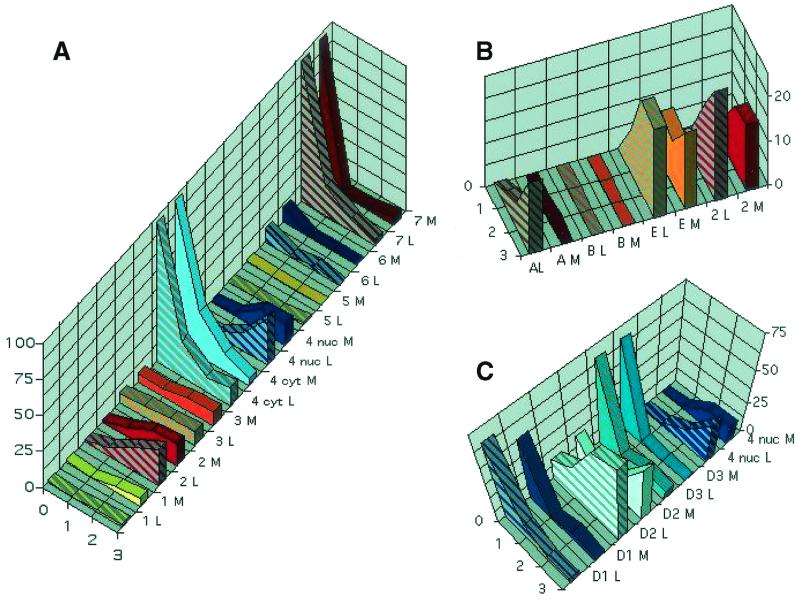
FIG. 2.
Percentage of trigeminal ganglia neurons expressing cdks and cyclins before and after explant. Sections from trigeminal ganglia fixed immediately after necropsy and at 1, 2, and 3 days postexplant (four trigeminal ganglia per time point) were processed by immunohistochemistry. At least 100 neurons in three sections of each of four trigeminal ganglia processed in at least two independent times were counted for each protein. The percentage of neurons positive for each protein is presented. Striped and plain bars indicate the percentages of neurons that expressed each protein in trigeminal ganglia from latently (L) and mock (M) HSV-infected mice, respectively. (A) cdks 1 through 7. Except for cdk4, the bars indicate the percentage of neurons expressing the indicated cdk in any cellular compartment. For cdk4, light and dark blue bars indicate the percentage of neurons expressing cytoplasmic (cyt) and nuclear (nuc) cdk4, respectively. (B) Cyclins that activate roscovitine-sensitive cdks. The percentage of neurons expressing cyclins A, B, and E is indicated. The percentage of neurons expressing cdk2 (from panel A) is included for comparison. The y axis has been truncated at 20% to facilitate comparisons among percentages of neurons expressing each protein. (C) Expression of cyclins that activate roscovitine-insensitive cdks. The percentage of neurons expressing cyclins D1, D2, and D3 is indicated. The percentage of neurons expressing nuclear cdk4 (from panel A) is included for comparison. The y axis scale has been truncated at 75% to facilitate comparisons among percentages of neurons expressing each protein.
The percentage of neurons expressing detectable levels of cdks 1, 3, 5, and 6 did not change significantly during the 3-day period postexplant (Fig. 1 and 2A). Thus, cdk1 and cdk6 were not detected in trigeminal ganglia neurons on days 1 and 2 postexplant, although on day 3 postexplant, a small percentage of neurons (∼5%) expressed cdk1 but not cdk6. cdk5 was not detected in neuronal cell bodies at any time postexplant, and the small percentage of neurons showing cdk3 immunoreactivity did not change during explant cultivation.
Equivalent changes in the percentage of neurons expressing different cdks were observed in neurons from mock- and latently infected animals (Fig. 2 and 3). It appears that the levels of expression of the different cdks were higher in neurons of trigeminal ganglia explanted from latently infected animals, but the intensity of the immunohistochemistry signal cannot be quantitated reliably. Thus, we conclude that as a consequence of the stress associated with explant cultivation, expression of cdk2 in neurons is induced and that of cdk7 declines, whereas cdk4 translocates from the cytoplasm to the nucleus.
A subset of cell cycle-associated cyclins is expressed constitutively or inducibly in neurons of trigeminal ganglia.
We next examined the patterns of neuronal expression of the cyclins that activate cdks 2 and 4: cyclins A, E, D1, D2, and D3. Consistent with previous reports (13, 43, 44, 67), cyclin A was not detected in nonexplanted trigeminal ganglia neurons or in neurons of trigeminal ganglia explanted from mock-infected animals (Fig. 1 and 2B). A homogeneous pinkish background is observed in this figure because these sections were slightly overdeveloped in an attempt to maximize the possibilities of detecting neuronal cyclin A. This background is homogeneous throughout the section and not always cell associated (data not shown). Weak nuclear cyclin A immunoreactivity was detected, however, on days 2 and 3 postexplant in neurons of trigeminal ganglia explanted from latently infected mice, primarily in neuronal nuclei (Fig. 1 and 2B).
Cyclin E has been detected in resting neurons in some models (41) but not in others (8, 43). We detected no cyclin E immunoreactivity in nonexplanted trigeminal ganglia neurons, but neuronal expression of cyclin E was detected when trigeminal ganglia from mock- and latently infected mice were explanted (Fig. 1 and 2B). Thus, ∼10% of trigeminal ganglia neurons exhibited strong cyclin E immunoreactivity on day 1 postexplant and ∼26% of neurons were cyclin E+ on day 3 postexplant (Fig. 1 and 2B). Cyclin E immunoreactivity localized to both the nucleus and cytoplasm.
Cyclin D1 is induced in sympathetic and central nervous system neurons undergoing apoptosis in vitro and in vivo (3, 40, 47, 55, 56, 65), and mRNAs for cyclins D2 and D3 have been detected by RT-PCR and by in situ hybridization in resting sympathetic and central nervous system neurons that have and have not been cultured (13, 54, 65). We detected cyclins D1, D2, and D3 in approximately 60, 25, and 60% of nonexplanted trigeminal ganglia neurons, respectively (Fig. 1 and 2C). Cyclins D1 and D2 were primarily cytoplasmic, whereas cyclin D3 was primarily nuclear, and the signal was weak (Fig. 1). Cyclin D1 was consistently detected in nonneuronal cells as well as in neurons (Fig. 1). The percentage of neurons expressing cyclins D1 and D3 decreased rapidly after explant. Thus, on day 1 postexplant, only 5 to 10% of neurons expressed detectable levels of these cyclins (Fig. 2C). In contrast, the percentage of neurons expressing cyclin D2 increased after explant so that ∼50% of neurons expressed strong, nuclear cyclin D2 on day 3 postexplant (Fig. 1 and 2B). The same patterns of expression of cyclins D1, D2, and D3 were observed in neurons of trigeminal ganglia from mock- and latently infected mice. Finally, cyclin B1, a partner of cdk1, was not detected in neurons (Fig. 1 and 2B).
HSV-1 antigen expression correlates with expression of cdk2 and nuclear cdk4 but not cdk7.
If cdks expressed in neurons are required for HSV reactivation, HSV proteins should be detected only in neurons that express the required cdks. To test this hypothesis, latently infected mice were euthanized, their trigeminal ganglia were explanted, and the explanted trigeminal ganglia were fixed at selected times postexplant. Trigeminal ganglia sections were then subjected to two-color immunohistochemistry. HSV antigen was developed in red, and individual cdks were developed in blue (Fig. 4). Because these sections were from trigeminal ganglia explanted for several days and were not counterstained, neurons that are double negative and trigeminal ganglia cells other than neurons are difficult to identify. Therefore, we limited these analyses to neurons, although nonneuronal cells positive for HSV antigen and cdks were also detected.
HSV antigen immunoreactivity localized to the periplasm and membranes of neuronal cell bodies, as clearly visible in sections stained with anti-HSV antibody only (Fig. 4A). This staining is characteristic of the antibody used. HSV antigen was detected in less than one neuron per section when neurons were explant cultivated for less than 4 days postexplant (Fig. 5). The scarcity of HSV antigen-positive neurons and the technical complexities inherent in two-color immunohistochemistry made it impossible to determine with certainty if HSV antigen and cdk2 immunoreactivity colocalized to the same neurons at these early times. Thus, we analyzed HSV antigen and cdk immunoreactivity in trigeminal ganglia that had been explanted 4 to 6 days previously. At these relatively late times after infection, however, we could not ascertain whether the HSV-1 antigen detected in a given neuron indicated primary reactivation of the virus in this specific neuron or secondary infection of the neuron by virus previously reactivated in other cells.
A subpopulation of cdk2+ and no cdk2− neurons were also HSV antigen positive at any time postexplant (Fig. 4B). Thus, when the nucleus of an HSV antigen-positive neuron was visible, cdk2 was detected in the same cell. Because these sections had been explant cultivated for 4 and more days and were not counterstained, it was not possible in many instances to ascertain whether the HSV antigen immunoreactivity localized to the periphery of a neuron or to the satellite cells adjacent to it (for examples, see the two top left panels in Fig. 4B). In several neurons, however, HSV antigen immunoreactivity clearly localized to the periphery of cdk2+ neurons (for examples, see the two bottom left panels in Fig. 4B). Many cdk2+ neurons did not express HSV antigen (Fig. 4B). We did not attempt to identify whether these neurons were not infected or contained latent HSV-1 which failed to reactivate in the presence of cdk2 expression.
In cells in which it was possible to evaluate colocalization, the vast majority of HSV antigen-positive neurons also exhibited nuclear cdk4 staining (Fig. 4C). As with cdk2, in many cases it was difficult to ascertain whether HSV antigen immunoreactivity localized to the periphery of a neuron or to the satellite cells adjacent to it. In contrast to cdk2 and nuclear cdk4, however, HSV antigen seldom localized to cdk7+ neurons (Fig. 4D). Moreover, HSV antigen and cdk7 were more likely to colocalize to the same neurons at earlier times (when many cells expressed detectable cdk7) than at later times postexplant (when few cells expressed detectable cdk7). Thus, colocalization of HSV antigen and cdk7 appears to be based on probability.
The results from these experiments suggest that induction of expression of cdk2, nuclear translocation of cdk4, or reduced expression of cdk7 permits expression of HSV proteins in neurons.
Roscovitine, an inhibitor specific for cdks 1, 2, 5, and 7, inhibits HSV-1 reactivation.
Expression of HSV proteins in neurons correlated closely with expression of cdk2 and nuclear cdk4, and expression of HSV proteins in other cell types requires cdk activity (57, 58, 60). However, neuronal expression of HSV antigen also correlated with decreased levels of expression of cdk7. If expression of a roscovitine-sensitive cdk, such as cdk2, were required for HSV-1 reactivation, then roscovitine should inhibit HSV-1 reactivation in neurons. In contrast, if decreased levels of a roscovitine-sensitive cdk, such as cdk7, were required for HSV reactivation, then roscovitine should not inhibit and might even promote HSV-1 reactivation in neurons.
To discriminate between these alternatives, latently infected trigeminal ganglia were explanted in the presence of 30 and 40 μM roscovitine, which inhibits the activity of cdks 2 and 7 but not cdk4, and trigeminal ganglia supernatants were tested for the presence of infectious virus. We have shown previously that in nonneuronal cells, roscovitine inhibits HSV transcription and replication, most likely via inhibition of cell cycle cdks (58, 60). Roscovitine inhibited HSV reactivation efficiently in four independent experiments, suggesting that active cdks are expressed in neurons and that their activities are required for HSV reactivation (Table 1).
TABLE 1.
HSV-1 reactivation in the presence and absence of roscovitinea
| Expt no. | Roscovitine concn (μM) in treated group | Group | No. of trigeminal ganglia yielding infectious (reactivated) virus/no. in group | % of trigeminal ganglia yielding reactivated virus |
|---|---|---|---|---|
| 1 | 30 | Control | 8/12 | 66.7 |
| Treated | 1/12 | 8.3 | ||
| 2 | 40 | Control | 19/20 | 95.0 |
| Treated | 0/20 | 0.0 | ||
| 3 | 40 | Control | 16/16 | 100.0 |
| Treated | 0/16 | 0.0 | ||
| 4 | 30 | Control | 6/6 | 100.0 |
| Treated | 1/22 | 4.5 |
In experiments 1 and 2, all trigeminal ganglia were explanted for at least 4 additional days after the control (untreated) explants had reached maximum reactivation. In experiments 3 and 4, all trigeminal ganglia were explanted for at least 10 additional days after the control explants had reached 100% reactivation. The specific times in explant were 10, 7, 21, and 15 days for experiments 1 to 4, respectively.
Roscovitine could inhibit reactivation per se, indicating that cdk activity in neurons is required for HSV reactivation or secondary replication of reactivated virus in other trigeminal ganglia cells. To differentiate between these alternatives, trigeminal ganglia were explant cultivated in the presence of roscovitine and fixed 1, 2, and 3 days postexplant. Reactivation per se (defined as the production of infectious virus in a previously latently infected neuron in the absence of superinfection with extracellular virus) was then evaluated by examining expression of HSV proteins in neurons. If roscovitine inhibited secondary amplification of reactivated virus, only the neurons in which HSV reactivated from latency should express HSV antigen. If roscovitine inhibited primary reactivation of HSV in a given neuron, no HSV antigen should be detected in any neuron.
No HSV antigen-positive neurons were detected at any time in the presence of roscovitine. In contrast, a small number of HSV antigen-positive neurons were detected in trigeminal ganglia cultivated for 2 and 3 days postexplant in the absence of drug (Fig. 5). In two additional experiments, we fixed trigeminal ganglia cultivated for 6 and 14 days in the presence and absence of drug. No HSV antigen-positive cells were observed in trigeminal ganglia cultivated in the presence of roscovitine, even though large HSV foci were readily detected in cells of trigeminal ganglia at 6 and 14 days postexplant in the absence of drug, and infection had even spread to nonneuronal cells at these late times (Fig. 5). Collectively, these results thus suggest that a subpopulation of explant-cultivated neurons express roscovitine-sensitive cdk activity and that this activity mediates primary reactivation of HSV-1. These studies, however, did not attempt to address at what specific stage reactivation was inhibited in latently infected cells.
DISCUSSION
In the experiments presented herein, we have shown that neurons do not express cdk2, nuclear cdk4, or their cyclin partners in conditions under which neurons support latent but not productive HSV infection. In contrast, neurons express nuclear cdks 2 and 4 and their cyclin partners in conditions under which neurons support productive HSV replication. Furthermore, expression of HSV-1 proteins (antigen) during reactivation was restricted to neurons expressing cdk2 and nuclear cdk4, and roscovitine, which inhibits cdk2 (and other kinases), inhibited HSV-1 reactivation in neurons. Thus, neuronal cdk2 activity appears to be required for HSV-1 reactivation.
In the course of these experiments, we also found that terminally differentiated sensory neurons can express cdks whose primary function in nonneuronal cell types is to regulate cell cycle progression. Thus, the great majority of nonexplanted trigeminal ganglia neurons expressed cdk7 and cytoplasmic cdk4, whereas a subpopulation of explant-stressed trigeminal ganglia neurons from both mock- and latently infected mice expressed cdk2 and nuclear cdk4. Moreover, expression of a subset of the cyclin partners of cdks2 and 4, cyclins E and D2, was induced in parallel with their respective cdks in neurons of both mock- and latently infected mice. In contrast, cyclin A was detected only in neurons that were explant cultivated from latently infected animals. Although it may be tempting to speculate that cyclin A expression determines the ability of neurons to support productive HSV-1 replication, this hypothesis has yet to be tested.
Interestingly, expression of cyclins A and E has also been observed in neurons of trigeminal ganglia of calves acutely infected with bovine herpesvirus 1, a virus that is closely related to HSV-1 (72). Consistent with our results, Winkler and colleagues observed that cyclin A was primarily nuclear, whereas cyclin E was both nuclear and cytoplasmic. Although Winkler and colleagues did not observe induction of cyclin D2 expression, they did observe induction of cyclin D1 expression. It is possible that the functions of these cyclins during viral replication in neurons overlap, at least partially, to the point that expression of either cyclin D1 or D2 can provide the functions required for viral replication in neurons.
In sum, expression of cyclins A and E has now been observed in two distinct models of alphaherpesvirus replication in neurons. Thus, it is probable that these proteins are involved in, if not required for, replication of alphaherpesviruses in neurons. Because we also detected expression of cdk2 in the neurons where HSV reactivates from latency, it is logical to hypothesize that one function of cyclins A and E during HSV reactivation is to activate cdk2. We have found previously that pharmacological inhibition of cdks 1, 2, and 7 in nonneuronal cells results in inhibition of HSV-1 transcription and DNA replication (25, 57-60). Thus, cdk 1, 2, or 7 is required for HSV transcription (and DNA replication) in nonneuronal cells. In explanted neurons, we now found that HSV-1 proteins (antigen) are expressed only in neurons that express cdk2 and that roscovitine inhibits expression of HSV-1 proteins in neurons (Fig. 4 and 5). Thus, cdk2 appears to be required for HSV-1 gene expression in all cell types. The mechanisms by which cdks activate HSV-1 gene expression, however, remain to be elucidated and may or may not be the same in neuronal and nonneuronal cells.
A roscovitine-sensitive cdk is required for posttranslational modifications of ICP0 and ICP4 (1, 7), and ICP0 does not activate HSV-1 promoters in the presence of roscovitine (7, 60). These results may indicate that cdk2-induced modifications of ICP0 are required to activate the transactivating function of ICP0. Interestingly, ICP0 plays an important role in HSV reactivation (16, 17, 30). Thus, if ICP0 were expressed in neurons in the absence of cdk2 activity, it could be incapable of activating expression of other HSV genes and consequently of activating HSV reactivation. In this model, HSV-1 reactivation would be triggered when cdk2 activity is induced in neurons that are expressing ICP0. Then, cdk2 would activate ICP0, which would trigger reactivation. Alternatively, cdk2 activity may be required to phosphorylate some as yet unidentified cellular proteins that are required to initiate the process of HSV reactivation. Future experiments are aimed at addressing these issues.
Neuronal expression of cdks has been reported previously to be deregulated in certain pathologies. For example, cdks 1, 2, 4, and 6 have been detected in neurons of patients with Alzheimer's disease, Parkinson's disease, and cerebral ischemia (4, 18, 37, 40, 56, 69). However, whether cdk expression is a cause, a consequence, or simply a correlate of these pathologies is unclear (18, 69). Expression of cdks 1, 2, 4, and 6 and their cyclin partners has also been observed in neurons during apoptosis in vitro (13, 47). Moreover, neuronal apoptosis in vitro is repressed by cdk inhibitors, including olomoucine, a drug very closely related to roscovitine (47-49), indicating that cdk activities are required for neuronal apoptosis. cdk4, cyclin A, and the D-type cyclins have also been detected in central nervous system neurons programmed to die by apoptosis in several animal models (4, 6, 21, 24, 29, 40, 46). Thus, functional expression of cdks in neurons had been documented previously, yet this is the first report of a function for cdks in neurons other than apoptosis (i.e., viral replication).
Expression of cdks 1 and 6 and cyclin B1 was not detected in explanted and nonexplanted neurons in our studies. The antibodies used to detect these proteins recognized their cognate proteins by immunohistochemistry in nonneuronal cells and even in a low percentage (i.e., 1 to 5%) of heat-stressed neurons (data not shown). It should be noted, however, that these antibodies may not be sufficiently sensitive to detect very low levels of their cognate proteins in neurons, or alternatively, they may be directed against epitopes that are modified differentially in non-heat-stressed neurons versus heat-stressed neurons and other cell types. Similarly, the levels of expression of cdk7, cytoplasmic cdk4, and cyclins D1 and D3 in neurons fell below the limit of detection of our technique during explant culture, but these proteins may still be expressed in these cells to very low levels. With respect to cdk1, however, we obtained these negative results with two different antibodies (Fig. 1 and 2 and data not shown).
The low percentage of neurons expressing cdk3 immunoreactivity detected did not change under any of the conditions tested, suggesting that cdk3 does not play a major role in HSV-1 reactivation. This hypothesis is further supported by the recent finding that the cdk3 gene in several strains of the “Castle” group of mice, such as BALB/c, contains a stop codon before the kinase domain (73). Thus, cdk3 in these mice is not likely to possess kinase activity. Because HSV-1 replicates in neurons (and other cells) of BALB/c mice, these results support the conclusion that neuronal cdk3 likely has no major relevance for HSV-1 reactivation. The mice used in our experiments, ICR, are distantly related to the Castle group, and it is unknown whether the cdk3 gene of ICR mice contains a stop codon before the kinase domain.
During the preparation of this paper, Berger and colleagues reported that several genes that regulate the cell cycle are expressed in explanted trigeminal ganglia, but not in nonexplanted trigeminal ganglia. These genes included cyclin F and a gene that has significant homology to those for cdks 1 and 2 (68). These investigators reached these conclusions following large-scale gene expression analysis of RNA extracted from whole trigeminal ganglia, which contain a variety of cell types. Because they focused their efforts on the analyses of other cellular genes, Berger and colleagues did not determine whether cyclin F and the cdk-homologous protein were expressed in neurons or in other trigeminal ganglia cell types. The fact that these investigators did not detect induction of cdk2 or cyclin E is hardly surprising, as only a minority of neurons (which themselves are only a small minority of cells in trigeminal ganglia) express cdk2 and cyclin E in explant-cultured trigeminal ganglia (Fig. 1, 2, 3, and 4). Therefore, expression of these genes would be difficult to detect by analysis of gene expression in mixed cell populations. In contrast, expression of these genes may be detectable by analyzing gene expression of purified neurons. For example, by performing gene array analysis of gene expression in cultures of purified neurons, Chiang and colleagues recently detected induction of cyclin A expression in neurons by several apoptotic and nonapoptotic stimuli (5).
In sum, we have shown that during explant-induced reactivation, expression of cdk2 and nuclear cdk4 (but not cdks 1, 3, 5, or 7) in trigeminal ganglia neurons correlates with induction of HSV-1 antigen expression and viral reactivation and that an inhibitor specific for cdks 1, 2, 5, and 7 (but not cdks 4 or 6) blocked both expression of HSV-1 proteins in neurons and viral reactivation. We infer, therefore, that cdk2 is likely required for ex vivo explant-induced HSV-1 reactivation. Whether cdk2 is also required for HSV-1 reactivation in vivo remains to be determined.
Acknowledgments
This work was supported by Public Health Service grants R01CA20260 from the National Cancer Institute and PO1NS35138 from the National Institute of Neurological Disorders and Stroke.
We thank Elsa Aglow for preparing the tissue sections and for excellent technical assistance with histological techniques in general and all the members of the Schaffer lab for stimulating discussions and suggestions. S. L. Berger kindly provided us with detailed information on the gene array system used in their analyses of gene expression in explanted trigeminal ganglia.
REFERENCES
- 1.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, F., S. Quarta, M. Savio, F. Riva, L. Rossi, L. A. Stivala, A. I. Scovassi, L. Meijer, and E. Prosperi. 1998. The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp. Cell Res. 245:8-18. [DOI] [PubMed] [Google Scholar]
- 3.Boutillier, A. L., P. Kienlen-Campard, and J. P. Loeffler. 1999. Depolarization regulates cyclin D1 degradation and neuronal apoptosis: a hypothesis about the role of the ubiquitin/proteasome signalling pathway. Eur. J. Neurosci. 11:441-448. [DOI] [PubMed] [Google Scholar]
- 4.Busser, J., D. S. Geldmacher, and K. Herrup. 1998. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J. Neurosci. 18:2801-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang, L. W., J. M. Grenier, L. Ettwiller, L. P. Jenkins, D. Ficenec, J. Martin, F. Jin, P. S. DiStefano, and A. Wood. 2001. An orchestrated gene expression component of neuronal programmed cell death revealed by cDNA array analysis. Proc. Natl. Acad. Sci. USA 98:2814-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, A. R., E. R. Maandag, M. van Roon, N. M. van der Lugt, M. van der Valk, M. L. Hooper, A. Berns, and H. te Riele. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359:328-330. [DOI] [PubMed] [Google Scholar]
- 7.Davido, D. J., D. A. Leib, and P. A. Schaffer. 2002. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 76:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delalle, I., T. Takahashi, R. S. Nowakowski, L. H. Tsai, and V. S. Caviness, Jr. 1999. Cyclin E-p27 opposition and regulation of the G1 phase of the cell cycle in the murine neocortical PVE: a quantitative analysis of mRNA in situ hybridization. Cerebral Cortex 9:824-832. [DOI] [PubMed] [Google Scholar]
- 9.Deng, M. Q., and S. S. Shen. 2000. A specific inhibitor of p34cdc2/cyclin B suppresses fertilization-induced calcium oscillations in mouse eggs. Biol. Reprod. 62:873-878. [DOI] [PubMed] [Google Scholar]
- 10.Devi-Rao, G. B., D. C. Bloom, J. G. Stevens, and E. K. Wagner. 1994. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J. Virol. 68:1271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ecob-Prince, M., and K. Hassan. 1994. Reactivation of latent herpes simplex virus from explanted dorsal root ganglia. J. Gen. Virol. 75:2017-2028. [DOI] [PubMed] [Google Scholar]
- 12.Feaver, W. J., J. Q. Svejstrup, N. L. Henry, and R. D. Kornberg. 1994. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell 79:1103-1109. [DOI] [PubMed] [Google Scholar]
- 13.Freeman, R. S., S. Estus, and E. M. Johnson, Jr. 1994. Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of cyclin D1 during programmed cell death. Neuron 12:343-355. [DOI] [PubMed] [Google Scholar]
- 14.Giovanni, A., F. Wirtz-Brugger, E. Keramaris, R. Slack, and D. S. Park. 1999. Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F x DP in B-amyloid-induced neuronal death. J. Biol. Chem. 274:19011-19016. [DOI] [PubMed] [Google Scholar]
- 15.Gray, N., L. Detivaud, C. Doerig, and L. Meijer. 1999. ATP-site directed inhibitors of cyclin-dependent kinases. Curr. Med. Chem. 6:859-875. [PubMed] [Google Scholar]
- 16.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, and VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 75:6143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi, T., M. Sakurai, K. Abe, and Y. Itoyama. 1999. DNA fragmentation precedes aberrant expression of cell cycle-related protein in rat brain after MCA occlusion. Neurol. Res. 21:695-698. [DOI] [PubMed] [Google Scholar]
- 19.Hayes, T. E., N. L. Valtz, and R. D. McKay. 1991. Downregulation of CDC2 upon terminal differentiation of neurons. New Biol. 3:259-269. [PubMed] [Google Scholar]
- 20.Hellmich, M. R., H. C. Pant, E. Wada, and J. F. Battey. 1992. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc. Natl. Acad. Sci. USA 89:10867-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrup, K., and J. C. Busser. 1995. The induction of multiple cell cycle events precedes target-related neuronal death. Development 121:2385-2395. [DOI] [PubMed] [Google Scholar]
- 22.Ino, H., T. Ishizuka, T. Chiba, and M. Tatibana. 1994. Expression of CDK5 (PSSALRE kinase), a neural cdc2-related protein kinase, in the mature and developing mouse central and peripheral nervous systems. Brain Res. 661:196-206. [DOI] [PubMed] [Google Scholar]
- 23.Iseki, H., T. C. Ko, X. Y. Xue, A. Seapan, and C. M. Townsend, Jr. 1998. A novel strategy for inhibiting growth of human pancreatic cancer cells by blocking cyclin-dependent kinase activity. J. Gastrointest. Surg. 2:36-43. [DOI] [PubMed] [Google Scholar]
- 24.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 25.Jordan, R., L. Schang, and P. A. Schaffer. 1999. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J. Virol. 73:8843-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaya, S. S., A. Mahmood, Y. Li, E. Yavuz, and M. Chopp. 1999. Expression of cell cycle proteins (cyclin D1 and cdk4) after controlled cortical impact in rat brain. J. Neurotrauma 16:1187-1196. [DOI] [PubMed] [Google Scholar]
- 27.Kosz-Vnenchak, M., J. Jacobson, D. M. Coen, and D. M. Knipe. 1993. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J. Virol. 67:5383-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leclerc, V., J. P. Tassan, P. H. O'Farrell, E. A. Nigg, and P. Leopold. 1996. Drosophila Cdk8, a kinase partner of cyclin C that interacts with the large subunit of RNA polymerase II. Mol. Biol. Cell 7:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, E. Y., C. Y. Chang, N. Hu, Y. C. Wang, C. C. Lai, K. Herrup, W. H. Lee, and A. Bradley. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288-294. [DOI] [PubMed] [Google Scholar]
- 30.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lew, J., Q. Q. Huang, Z. Qi, R. J. Winkfein, R. Aebersold, T. Hunt, and J. H. Wang. 1994. A brain-specific activator of cyclin-dependent kinase 5. Nature 371:423-426. [DOI] [PubMed] [Google Scholar]
- 32.Maas, J. W., Jr., S. Horstmann, G. D. Borasio, J. M. Anneser, E. M. Shooter, and P. J. Kahle. 1998. Apoptosis of central and peripheral neurons can be prevented with cyclin-dependent kinase/mitogen-activated protein kinase inhibitors. J. Neurochem. 70:1401-1410. [DOI] [PubMed] [Google Scholar]
- 33.Makela, T. P., J. P. Tassan, E. A. Nigg, S. Frutiger, G. J. Hughes, and R. A. Weinberg. 1994. A cyclin associated with the CDK-activating kinase MO15. Nature 371:254-257. [DOI] [PubMed] [Google Scholar]
- 34.Margolis, T. P., F. Sedarati, A. T. Dobson, L. T. Feldman, and J. G. Stevens. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150-160. [DOI] [PubMed] [Google Scholar]
- 35.Matoba, R., K. Kato, C. Kurooka, C. Maruyama, Y. Sakakibara, and K. Matsubara. 2000. Correlation between gene functions and developmental expression patterns in the mouse cerebellum. Eur. J. Neurosci. 12:1357-1371. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto, Y., K. Hayashi, and E. Nishida. 1999. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 9:429-432. [DOI] [PubMed] [Google Scholar]
- 37.McShea, A., P. L. Harris, K. R. Webster, A. F. Wahl, and M. A. Smith. 1997. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. Am. J. Pathol. 150:1933-1939. [PMC free article] [PubMed] [Google Scholar]
- 38.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 39.Meyerson, M., G. H. Enders, C. L. Wu, L. K. Su, C. Gorka, C. Nelson, E. Harlow, and L. H. Tsai. 1992. A family of human cdc2-related protein kinases. EMBO J. 11:2909-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Migheli, A., R. Piva, S. Casolino, C. Atzori, S. R. Dlouhy, and B. Ghetti. 1999. A cell cycle alteration precedes apoptosis of granule cell precursors in the weaver mouse cerebellum. Am. J. Pathol. 155:365-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyajima, M., H. O. Nornes, and T. Neuman. 1995. Cyclin E is expressed in neurons and forms complexes with cdk5. Neuroreport 6:1130-1132. [DOI] [PubMed] [Google Scholar]
- 42. Murray, A. W., and D. Marks. 2001. Can sequencing shed light on cell cycling? Nature 409:844-846. [DOI] [PubMed] [Google Scholar]
- 43.Nagy, Z., and M. M. Esiri. 1998. Neuronal cyclin expression in the hippocampus in temporal lobe epilepsy. Exp. Neurol. 150:240-247. [DOI] [PubMed] [Google Scholar]
- 44.Nagy, Z., M. M. Esiri, A. M. Cato, and A. D. Smith. 1997. Cell cycle markers in the hippocampus in Alzheimer's disease. Acta Neuropathol. 94:6-15. [DOI] [PubMed] [Google Scholar]
- 45.Okano, H. J., D. W. Pfaff, and R. B. Gibbs. 1993. Rb and Cdc2 expression in brain: correlations with 3H-thymidine incorporation and neurogenesis. J. Neurosci. 13:2930-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osuga, H., S. Osuga, F. Wang, R. Fetni, M. J. Hogan, R. S. Slack, A. M. Hakim, J.-E. Ikeda, and D. S. Park. 2000. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 97:10254-10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padmanabhan, J., D. S. Park, L. A. Greene, and M. L. Shelanski. 1999. Role of cell cycle regulatory proteins in cerebellar granule neuron apoptosis. J. Neurosci. 19:8747-8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park, D. S., S. E. Farinelli, and L. A. Greene. 1996. Inhibitors of cyclin-dependent kinases promote survival of postmitotic neuronally differentiated PC12 cells and sympathetic neurons. J. Biol. Chem. 271:8161-8169. [DOI] [PubMed] [Google Scholar]
- 49.Park, D. S., B. Levine, G. Ferrari, and L. A. Greene. 1997. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J. Neurosci. 17:8975-8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng, J., N. F. Marshall, and D. H. Price. 1998. Identification of a cyclin subunit required for the function of Drosophila P-TEFb. J. Biol. Chem. 273:13855-13860. [DOI] [PubMed] [Google Scholar]
- 51.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 52.Raina, A. K., M. J. Monteiro, A. McShea, and M. A. Smith. 1999. The role of cell cycle-mediated events in Alzheimer's disease. Int. J. Exp. Pathol. 80:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rickert, P., W. Seghezzi, F. Shanahan, H. Cho, and E. Lees. 1996. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene 12:2631-2640. [PubMed] [Google Scholar]
- 54.Ross, M. E., and M. Risken. 1994. MN20, a D2 cyclin found in brain, is implicated in neural differentiation. J. Neurosci. 14:6384-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakai, K., K. Suzuki, S. Tanaka, and T. Koike. 1999. Up-regulation of cyclin D1 occurs in apoptosis of immature but not mature cerebellar granule neurons in culture. J. Neurosci. Res. 58:396-406. [PubMed] [Google Scholar]
- 56.Sakurai, M., T. Hayashi, K. Abe, Y. Itoyama, K. Tabayashi, and W. I. Rosenblum. 2000. Cyclin D1 and Cdk4 protein induction in motor neurons after transient spinal cord ischemia in rabbits. Stroke 31:200-207. [DOI] [PubMed] [Google Scholar]
- 57.Schang, L. M., A. Bantly, M. Knockaer, F. Shaheen, L. Meijer, M. H. Malim, N. S. Gray, and P. A. Schaffer. 2002. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J. Virol. 76:7874-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serizawa, H., T. P. Makela, J. W. Conaway, R. C. Conaway, R. A. Weinberg, and R. A. Young. 1995. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374:280-282. [DOI] [PubMed] [Google Scholar]
- 62.Shiekhattar, R., F. Mermelstein, R. P. Fisher, R. Drapkin, B. Dynlacht, H. C. Wessling, D. O. Morgan, and D. Reinberg. 1995. Cdk-activating kinase complex is a component of human transcription factor. Nature 374:283-287. [DOI] [PubMed] [Google Scholar]
- 63.Stefanis, L., D. S. Park, W. J. Friedman, and L. A. Greene. 1999. Caspase-dependent and -independent death of camptothecin-treated embryonic cortical neurons. J. Neurosci. 19:6235-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tal-Singer, R., T. M. Lasner, W. Podrzucki, A. Skokotas, J. J. Leary, S. L. Berger, and N. W. Fraser. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J. Virol. 71:5268-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timsit, S., S. Rivera, P. Ouaghi, F. Guischard, E. Tremblay, Y. Ben-Ari, and M. Khrestchatisky. 1999. Increased cyclin D1 in vulnerable neurons in the hippocampus after ischaemia and epilepsy: a modulator of in vivo programmed cell death? Eur. J. Neurosci. 11:263-278. [DOI] [PubMed] [Google Scholar]
- 66.Tsai, L. H., I. Delalle, V. S. Caviness, Jr., T. Chae, and E. Harlow. 1994. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371:419-423. [DOI] [PubMed] [Google Scholar]
- 67.Tsai, L. H., T. Takahashi, V. S. Caviness, Jr., and E. Harlow. 1993. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development 119:1029-1040. [DOI] [PubMed] [Google Scholar]
- 68.Tsavachidou, D., W. Podrzucki, J. Seykora, and S. L. Berger. 2001. Gene array analysis reveals changes in peripheral nervous system gene expression following stimuli that result in reactivation of latent herpes simplex virus type 1: induction of transcription factor Bcl-3. J. Virol. 75:9909-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsujioka, Y., M. Takahashi, Y. Tsuboi, T. Yamamoto, and T. Yamada. 1999. Localization and expression of cdc2 and cdk4 in Alzheimer brain tissue. Dement. Geriatr. Cogn. Disord. 10:192-198. [DOI] [PubMed] [Google Scholar]
- 70.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe, Y., T. Watanabe, M. Kitagawa, Y. Taya, K. Nakayama, and N. Motoyama. 1999. pRb phosphorylation is regulated differentially by cyclin-dependent kinase (Cdk) 2 and Cdk4 in retinoic acid-induced neuronal differentiation of P19 cells. Brain Res. 842:342-350. [DOI] [PubMed] [Google Scholar]
- 72.Winkler, M. T., L. S. Schang, A. Doster, T. Holt, and C. Jones. 2000. Analysis of cyclins in trigeminal ganglia of calves infected with bovine herpesvirus-1. J. Gen. Virol. 81:2993-2998. [DOI] [PubMed] [Google Scholar]
- 73.Ye, X., C. Zhu, and J. W. Harper. 2001. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc. Natl. Acad. Sci. USA 98:1682-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zindy, F., H. Soares, K. H. Herzog, J. Morgan, C. J. Sherr, and M. F. Roussel. 1997. Expression of INK4 inhibitors of cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 8:1139-1150. [PubMed] [Google Scholar]