Abstract
In this study, we evaluated the potency of a human papillomavirus (HPV) virus-like particle (VLP)-based vaccine at generating HPV type 11 (HPV-11)-specific cellular and humoral immune responses in seronegative women. The vaccine was administered by intramuscular immunizations at months 0, 2, and 6. A fourth immunization was administered to approximately half of the women at month 12. All vaccine recipients had positive HPV-11 VLP-specific lymphoproliferative responses at month 3 following the second immunization (geometric mean lymphoproliferative stimulation index [SI] = 28.4; 95% confidence interval [CI] = 16.9 to 48.0) and HPV-11 VLP-specific antibody titers following the first immunization at month 1 (geometric mean antibody titer = 53.9 milli-Merck units/ml, 95% CI, 34.8 to 83.7). In contrast, lymphoproliferative and antibody titer responses were never detected in the participants who received placebo. Relatively homogeneous lymphoproliferative responses were observed in all vaccinated women. The mean lymphoproliferative SI of the vaccinated group over the first 12 months of the study was 7.6-fold greater than that of the placebo group following the initial immunization. The cellular immune responses generated by VLP immunization were both Th1 and Th2, since peripheral blood mononuclear cells from vaccinees, but not placebo recipients, secreted interleukin 2 (IL-2), IL-5, and gamma interferon (IFN-γ) in response to in vitro stimulation with HPV-11 VLP. The proliferation-based SI was moderately correlated with IFN-γ production and significantly correlated with IL-2 production after the third immunization (P = 0.078 and 0.002, respectively). The robust lymphoproliferative responses were specific for HPV-11, since SIs generated against bovine papillomavirus and HPV-16 VLPs were not generally observed and when detected were similar pre- and postimmunization.
Human papillomaviruses (HPVs) are small double-stranded DNA viruses that infect cutaneous and mucosal epithelial cells and cause benign and malignant hyperproliferative lesions, such as genital warts and cervical cancer (56). Condylomata acuminatum (genital warts) is the most commonly diagnosed sexually transmitted viral disease in the United States (29), and approximately 95% of genital warts are caused by infection with the low-risk HPV types 6 and 11 (HPV-6 and -11) (5, 16). Although genital warts do not have a propensity for malignant transformation, they are a cause of great psychosocial morbidity. Since all available therapies are associated with high rates of recurrence, the development of a vaccine to prevent the occurrence of HPV-6 and -11-induced lesions is needed. The present study was designed to evaluate the immunogenicity of a virus-like particle (VLP)-based HPV-11 vaccine in a phase I human trial.
While early clinical studies of HPV VLP-based vaccines are presently under way, little is understood about the immune responses generated in vaccine recipients and the specific types of cellular immunity that will be required for long-term protection are unknown. Based on the pathogenesis of HPV infection and disease, two main strategies have been proposed for the development of a successful HPV vaccine. The first strategy is to prime neutralizing antibodies, preferentially at the mucosal (and cutaneous) sites, so that infection of epithelial cells can be prevented. A second strategy is to elicit HPV-specific T cells, as virus-specific T cells have been shown to be important for effectively controlling and eradicating numerous viral infections (26, 33, 34, 41, 46).
When expressed in bacteria or eukaryotic cells, the papillomavirus capsid protein L1, alone or in combination with L2, autoassembles to form intact VLPs that morphologically and antigenically resemble native virion (17). Immunization of animals with various papillomavirus VLP-based vaccines has been shown to elicit high antibody titer (4, 22, 23, 27, 32, 52) and durable T-cell responses (10, 31, 37, 40). The presence of vaccine-induced neutralizing antibodies was shown to correlate with complete protection against viral challenge in the cottontail rabbit papillomavirus rabbit model (4, 22), the canine oral papillomavirus dog model (52), and the bovine papillomavirus (BPV) cow model (23, 27).
There is clinical evidence that cellular immune responses play an important role in the outcome of HPV infection and disease (25, 48). Specifically, infiltrating CD4+ and CD8+ T cells have been observed in spontaneously regressing warts (9, 21, 36). In addition, the prevalence of HPV-associated lesions is increased in human immunodeficiency virus-infected patients (28) and transplant recipients (18, 45), both of whom are known to have impaired cell-mediated immunity. In this study, we measured HPV-11 VLP vaccine priming of humoral and cellular immune responses in seronegative, HPV DNA-negative, college-aged women.
MATERIALS AND METHODS
Study participants.
Fifty-five college-aged women (ages, 18 to 25 years) attending the University of New Mexico (UNM) were enrolled in a phase I HPV-11 VLP vaccine study between 30 March and 30 June 1998. These participants represent a subset of women enrolled in a multisite trial conducted at Indiana University and UNM. The vaccine study was designed as a randomized, double-blind, placebo-controlled trial. Doses of 10, 20, 50, and 100 μg of VLP vaccine were administered in 0.5-ml intramuscular injections over 6 months in which three immunizations were given at months 0, 2, and 6. Half of consenting participants received a fourth immunization at month 12. Eligibility criteria for study participants required that all volunteers be in general good health and have no history of genital warts or abnormal cervical cytology. Additionally, at screening, women were ineligible for the vaccine study if they demonstrated positive results for HPV-6 or -11 DNA at any of four anogenital sites or if they tested positive for HPV-6 or -11 antibodies (6).
Of the 55 participants enrolled at UNM, 30 individuals consented to donate blood for the studies presented here. Only participants in the 50- or 100-μg dose groups were evaluated for HPV-11 VLP-specific cellular immune responses over the 36-month trial. Six women who were not part of the vaccine study and who reported no previous sexual intercourse (i.e., virginal) were also included in this study. These women were considered a reference control group when the specificity of the vaccine-induced proliferative response was assessed (age range, 17 to 26 years; median age, 22 years). Informed written consent was obtained from all women before participation in the study. Laboratory assays were performed with no knowledge of the placebo or vaccine status of the individual participants. This study was approved by the UNM School of Medicine Human Research Review Committee.
HPV-11 VLP vaccine.
The HPV-11 VLP vaccine, consisting of the HPV-11 L1 protein only, was produced in Saccharomyces cerevisiae (32), and VLPs were adsorbed to aluminum hydroxyphosphate adjuvant. The VLP was highly purified (>98%) as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and colloidal Coomassie staining. The placebo consisted of aluminum hydroxyphosphate adjuvant alone.
Specimen collection.
Before the receipt of vaccine or clinical examination, blood was collected into Vacutainer Cell Preparation Tubes containing sodium citrate (Becton Dickinson, Bridgeport, N.J.) and was centrifuged at 1,600 × g for 25 min at room temperature (RT) within 4 h of blood collection. Peripheral blood mononuclear cells (PBMCs) were washed twice prior to being resuspended in culture medium AIM-V (Gibco, Rockville, Md.) supplemented with 1% penicillin-streptomycin (Sigma, St. Louis, Mo.) and 1% human AB serum (Gemini Bio-Products, Calabasas, Calif.). PBMCs were used directly or were cryopreserved for later analysis. All assays were performed with no knowledge of the vaccine or placebo status of the study participant specimens tested.
Antibody titer/RIA.
A competitive radioimmunoassay (cRIA) was used to measure titers of serum antibodies to HPV-11 VLPs as previously described (6). Antibody titers were quantified against a serum standard and were expressed in arbitrary units as milli-Merck units (mMU)/milliliter. The HPV-11 antibody cRIA measures the competition between HPV-11 antibodies in participant serum against an HPV-11 monoclonal antibody (MAb) (MAB8740; Chemicon International, Inc., Temecula, Calif.) that was shown to neutralize HPV-11 and does not cross-react with HPV-6 conformationally dependent, neutralizing epitopes (7).
Immunoglobulin-specific covalent coupling of HPV-11 VLPs to Luminex microspheres.
HPV-11 VLPs were coupled to Luminex microspheres (Luminex Corp., Austin, Tex.) using an N-hydroxysuccinimide-enhanced, carbodiimide-mediated coupling reaction (50). Luminex microspheres are fluorescent polystyrene beads approximately 5,000 nm in diameter with functional carboxyl groups that covalently attach proteins for use in multiplex fluorescence-based immunoassay (54). The carboxylated sites on the surface of the microspheres were activated by adding 50 μl of a 50-mg/ml solution of N-hydroxysuccinimide and 50 μl of a 50-mg/ml solution of 1-ethyl-3-[3-dimethylaminopropyl]-carbodiimide hydrochloride. Following the activation step, the microspheres were washed once in 500 μl of 50 mM 2-(N-morpholino)ethanesulfonic acid (pH 6.2)-buffered saline before the addition of the HPV-11 VLPs to microsphere 53. VLP microspheres were stored in a histidine buffer (20 mM histidine, 0.5 M NaCl, pH 6.2) with 1% bovine serum albumin (BSA) in light-resistant vials.
Luminex serology assay.
Immunoglobulin-specific antibodies to HPV-11 L1 VLP were detected in 40 study participant serum samples by use of a fluorescence-based capture assay performed in a 96-well format on a Luminex100. A panel of murine anti-human isotype-specific MAbs specific to immunoglobulin M (IgM), IgA1 and IgA2, IgG1, IgG2, IgG3, IgG4, and IgE were used to detect human isotype-specific antibodies bound to the HPV-11 VLPs. Murine anti-human IgM (G20-127), IgA1/IgA2 (G20-359), IgG1 (G17-1), IgG2 (G18-21), IgG4 (JDC14), and mouse anti-human total IgG were purchased from PharMingen, San Diego, Calif. The murine anti-human IgG3 (HP-6050) and human Ig-adsorbed, fluorescently labeled goat anti-mouse IgG-phycoerythrin were purchased from Sigma. Participant sera were diluted in phosphate-buffered saline (PBS)-1% BSA at 1:10, 1:50, 1:250, and 1:1,250 to detect IgM, IgA, IgE, IgG2, IgG3, and IgG4 and additional dilutions of 1:6,250, 1:31,250, and 1:156,250 to detect IgG1 and total IgG levels. Sera were incubated with 5,000 HPV-11 VLP microspheres overnight at RT, at which time the HPV-11 VLP microspheres were washed three times with PBS and resuspended in 50 μl of PBS-1% BSA. Mouse anti-human Ig isotype-specific MAbs (50 μg/ml) were added for 2 h at RT; the samples were washed three times with PBS and resuspended in 50 μl of PBS-1% BSA, and the fluorescent goat anti-murine phycoerythrin antibody (50 μg/ml) was added in 50 μl of PBS-1% BSA and incubated for 30 min at RT. The samples were washed three times with PBS-1% BSA and were analyzed on the Luminex100. At least 50 VLP microspheres were analyzed per sample at each dilution. Sera were designated positive, at a given dilution, if the absolute median fluorescent intensity was greater than the mean plus 5 standard deviations from the mean of a panel of known HPV-negative antibody sera.
Lymphocyte proliferation assay.
PBMCs were plated in 96-well round-bottomed plates at 2 × 105 and 5 × 104 cells/well in four or six replicates. Cells were cultured in the absence or presence of 5.0 μg of HPV-11 VLP/ml or with 0.75 μg of phytohemagglutinin (PHA; Murex Diagnostics Limited, Dartford, England)/ml. Stimulation of PBMCs in four or six replicates with 0.05 μg of yeast lysate/ml was included in these assays to monitor possible T-cell priming by any potential contamination of yeast protein following VLP purification. After 5 days of culture, plates were pulsed with 1.0 μCi of [3H]thymidine per well, and cells were harvested 6 to 8 h later. For type-specific analyses 5.0 μg of the following antigen preparations per ml was used in proliferation assays: BPV and HPV-16 VLP (VLP-16 [bac]) produced by recombinant baculovirus (DynCorp, Rockville, Md.) and HPV-16 VLP (VLP-16 [yeast]) produced in yeast as well as 0.05 μg of baculovirus and yeast extracts per ml. Lymphoproliferation results were presented as a stimulation index (SI) and were calculated as the geometric mean of counts per minute of cells cultured in the presence of VLP divided by the geometric mean of cells cultured in the absence of VLP antigen (culture media alone). An SI of 5.0 or greater was scored positive. All positive responses had a change in counts per minute of ≥1,000 (calculated as the experimental counts per minute minus the background counts per minute; data not shown).
Cytokine detection.
The levels of gamma interferon (IFN-γ), interleukin 5 (IL-5), and IL-2 present in the 48-h supernatants of HPV-11 VLP (5.0 μg/ml)-stimulated PBMCs were determined by enzyme-linked immunosorbent assays (ELISAs) and HT-2 bioassays. The OptEIA Human IFN-γ and IL-5 sets (PharMingen) were used to detect IFN-γ and IL-5, and the ELISAs were essentially performed per the manufacturer's protocol. ELISAs were performed in duplicate. Data were analyzed as the mean experimental measurement minus the mean background measurement. IL-2 production in culture supernatant was measured in duplicate in a bioassay with the IL-2-dependent cell line HT-2 (12), and results were presented as SIHT-2.
ELISPOT assay.
ImmunoSpot P50 plates (Cellular Technologies Ltd., Cleveland, Ohio) were coated with IFN-γ or IL-5 capture antibodies (PharMingen, at 1.5 and 2.0 μg/ml, respectively) in 50 μl of 1× PBS overnight at 4°C. The following day plates were washed three times with PBS and then three times with PBS containing 0.05% (vol/vol) Tween 20 (PBS/T). The plates were then blocked with BSA (10 g/liter in PBS/T) (BSA from Sigma) for 2 h at RT. Plates were washed three times with PBS before cells and antigens were added as specified. Cryopreserved PBMCs from study participants were plated in triplicate at 5 × 104 cells/well in a 96-well plate with 5.0 μg of HPV-11 L1 VLP and HPV-16 L1 VLP per ml expressed in S. cerevisiae and HPV-16 L1 VLP and BPV L1 VLP produced by baculovirus vectors in Tricholusia ni. Negative wells containing only medium (10% fetal calf serum, 1% penicillin-streptomycin in RPMI medium 1640; Gibco) and positive control wells containing PHA were included. Wells containing 0.05 μg of yeast or insect lysate per ml were added as control wells to test any potential expression system contaminants. T-cell subsets were isolated using anti-CD8+ antibodies bound to magnetic beads (Dynal, Oslo, Norway) according to the manufacturer's instructions. Cells were cultured at 37°C and 5% CO2 in the incubator for 24 or 48 h when testing for the production of IFN-γ and IL-5, respectively.
After antigen activation for IFN-γ and IL-5, cells were discarded and the enzyme-linked immunospot (ELISPOT) plates were washed three times with PBS and three times with PBS/T. Biotinylated detection antibody was added (0.75 μg/ml for both biotin anti-human IFN-γ and IL-5) and was incubated overnight at 4°C. The following day, plates were washed three times with PBS/T and their contents were subsequently incubated for 2 h at RT with the tertiary reagent horseradish peroxidase-conjugated streptavidin (DAKO, Carpinteria, Calif.) diluted 1:2,000 in PBS/T containing 1% BSA. Plates were washed three times with PBS/T and three times with PBS. Spots were developed by adding 200 μl of freshly made development solution to each well. This solution consists of 3-amino-9-ethylcarbazole (AEC; Pierce Pharmaceuticals, Rockford, Ill.; 10 mg per ml of N,N′-dimethylformamide) freshly diluted 1:30 in 0.1 M sodium acetate, pH 5.0. To remove particulates, the AEC solution was filtered (0.2 μm) and H2O2 was then added to a final concentration of 0.015% before immediate use. Once the development solution was added, the plates were kept at RT for 15 to 45 min until spots became macroscopically visible. Rinsing with distilled water stopped the colorimetric reaction. The plates were air dried overnight before they were subjected to image analysis on a Series 1 ImmunoSpot Image Analyzer (Cellular Technologies Ltd.). ELISPOTs were counted using an ImmunoSpot Analyzer (Cellular Technologies Ltd.) with set criteria applied to the software to assess spot size and density. The number of spots generated against each antigen was calculated as the average number of spots per experiment minus the average number of spots per background wells. ELISPOT results are presented as spots per 106 PBMCs.
Statistical methods.
All comparisons of lymphoproliferation SI, antibody titer, and cytokine responses between the two vaccine dose groups (50 and 100 μg of HPV-11 VLP) or between the combined vaccine group and the placebo group were evaluated for statistical significance using the nonparametric Wilcoxon test and exact two-sided P values. A P of <0.050 was used for statistical significance in all cases. All correlations between antibody titer, SI, and cytokine production are Spearman rank correlations. SI and antibody titer levels were log transformed before calculation of means and confidence intervals (CIs). A mixed model analysis was performed to compute within and between subject variances in SIs after the administration of the first immunization (months 1 to 12).
RESULTS
HPV-11 VLP vaccine-induced lymphoproliferation.
Cellular immune responses were monitored in 30 participants: 8 women received the 50-μg vaccine dose, 13 received the 100-μg vaccine dose, and 9 received the placebo (Table 1). Geometric mean SIs against the positive control mitogen, PHA, over all study visits were 41.0 (95% CI, 30.7 to 54.8) for placebo and 50.4 (95% CI, 40.9 to 62.3) for vaccine recipients. Prior to vaccination, no study participants had detectable HPV-11VLP-specific lymphoproliferative responses (SI cutoff of 5.0; Fig. 1, top panel), suggesting that none of these women had been previously exposed to HPV-11. Throughout the duration of the study, HPV-11 VLP-induced SI responses of placebo recipients remained below the assay cutoff. In contrast, positive proliferative T-cell responses against HPV-11 VLP (SI ≥ 5.0) were detected in women who received the VLP immunization (Fig. 1, top panel).
TABLE 1.
Immunization scheme for participants of an HPV-11 VLP-based vaccine study
| Vaccine dose | No. of participants | No. who received 3 dosesa | No. who received 4 dosesa |
|---|---|---|---|
| 50 μg | 8 | 3 | 5 |
| Placebo for 50-μg group | 5 | 4 | 1 |
| 100 μg | 13 | 6 | 7 |
| Placebo for 100-μg group | 4 | 2 | 2 |
Vaccine was administered in three or four intramuscular injections at months 0, 2, and 6 or 0, 2, 6, and 12, respectively. VLP was given in a 50- or 100-μg dose in aluminum adjuvant. Placebo recipients received adjuvant alone.
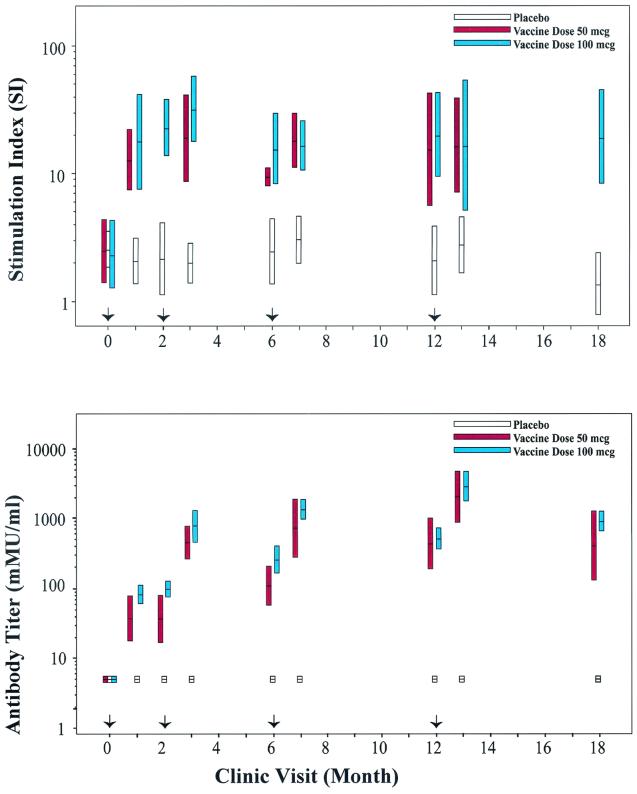
FIG. 1.
Lymphoproliferative responses and antibody titers in HPV-11 VLP vaccine participants. Women in both dose groups (50 and 100 μg) are represented separately, while placebo recipients from both dose groups are combined. The bars indicate the 95% CIs for the geometric mean SI and antibody titer. Arrows at months 0, 2, 6, and 12 represent when vaccine was administered. Top panel, T-cell proliferation was measured by standard lymphoproliferation assays at designated times throughout the vaccination regime. Lymphoproliferation results are presented as SIs. Participants who received a 50-μg HPV-11 VLP vaccine dose regime were not monitored for lymphoproliferation at months 2 and 18. Bottom panel, HPV-11 VLP-specific antibody titers were measured by standard RIA methods at designated times throughout the vaccination regime. The data were quantified against a serum standard and expressed in arbitrary mMU per milliliter.
A Wilcoxon nonparametric test of significance at each time point provided no evidence for a difference in SI levels between the 50-μg and 100-μg dose groups; therefore; all analyses were performed on data combined from both dose groups. At 1 month following the initial priming immunization, PBMCs from all but two of the vaccinated women proliferated (SI ≥ 5.0) in response to VLP (Fig. 2, month 1). All vaccine recipients demonstrated robust proliferative T-cell responses following the second and third immunizations, and the mean SI did not change significantly once the first immunization was received (Fig. 2, months 3 and 7, respectively). After the initial immunization, the ratio of between-subject variance to within-subject variance for vaccine recipients was 0.14, indicating that subjects were relatively homogenous in their lymphoproliferative responses. Using the between-subject variance and the vaccine group SI of 17.4, it was estimated that 99% of the subjects had a positive SI within the interval (7.9 to 38.1). For placebo recipients, the mean SI was 2.3 and the ratio of between- to within-subject variance was 0.19 (99% interval for the SI was 1.3 to 4.0). Thus, in the first 12 months of this study the SI responses within the vaccine and placebo groups varied 4.8- and 3.1-fold, respectively, and the mean SI response of the vaccine recipients was 7.6-fold greater than that of the placebo recipients.
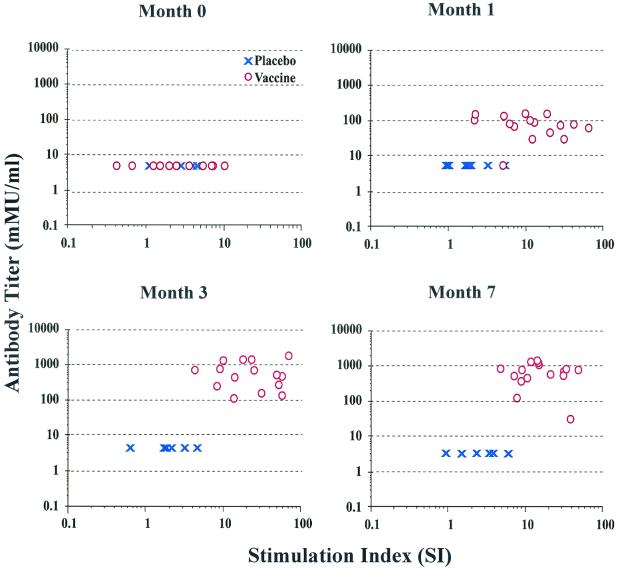
FIG. 2.
HPV-11 VLP-specific antibody titer versus SI. Measurements of humoral and cellular immunity are presented at baseline (month 0) and after first, second, and third immunizations (months 1, 3, and 7, respectively) in vaccine and placebo recipients. Lymphoproliferation results are presented as SI, and antibody data are presented in mMU per milliliter.
Kinetics of HPV-11 VLP-specific antibody and lymphoproliferative responses following vaccination.
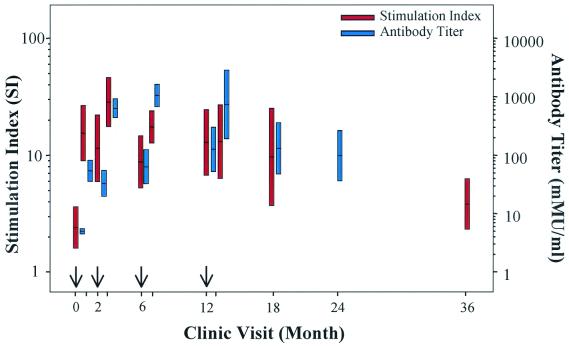
Prior to immunization, none of the participants had a detectable HPV-11-specific antibody titer (cutoff value of 10 mMU/ml). Similarly, the HPV-11 antibody titers assessed by cRIA for the placebo group were never above 10 mMU/ml (Fig. 1, bottom panel). HPV-11 VLP-specific, immunization-dependent cRIA titers were observed immediately following the first priming immunization, and significant titers (>200 mMU/ml) were achieved in most patients following the third immunization (geometric mean titer = 1,052.7 mMU/ml; 95% CI, 656.5 to 1,688.0). These cRIA titers correlated well with neutralization of HPV-11 virions as measured by an athymic mouse xenograft model (6); 63 of 69 (91.3%) postimmunization serum specimens with cRIA titers of >200 mMU/ml were 100% neutralizing when tested with this assay. In contrast to the absolute SI measurements that remained relatively constant following vaccination, an increase in antibody titer was observed following consecutive immunizations (Fig. 2). No association was observed between the magnitude of antibody titers and SI in the vaccine group. While humoral and cellular immune responses were not correlated within each individual, an obvious similarity was apparent in the global responses of the B- and T-cell populations to vaccination over time with a gradual decline in the absolute values of antibody titer and SIs 24 to 30 months following the last vaccine administration (Fig. 3).
FIG. 3.
Persistence of HPV-11-specific humoral and cellular immune responses. Lymphoproliferative responses and antibody titers in HPV-11 VLP-immunized women are presented for 36 months of the vaccine study. The left y axis represents the T-cell lymphoproliferative response (SI), while the right y axis represents antibody titers (mMU/milliliter). The bars indicate the 95% CIs for the geometric mean SI and antibody titer. Arrows at months 0, 2, 6, and 12 represent when vaccine was administered. SIs and antibody titers are not presented for the 24- and 36-month time points, respectively.
Immunoglobulin isotype and subclass responses to HPV-11 VLP vaccination.
The sera of 40 study participants (n = 10 placebo and n = 30 vaccine recipients) were analyzed at month 7, 1 month following three immunizations, for HPV-11 VLP-specific IgM, IgA, total IgG, IgG1, IgG2, IgG3, IgG4, and IgE. Results are presented in Table 2. Twenty-nine of 30 (96.7%) sera from women receiving the vaccine (cRIA titers > 10 mMU/ml) were seropositive for total IgG antibodies, and 25 of 30 (83%) were seropositive for IgA subclass antibodies. Total IgG seropositive responses were of the IgG1 isotype, and over half (17 of 30) of the vaccine recipient sera tested were also seropositive for IgG2 isotype antibodies. In addition, HPV-11 VLP-specific IgG3 and IgG4 antibodies were detected in the majority of sera tested, 86.7 and 100%, respectively. HPV-11 VLP-specific IgM antibodies were also detected in 5 of 30 (16.7%) vaccine recipients. No IgE was detected in either vaccine or placebo recipients. Minimal HPV-11 VLP-specific subclass or isotype immunoglobulins were detected in sera from women receiving placebo; two placebo recipients were seropositive for IgA, and a third placebo recipient was seropositive for IgG2. It should be noted that the one vaccine recipient who was seronegative for both total IgG and IgG1 when assayed at the 1:31,250 dilution was seropositive when tested at the 1:250 dilution (data not shown). At this lower dilution, none of the placebo recipients were seropositive for either total IgG or IgG1; the dilution was raised, however, in order to measure other vaccine recipient responses in the linear range of the assay.
TABLE 2.
Number of patient sera that were seropositive for HPV-11 VLP immunoglobulin isotype or subclass at month 7
| Groupd (no. of patients) | No. of sera positive for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IgMb | IgAb | Total IgGc | IgG1b | IgG2a | IgG3a | IgG4a | IgEa | |
| Placebo* (n = 10) | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 |
| Vaccine** (n = 30) | 5 | 25 | 29 | 29 | 17 | 26 | 30 | 0 |
Serum dilution at 1:25.
Serum dilution at 1:1,250.
Serum dilution at 1:31,250.
∗, cRIA ≤ 10 mMU/ml; ∗∗, cRIA > 10 mMU/ml.
Antigen specificity of HPV-11 VLP-lymphoproliferative responses.
Lymphoproliferative responses against the yeast lysate control were uncommon and were observed in both vaccine and placebo recipients alike over the duration of the 36-month study (data not shown). When yeast lysate responses were used as the denominator in the SI calculations (rather than culture media alone), the comparison of vaccine to placebo responses was not changed; therefore, these analyses are not presented. It is likely that most responses to yeast lysate resulted from prior exposure to yeast rather than proliferation of PBMCs that was specific for yeast proteins potentially contaminating the VLP vaccine preparation.
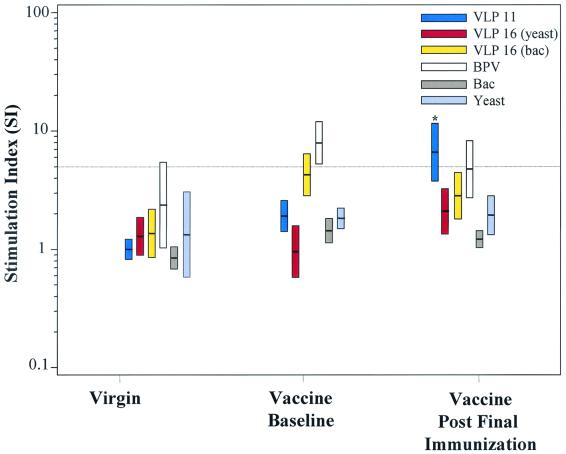
We further measured the HPV type specificity of the vaccine-induced response by using multiple papillomavirus VLPs to stimulate PBMCs from 12 vaccine recipients (at baseline and 1 month following the last immunization [month 7 or 13]) and from six women with no previous sexual history (virginal women). HPV-16 VLPs produced by both recombinant baculovirus and yeast expression systems were tested, as were BPV1 VLPs produced by a baculovirus expression system. Yeast lysate and baculovirus extract were also included in these type specificity experiments as controls to measure lymphoproliferative responses to potential VLP expression system contaminants. As previously observed, yeast-activated SIs were occasionally measured, but baculovirus extract was never stimulatory in any of the virginal women or in the vaccine study participants at baseline or after complete immunization (Fig. 4). In the six virginal women tested, HPV-11, BPV, and HPV-16 VLPs generally did not activate PBMCs. Responses to BPV were greatest in the group of virginal women, although a mean SI of 2.4 and 95% CI (1.0 to 5.4) were below the assay cutoff.
FIG. 4.
Specificity of lymphoproliferative responses: evaluation of multiple papillomavirus antigens. Proliferation assays were performed using multiple papillomavirus VLPs and control lysates of the respective VLP expression systems. Six virginal women and 12 vaccinated women (at month 0 and month 7 or 13) were analyzed for proliferative responses against the following antigen preparations: BPV, HPV-16 VLP prepared in yeast (VLP16 [yeast]), HPV-16 VLP expressed by baculovirus (VLP16 [bac]), HPV-11 VLP, and yeast and baculovirus (Bac) lysates. The bars indicate the 95% CI for the geometric mean SI. Statistical significance is indicated (∗) from a comparison of baseline measurements for lymphoproliferation against HPV-11 VLP in vaccine recipients (P = 0.001).
HPV-11-pecific responses were not detected at baseline in vaccine study participants (geometric mean SI, 1.91; 95% CI, 1.42 to 2.6), but after the last immunization, significant increases from baseline measurements were observed only in response to HPV-11 VLP, geometric mean SI, 6.6 (95% CI 3.7 to 11.6); P = 0.001. In contrast, vaccine-independent baseline responses against HPV-16 and BPV VLPs produced by recombinant baculovirus were measured in vaccine study participants (geometric mean SI, 4.3 and 7.9; and 95% CI, 2.8 to 6.4 and 5.3 to 12.0, respectively). Mean SI values against BPV remained positive in the vaccinated group following the last immunization, while responses against HPV-16 VLP produced by baculovirus dropped below the assay cutoff. These data demonstrate that type-specific proliferative responses were induced by HPV-11 VLP vaccination.
HPV-11 VLP-specific immune responses and cytokine production primed by vaccination.
Having shown that HPV-11 VLP-specific cellular and humoral immune responses were induced by vaccination, we next investigated whether the VLP-based vaccine elicited a Th1 or Th2 (IFN-γ or IL-5) cytokine pattern of response. PBMCs from both vaccine and placebo recipients were tested by ELISA for their capacity to produce IL-2, IL-5, and IFN-γ in response to 48 h of restimulation with HPV-11 VLP. Both IFN-γ and IL-5 were produced in vitro by vaccine but not placebo recipients (Table 3). IL-2 production was more readily detectable in the vaccine versus the placebo group, but there were no statistically significant differences in levels detected. IFN-γ production by vaccine recipients compared to that by placebo recipients was significantly different at three of four time points measured (P = 0.036, 0.000, 0.051, and 0.005 after first, second, third, and fourth immunizations, respectively [Table 3]), and IL-5 production was significantly greater in the vaccine group following the third and fourth immunizations (P = 0.009 and 0.027, respectively [Table 3]).
TABLE 3.
Characterization of HPV-11 VLP cytokine responses primed by immunization
| Cytokine produced | Immunization after which response was measured | No. of participants and cytokine response in placebo groupb
|
No. of participants and cytokine response in vaccine group
|
P (Wilcoxon) | ||||
|---|---|---|---|---|---|---|---|---|
| na | Mean amt | Median amt | n | Mean amt | Median amt | |||
| IFN-γ | 1st | 9 | 1.4 | 0.0 | 11 | 67.7 | 14.0 | 0.036 |
| 2nd | 8 | 0.5 | 0.0 | 12 | 198.4 | 25.5 | 0.000 | |
| 3rd | 7 | 0.4 | 0.0 | 12 | 233.8 | 26.0 | 0.051 | |
| 4th | 7 | 0.0 | 0.0 | 10 | 141.9 | 12.0 | 0.005 | |
| IL-5 | 1st | 9 | 0.0 | 0.0 | 11 | 1.4 | 0.0 | 0.098 |
| 2nd | 8 | 0.4 | 0.0 | 12 | 2.7 | 0.5 | 0.287 | |
| 3rd | 7 | 0.3 | 0.0 | 12 | 19.4 | 6.5 | 0.009 | |
| 4th | 7 | 0.0 | 0.0 | 10 | 15.8 | 2.5 | 0.027 | |
| IL-2 | 1st | 9 | 2.4 | 2.0 | 12 | 3.0 | 2.0 | 0.866 |
| 2nd | 8 | 1.9 | 1.0 | 12 | 4.7 | 3.0 | 0.053 | |
| 3rd | 7 | 3.3 | 1.0 | 12 | 6.5 | 5.5 | 0.061 | |
| 4th | 7 | 4.3 | 5.0 | 11 | 10.5 | 3.0 | 0.844 | |
n = the number of participants evaluated. Cytokine production by PBMCs from a minimum of 7 placebo recipients and 10 vaccine recipients was examined at all time points. The same individuals plus one or two additional participants were evaluated at various time points.
Production of IFN-γ, picograms per milliliter; IL-5, picograms per milliliter; and IL-2, SIHT-2.
The observed cytokine secretion patterns detected by ELISA were supported by ELISPOT analyses of both IFN-γ and IL-5 production by PBMCs from vaccine recipients at month 0 and months 7 through 13. IFN-γ production was detected following the last immunization with a mean of 148.9 spots per million PBMCs (median, 58.3); no IFN-γ was detected at baseline (mean, 2.1; median, 0.0). This frequency of HPV-11 VLP-specific PBMCs represents approximately 0.015% of the total HPV-11 VLP-stimulated cells or 15 antigen-specific cells per 100,000 cultured PBMCs. Similar to what was observed in the ELISA results, IL-5 responses were detectable but at lower levels than those of IFN-γ. The mean value for IL-5 produced after the last immunization was 73.2 spots/million PBMCs (median, 21.7); no IL-5 was detected at baseline (mean, 8.2; median, 0.8). ELISPOT assays were also performed with CD8+-depleted and CD8+-enhanced T-cell subsets at 1 month following the last immunization. These experiments demonstrated that the T-cell subpopulation depleted of CD8+ T cells was the predominant population with detectable IFN-γ and IL-5 production (mean and median values of spots per million CD8+-depleted lymphocytes were 392.2 and 271.7 and 57.0 and 26.7, respectively). When calculated as a percentage of the CD8+-depleted cultures, these frequencies represent 0.039% of the stimulated cultures or approximately 40 antigen-specific cells per 100,000 CD8+ depleted cells. The percentage of IL-5-producing, CD8+-depleted lymphocytes was 0.027% or about 30 antigen-specific cells per 100,000 CD8+-depleted lymphocytes. Minimal IFN-γ was produced by the CD8+-enriched T-cell cultures; mean and median values of spots per million CD8+ T cells were 28.0 and 0.0, respectively, and no IL-5 was produced.
Associations were observed between the ELISA cytokine patterns produced by vaccine recipients and lymphoproliferation or antibody titers. SI levels were moderately correlated with IFN-γ production and were significantly correlated with IL-2 production after the third immunization (Spearman correlation, 0.53; P = 0.078; and 0.79, P = 0.002, respectively). IL-2 production was also significantly correlated with antibody titer following the fourth immunization (Spearman correlation, 0.68; P = 0.020). Overall, these data demonstrate a Th1/Th2 cytokine response induced by HPV-11 VLP vaccine in seronegative, college-aged women.
DISCUSSION
The data presented here demonstrate that a recombinant HPV-11 L1 VLP vaccine could elicit HPV-specific antibody and proliferative T-cell responses in 18- to 25-year-old women not exposed to HPV-11. All vaccine recipients had detectable proliferative responses following the second immunization, while vaccine-dependent antibody responses were observed immediately following the first priming immunization.
The results of this study do not indicate a correlation between the magnitude of antibody titer and proliferative T-cell responses in individual vaccine recipients. While it is thought that antibody production is facilitated by T-helper cells, the study results suggest that the dynamics of B- and T-cell populations within a single person cannot be analyzed in terms of a linear association between antigen-specific antibody secretion and lymphocyte proliferation. An increase in antibody titer was observed following each administration of the vaccine. This contrasted with lymphoproliferative responses that appear to achieve a maximum level of stimulation following initial immunization. Overall, HPV-specific T-cell activity was observed as a discrete proliferative response and there was no trend in average SI after the initial immunization. In other words, once the lymphoproliferative response was primed, the magnitude of that response did not change significantly with subsequent immunizations. This observation is consistent with the present understanding of the maintenance of homeostasis in memory T-cell populations (14) and may be due to a “physiologic limit” of antigen-specific T cells that can be present in a host at any given time. Although there was no significant effect of vaccination on SI levels following the administration of the first immunization, it remains possible that the subsequent immunizations played a role in conferring long-lived proliferative T-cell responses.
To investigate whether HPV-11 VLP immunization could prime cross-reactive T-cell responses against other papillomavirus types, we performed lymphoproliferation assays on PBMCs from a subset of study participants and virginal women. Multiple papillomavirus VLP sources, including HPV-16 and BPV VLPs, were used for antigenic stimulation. A statistically significant increase between baseline proliferative responses and those measured 1 month following the third immunization was observed only against the HPV-11 VLP in vaccine recipients. Neither HPV-11 nor HPV-16 VLPs elicited lymphoproliferative responses in PBMCs isolated from virginal women. In both vaccinated and virginal women, BPV VLP most commonly elicited responses independent of the receipt of vaccine. In addition, mean responses against BPV VLP in vaccine participants were higher at baseline than after the receipt of three or four HPV-11 VLP immunizations, indicating that this response was not vaccine induced. Similarly, one of two HPV-16 VLP preparations (i.e., HPV-16 VLP produced in recombinant baculovirus-infected insect cells) elicited vaccine-independent responses in vaccine study participants at baseline, although the mean SI level generated was not above the cutoff for a positive SI value (Fig. 4).
It is possible that some vaccine study participants had previous exposure to HPVs other than types 6 and 11 before their inclusion into this study; therefore, in vitro stimulation of PBMCs by BPV or HPV-16 VLPs may have induced a memory response against a conserved papillomavirus capsid epitope, albeit a putative common T-cell epitope has not been reported (20, 51). In fact, PCR-based HPV-16 DNA analysis of anogenital specimens from vaccine study participants over two years of the study revealed that two of the participants analyzed in these type-specific lymphoproliferation assays had been exposed to HPV-16 (data not shown). Both of these participants demonstrated HPV-16-specific lymphoproliferative memory responses and would therefore account for some of the HPV-16 type-specific proliferation observed.
The lymphoproliferative responses to BPV VLP by PBMCs from virginal women as well as from vaccine study participants pre-and post-HPV-11 VLP vaccination may be due to a general mitogenicity of the VLP structure, as has been suggested (30, 39). Other investigators have observed BPV VLP responses at baseline measurements in study participants of HPV-16 VLP-based vaccine trails (A. Hildesheim and L. Pinto, personal communication). Taken together, these data suggest that, whether due to a commonly recognized putative papillomavirus epitope or a general mitogenic stimulatory effect, use of BPV VLP as a “negative” VLP control appears inappropriate in VLP-based vaccine studies. Nonetheless, the robust increase in HPV-11 VLP lymphoproliferation observed after immunization with minimal vaccine-dependent responses to other VLPs supports the HPV type specificity of the measured proliferative response.
Our data on the type-specific nature of this VLP-based vaccine do not support recent findings by Evans and colleagues who reported cross-reactive cytokine and lymphoproliferative responses against HPV-6 and -16 following vaccination with HPV-11 VLP (13). However, baseline measurements in vaccine recipients were high in the study by Evans et al. This may have been a consequence of previous exposure to other HPVs, given that study exclusion criteria were limited to a history of abnormal Pap smears and seropositivity to HPV-11 by ELISA only. HPV-6 and HPV-11 have 93% amino acid identity in the L1 protein yet do not share conformationally dependent neutralizing epitopes (7); thus, it is not surprising that cross-type proliferative responses were not observed in the screening and ELISA evaluations. This may support future efforts to include both HPV-6 and HPV-11 in HPV VLP vaccines targeting low-risk HPV types.
Th1 and Th2 cytokines were detected in response to HPV-11 L1 VLP in vitro restimulation in all vaccine recipients and in none of the placebo recipients tested. In general, the quantitative responses of IFN-γ (picograms/milliliter) and IL-2 (per SIHT-2) measured in vaccine recipients were similar to or exceeded previously reported levels of these cytokines detected after mitogenic (8), E6/E7 peptide (53), or VLP (11) restimulation in women naturally infected with HPV-16. SIs against HPV-11 VLP were also higher than what is generally measured in naturally infected individuals (48), suggesting that HPV VLP vaccination primes stronger cellular immune responses than natural HPV infection. Although IL-2 production was greater in the immunized group versus the placebo group following the second immunization, the differences were not statistically significant. It is possible that levels of IL-2 detected in the vaccine recipient group were low due to IL-2 uptake by VLP-stimulated PBMCs during 48 h of cell culture. While some significant associations between cytokine production, lymphoproliferation, and antibody titer were observed, these relationships were not consistently observed over time. This may be the result of the small number of participants evaluated in this study. Nonetheless, the observed correlations support the Th1/Th2 cytokine pattern measured among vaccinated participants.
In our study, immunoglobulin isotype and subclass responses to HPV-11 VLP vaccination demonstrated the generation of both Th1 and Th2 responses in over half of women immunized with HPV-11 VLP. These data corroborate a similar response observed in our cytokine and ELISPOT studies. Our results differ from those in the work previously published by Harro et al., which examined the immunoglobulin isotypes induced by vaccination with an HPV-16 VLP produced from baculovirus (19). All vaccine recipients immunized with HPV-16 VLP (with or without aluminum adjuvant) were seropositive for HPV-16 VLP-specific IgG1 antibodies and minimal IgG2, IgG3, or IgG4 antibodies were detected 1 month following the first immunization. Similar frequencies of IgA were detected in women immunized with both HPV-11 and HPV-16 VLP vaccines, but IgM was more common in recipients of the HPV-16 vaccine, a likely consequence of the primary immune response generated immediately following vaccination. Several aspects of vaccine study design and the technologies employed may account for the differences in our results. We examined immune responses at month 7 after the vaccinees had received three doses of the HPV-11 VLP vaccine, whereas Harro et al. tested sera 1 month after the first vaccination. This may explain why we saw fewer individuals who had a detectable IgM response. This difference in the sampling time points may also explain why we were able to detect IgG2, IgG3, and IgG4 response in a higher percentage of individuals than that observed in the HPV-16 VLP vaccine study. The MAbs may have different affinities for the various immunoglobulin isotypes targeted and were obtained from different sources for the two studies. Lastly, our assay employed the use of Luminex microspheres and fluorescent detection technology as a readout, compared to a colorimetric detection used in an ELISA. The liquid phase kinetics of these microsphere-based binding assays, the increased sensitivity of fluorescence-based technology, and the increased precision afforded by analyzing multiple microspheres per well may account for the apparent increased sensitivity of the Luminex isotyping assay. Thus, the overall differences observed between our study and that reported by Harro et al. may be attributed to the laboratory technologies applied, the difference in HPV type-specific VLP selected for immunization, the vaccine formulation and doses administered, and the time point chosen for serologic measurement.
Although aluminum hydroxyphosphate is not known to be a potent cytotoxic-T-lymphocyte (CTL)-inducing adjuvant, immunization of VLPs without adjuvant has been shown to induce cell-mediated immune responses (10, 31, 37, 40, 55). Recent observations of strong and long-lived CTL responses using a human immunodeficiency virus-gag VLP (37) and Th1 delayed-type hypersensitivity responses following HPV-6 VLP (55), both in the absence of adjuvant, have been reported. In contrast, HPV-11 L1-specific CTLs were only weakly detectable by bulk CTL assays in our study after the fourth immunization in two of nine vaccine recipients monitored (data not shown). Our observations in humans corroborate the weak and infrequent CTLs measured in chimpanzees immunized with similar HPV VLP vaccines adsorbed to aluminum hydroxyphosphate (38).
Detection of CTLs using techniques such as major histocompatibility complex tetramers and ELISPOT analysis have been reported to be more sensitive than bulk CTL assays (1, 15, 42, 49) and may provide a more accurate assessment of HPV-specific CD8+-T-cell responses. Towards this aim we performed ELISPOT experiments on a subset of vaccine participants. The measurable IFN-γ cytokine response was generally restricted to the CD4+ population of T cells. It is possible that the addition of aluminum adjuvant to the HPV-11 VLP preparation interferes with the inherent ability of VLPs alone to prime a Th1-directed CTL response (10, 31, 37, 40, 44, 55).
Limited clinical investigations of human subjects with genital warts have revealed wart-infiltrating HPV-specific T cells to be modulators of disease outcome (2, 9, 16, 21, 36). More extensive evidence exists that virus-specific cellular immune responses are protective against HPV-16 persistence and oncogenic development (3, 8, 11, 24, 35, 47, 53), but the role of CD4+ and CD8+ activity in natural host protection remains uncertain. Although the generation of virus-specific cell-mediated immune responses appears to be beneficial for effective protection against chronic viral infections (33, 34, 43, 46), future work is needed to determine the respective role and relative contribution of HPV-specific host B and T cells in terms of protection against HPV-induced lesions. Similarly, the characterization of antibody, lymphoproliferative, and cytokine immune responses induced in patients by vaccination such as those presented here are potentially key to understanding the long-term efficacy of HPV VLP-based vaccines.
Acknowledgments
This work was supported by a grant to C.M.W. from the National Institutes of Health (AI/CA 32917).
We thank Harriette Barber, Carla Herman, and Melissa Schiff for the enrollment and clinical management of the women in the UNM vaccine study. Many thanks are extended to Cheri Peyton, Nicole Stephens, Norah Torrez-Martinez, and Meifen Zhou for their technical assistance and help in specimen collection. We thank Cellular Technologies Ltd. for technical support with the ELISPOT assays and A. Hildesheim and W. Koop for generously providing recombinant baculovirus-produced BPV1 L1 and HPV-16 L1 VLPs and baculovirus control lysate. Finally, we are grateful to the study participants who generously donated blood for these studies.
REFERENCES
- 1.Altman, J. D., P. Moss, P. Goulder, D. H. Barouch, M. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Arany, I., and S. K. Tyring. 1996. Status of local cellular immunity in interferon-responsive and -nonresponsive human papillomavirus-associated lesions. Sex. Transm. Dis. 23:475-480. [DOI] [PubMed] [Google Scholar]
- 3.Bontkes, H. J., T. D. deGruijl, A. J. C. van den Muysenberg, R. H. M. Verheijen, M. J. Stukart, C. J. L. M. Meijer, R. J. Scheper, S. N. Stacey, M. F. Duggan-Keen, P. L. Stern, S. Man, L. K. Borysiewicz, and J. M. M. Walboomers.2000. Human papillomavirus type16. E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int. J. Cancer 88:92-98. [PubMed] [Google Scholar]
- 4.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. R., J. M. Schroeder, J. T. Bryan, M. H. Stoler, and K. H. Fife. 1999. Detection of multiple human papillomavirus types in condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J. Clin. Microbiol. 37:3316-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. R., J. T. Bryan, J. M. Schroeder, T. S. Robinson, K. H. Fife, C. M. Wheeler, E. Barr, P. R. Smith, L. Chiaccherini, and K. U. Jansen. 2001. Neutralization of human papillomavirus type 11 (HPV11) by serum from women vaccinated with yeast-derived HPV11 virus-like particles: correlation with competitive radioimmunoassay titer. J. Infect. Dis. 184:1183-1186. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, N. D., R. Kirnbauer, J. T. Schiller, S.-J. Ghim, R. Schlegel, A. B. Jenson, and J. W. Kreider. 1994. Human papillomavirus types 6 and 11 have antigenically distinct strongly immunogenic conformationally dependent neutralizing epitopes. Virology 205:329-335. [DOI] [PubMed] [Google Scholar]
- 8.Clerici, M., M. Merola, E. Ferrario, D. Trabattoni, M. L. Villa, B. Stefanon, D. J. Venzon, G. M. Shearer, G. De Palo, and E. Clerici. 1997. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J. Natl. Cancer Inst. 89:245-250. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, N., H. D. L. Birley, A. M. Renton, N. F. Hanna, B. K. Ryait, M. Byrne, D. Taylor-Robinson, and M. A. Stanley. 1994. Immunological events in regressing genital warts. Am. J. Clin. Pathol. 102:768-774. [DOI] [PubMed] [Google Scholar]
- 10.De Bruijn, M. L. H., H. L. Greenstone, H. Vermeulen, C. J. M. Melief, D. R. Lowy, J. T. Schiller, and W. M. Kast. 1998. L1 specific protection from tumor challenge elicited by HPV16 virus-like particles. Virology 250:371-376. [DOI] [PubMed] [Google Scholar]
- 11.deGruijl, T. D., H. J. Bontkes, J. M. M. Walboomers, P. Coursaget, M. J. Stukart, C. Dupuy, E. Kueter, R. H. M. Verheijen, T. J. M. Helmerhorst, M. F. Duggan-Keen, P. L. Stern, C. J. L. M. Meijer, and R. J. Scheper. 1999. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia I. Differential T-helper and IgG responses in relation to HPV infection and disease outcome. J. Gen. Virol. 80:399-408. [DOI] [PubMed] [Google Scholar]
- 12.Ertl, H. C. J., B. Dietzschold, M. Gore, L. Otvos, J. K. Larson, W. H. Wunner, and H. Koprowski. 1989. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J. Virol. 63:2885-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans, T. G., W. Bonnez, R. C. Rose, S. Koenig, L. Demeter, J. A. Suzich, D. O'Brian, M. Campbell, W. I. White, J. Balsley, and R. C. Reichman. 2001. A Phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J. Infect. Dis. 183:1485-1493. [DOI] [PubMed] [Google Scholar]
- 14.Freitas, A. A., and B. B. Rocha. 1993. Lymphocyte lifespans: homeostasis, selection and competition. Immunol. Today 14:25-29. [DOI] [PubMed] [Google Scholar]
- 15.Fu, T. M., D. C. Freed, W. L. Trigona, L. M. Guan, L. Zhu, R. Long, N. V. Persaud, K. Manson, S. Dubey, and J. W. Shiver. 2001. Evaluation of cytotoxic T-lymphocyte responses in human and nonhuman primate subjects infected with human immunodeficiency virus type 1 or simian human immunodeficiency virus. J. Virol. 75:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greer, C. E., C. M. Wheeler, M. B. Ladner, K. Beutner, M. Y. Coyne, H. Liang, A. Langenberg, T. S. Benedict Yen, and R. Ralston. 1995. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J. Clin. Microbiol. 33:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagensee, M. E., N. Yaegashi, and D. A. Galloway. 1993. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 67:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpert, R., R. G. Fruchter, A. Sedlis, K. Butt, J. G. Boyce, and F. H. Sillman. 1986. Human papillomavirus and lower genital tract neoplasia in renal transplant patients. Obstet. Gynecol. 68:251-258. [PubMed] [Google Scholar]
- 19.Harro, C. D., Y. Y. S. Pang, R. B. S. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 20.Heino, P., B. Skyldberg, M. Lehtinen, I. Rantala, B. Hagmar, J. W. Kreider, R. Kirnbauer, and J. Dillner. 1995. Human papillomavirus type 16 capsids expose multiple type-restricted and type-common antigenic epitopes. J. Gen. Virol. 76:1141-1153. [DOI] [PubMed] [Google Scholar]
- 21.Hong, K., C. E. Greer, N. Ketter, G. Van Nest, and X. Paliard. 1997. Isolation and characterization of human papillomavirus type 6-specific T cells infiltrating genital warts. J. Virol. 71:6427-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen, K. U., M. Rosolowsky, L. D. Schultz, H. Z. Markus, J. C. Cook, J. J. Donnelly, D. Martinez, R. W. Ellis, and A. R. Shaw. 1995. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine 13:1509-1514. [DOI] [PubMed] [Google Scholar]
- 23.Jarrett, W. F. H., B. W. O'Neil, J. M. Gaukroger, K. T. Smith, H. M. Laird, and M. S. Campo. 1990. Studies on vaccination against papillomaviruses: the immunity after infection and vaccination with bovine papillomaviruses of different types. Vet. Rec. 126:473-475. [PubMed] [Google Scholar]
- 24.Kadish, A. S., G. Y. F. Ho, R. D. Burk, Y. Wang, S. L. Romney, R. Ledwidge, and R. H. Angeletti. 1997. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J. Natl. Cancer Inst. 89:1285-1293. [DOI] [PubMed] [Google Scholar]
- 25.Kast, W. M., M. C. W. Feltkamp, M. E. Ressing, M. P. M. Vierboom, R. M. P. Brandt, and C. J. M. Melief. 1996. Cellular immunity against human papillomavirus associated cervical cancer. Semin. Virol. 7:117-123. [Google Scholar]
- 26.Kaul, R., S. L. Rowland-Jones, J. Kimani, T. Dong, H. B. Yang, P. Kiama, T. Rostron, E. Njagi, J. J. Bwayo, K. S. MacDonald, and A. J. McMichael. 2001. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Investig. 107:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirnbauer, R., L. M. Chandrachud, B. W. O'Neil, E. R. Wagner, G. J. Grindlay, A. Armstrong, G. M. McGarvie, J. T. Schiller, D. R. Lowy, and M. S. Campo. 1996. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 219:37-44. [DOI] [PubMed] [Google Scholar]
- 28.Kiviat, N., A. Rompalo, R. Bowden, D. Galloway, K. K. Holmes, L. Corey, P. L. Roberts, and W. E. Stamm. 1990. Anal human papillomavirus infection among HIV-seropositive and negative men. J. Infect. Dis. 162:358-361. [DOI] [PubMed] [Google Scholar]
- 29.Koutsky, L. A., D. A. Galloway, and K. K. Holmes. 1988. Epidemiology of genital human papillomavirus infection. Epidemiol. Rev. 10:122-163. [DOI] [PubMed] [Google Scholar]
- 30.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 31.Liu, X. S., I. Abdul-Jabbar, Y. M. Qi, I. H. Frazer, and J. Zhou. 1998. Mucosal immunization with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology 252:39-45. [DOI] [PubMed] [Google Scholar]
- 32.Lowe, R. S., D. R. Brown, J. T. Bryan, J. C. Cook, H. A. George, K. J. Hofmann, W. M. Hurni, J. G. Joyce, E. D. Lehman, H. Z. Markus, M. P. Neeper, and L. D. Schultz. 1997. Human papillomavirus type II (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J. Infect. Dis. 176:1141-1145. [DOI] [PubMed] [Google Scholar]
- 33.McMichael, A. J., M. Callan, V. Appay, T. Hanke, G. Ogg, and R. S. Jones. 2000. The dynamics of the cellular immune response to HIV infection: implications for vaccination. Philos. Trans. R. Soc. Lond. Ser. B 355:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musey, L., Y. X. Hu, L. Eckert, M. Christensen, T. Karchmer, and M. J. McElrath. 1997. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J. Exp. Med. 185:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa, M., D. P. Stites, S. Patel, S. Farhat, M. Scott, N. K. Hills, J. M. Palefsky, and B.-A. Moscicki. 2000. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte responses to the E6 antigens. J. Infect. Dis. 182:595-598. [DOI] [PubMed] [Google Scholar]
- 36.Opaneye, A. A. 1999. The cellular immune system in female patients with or without genital warts: a study of peripheral white blood components. Int. J. STD AIDS 10:815-816. [DOI] [PubMed] [Google Scholar]
- 37.Paliard, X., Y. Liu, R. Wagner, H. Wolf, J. Baenziger, and C. M. Walker. 2000. Priming of strong, broad, and long-lived HIV type 1 p55(gag)-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res. Hum. Retrovir. 16:273-282. [DOI] [PubMed] [Google Scholar]
- 38.Palker, T. J., J. M. Monteiro, M. M. Martin, C. Kakareka, J. F. Smith, J. C. Cook, J. G. Joyce, and K. U. Jansen. 2001. Antibody, cytokine and cytotoxic T lymphocyte responses in chimpanzees immunized with human papillomavirus virus-like particles. Vaccine 19:3733-3743. [DOI] [PubMed] [Google Scholar]
- 39.Payne, E., M. R. Bowles, A. Don, J. F. Hancock, and N. A. J. McMillan. 2001. Human papillomavirus type 6b virus-like particles are able to activate the Ras-MAP kinase pathway and induce cell proliferation. J. Virol. 75:4150-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng, S., I. H. Frazer, G. J. Fernando, and J. Zhou. 1998. Papillomavirus virus-like particles can deliver defined CTL epitopes to the MHC class I pathway. Virology 240:147-157. [DOI] [PubMed] [Google Scholar]
- 41.Reusser, P., S. Riddell, J. Meyers, and P. Greenberg. 1991. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78:1373-1380. [PubMed] [Google Scholar]
- 42.Rinisland, F. H., T. Helms, R. J. Assad, B. O. Boehm, and M. Tary-Lehmann. 2000. Granzyme B ELISPOT assay for ex vivo measurements of T cell immunity. J. Immunol. Methods 240:143-155. [DOI] [PubMed] [Google Scholar]
- 43.Roy, M. J., M. S. Wu, L. J. Barr, J. T. Fuller, L. G. Tussey, S. Speller, J. Culp, J. K. Burkholder, W. F. Swain, R. M. Dixon, G. Widera, R. Vessey, and A. King. 2000. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764-778. [DOI] [PubMed] [Google Scholar]
- 44.Rudolf, M. P., J. D. Nieland, D. M. daSilva, M. P. Velders, M. Mueller, H. L. Greenstone, J. T. Schiller, and W. M. Kast. 1999. Induction of HPV16 capsid protein-specific human T cell responses by virus-like particles. Biol. Chem. 380:335-340. [DOI] [PubMed] [Google Scholar]
- 45.Ruedlinger, R., I. W. Smith, M. H. Bunney, and J. A. A. Hunter. 1986. Human papillomavirus infections in a group of renal transplant recipients. Br. J. Dermatol. 115:681-692. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 47.Shepherd, P. S., A. J. Rowe, J. C. Cridland, T. Coletart, P. Wilson, and J. C. Luxton. 1996. Proliferative T cell responses to the human papillomavirus type 16 L1 peptides in patients with cervical dysplasia. J. Gen. Virol. 77:593-602. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd, P. S., and J. Luxton. 1999. T-cell responses to human papillomavirus in cervical dysplasia. Papillomavir. Rep. 10:53-78. [Google Scholar]
- 49.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staros, J. V., R. W. Wright, and D. M. Swingle. 1986. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220-222. [DOI] [PubMed] [Google Scholar]
- 51.Strike, D. G., W. Bonnez, R. C. Rose, and R. C. Reichman. 1989. Expression in Escherichia coli of seven DNA fragments comprising the complete L1 and L2 open reading frames of human papillomavirus type 6b and localization of the "common antigen' region. J. Gen. Virol. 70:543-555. [DOI] [PubMed] [Google Scholar]
- 52.Suzich, J. A., S. J. Ghim, F. J. Palmerhill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsukui, T., A. Hildesheim, M. H. Schiffman, J. Lucci, D. Contois, P. Lawler, B. B. Rush, A. Lorincz, A. Corrigan, R. D. Burk, W. Qu, M. A. Marshall, D. Mann, M. Carrington, M. Clerici, G. M. Shearer, D. P. Carborne, D. R. Scott, R. A. Houghten, and J. A. Berzofsky. 1996. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res. 56:3967-3974. [PubMed] [Google Scholar]
- 54.Vignali, D. A. 2000. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243:243-255. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, L. F., J. Zhou, S. Chen, L. L. Cai, F. Y. Zheng, J. Q. Lu, J. Padmanabha, K. Hengst, K. Malcolm, and I. H. Frazer. 2000. HPV6b virus-like particles are potent immunogens without adjuvant in man. Vaccine 18:1051-1058. [DOI] [PubMed] [Google Scholar]
- 56.zur Hausen, H. 1991. Viruses in human cancer. Science 254:1167-1172. [DOI] [PubMed] [Google Scholar]