Abstract
We investigated long-term memory and recall cellular immune responses to human immunodeficiency virus type 1 (HIV-1) Env and Gag proteins elicited by recombinant vesicular stomatitis viruses (VSVs) expressing Env and Gag. More than 7 months after a single vaccination with VSV-Env, ∼6% of CD8+ splenocytes stained with major histocompatibility complex class I tetramers containing the Env p18-I10 immunodominant peptide and showed a memory phenotype (CD44Hi). The level of tetramer-positive cells in memory was about 14% of the peak primary response. Recall responses elicited in these mice 5 days after boosting with a heterologous recombinant vaccinia virus expressing HIV-1 Env showed that 40 to 45% of CD8+ splenocytes were tetramer positive and activated (CD62LLo), and these cells produced gamma interferon after stimulation with Env peptide, indicating that they were functional. Five months after the boost, the long-term memory cell population (tetramer positive, CD44Hi) constituted 30% of the CD8+ splenocytes. Recall responses to HIV-1 Gag were examined in mice primed with VSV recombinants expressing HIV-1 Gag protein and boosted with a vaccinia virus recombinant expressing Gag. Using this protocol, we found that ∼40% of CD8+ splenocytes were activated (CD62LLo) and specific for a Gag immunodominant peptide (tetramer positive). The high-level Gag recall response elicited by the vaccinia virus-Gag was greater than that obtained by boosting with a VSV-Gag vector with a different VSV glycoprotein. The corresponding levels of CD44Hi memory cells were also higher long after boosting with vaccinia virus-Gag than after boosting with a glycoprotein exchange VSV-Gag. Our results show that VSV vectors elicit high-level memory CTL responses and that these can be amplified as much as six- to sevenfold using a heterologous boosting vector.
Cytotoxic T lymphocytes (CTL) play a major role in adaptive immunity to viral infections (reviewed in reference 49). CD8+ CTL recognize peptides derived from foreign viral antigens bound to major histocompatibility complex class I (MHC-I) molecules on the surface of infected cells. Upon first encountering antigen, CTL develop into mature effectors capable of eliminating virally infected cells while undergoing massive clonal expansion and proliferation (1, 11, 50). After clearing the infection, a significant proportion (often 95% or more) of activated CTL undergo activation-induced cell death (1, 38). However, from the original effector population is also formed a stable memory population of CTL (37). Memory CTL are capable of responding more rapidly and mediating faster viral clearance upon reexposure to antigen (5, 6, 25, 33).
Several lines of evidence indicate that a strong CTL response is critical for the control of human immunodeficiency virus type 1 (HIV-1) infection. During acute HIV-1 infection, the initial rise in HIV-specific CTL, which occurs before the production of neutralizing antibody, is associated with control of the initial viremia (8, 26). It has been reported that later in infection, viral load is inversely related to the level of HIV-1 CTL (36), although a more complicated relationship likely exists (7). Moreover, the frequency of HIV-specific effector and memory CTL is associated with a lower median level of viral RNA in plasma and slower disease progression (34, 36). Long-term nonprogressors also have consistently higher levels of HIV CTL than do progressors (17). Therefore, the ability of potential vaccines for HIV to elicit strong CTL responses is likely very important.
A recent focus in our laboratory has been on the development of recombinant vesicular stomatitis virus (rVSV) as a live viral vaccine vector (39, 40, 42). VSV is a nonsegmented, negative-stranded RNA virus that can be easily manipulated to express foreign genes from additional transcription units (27, 44). Two recent modifications to rVSVs have added further utility to rVSV as a vaccine vector. First, attenuated rVSVs have been created by shortening the cytoplasmic domain of the VSV glycoprotein (G) or completely eliminating G from the genome (39). Second, rVSVs in which different serotypes of the VSV G are exchanged for the standard Indiana serotype G (glycoprotein exchange vectors) have been developed to eliminate neutralization of the VSV vector upon boosting (42, 43).
It was shown recently that vaccination with rVSVs can be used to protect rhesus macaques from AIDS following challenge with a highly pathogenic simian immunodeficiency virus/HIV hybrid (42). Sequential vaccinations with rVSVs containing Indiana, Chandipura, and New Jersey serotype VSV Gs were used to prime and boost responses to the simian immunodeficiency virus Gag and HIV Env proteins expressed by these viruses. This vaccination protocol elicited antibody responses to all serotypes of VSV G as well as increasing antibody responses to Env proteins. A significant population of CTL was also elicited by rVSV vaccination and boosting, as quantitated by enzyme-linked immunospot and flow cytometric analysis of peripheral blood mononuclear cells. Others have shown that a prime-boost regimen using DNA and viral vaccine vectors has proven more successful than successive boosts with the same vector (2, 31, 41). Therefore, in the context of examining the memory and recall CTL responses to Gag and Env in this study, we have analyzed the relative efficacy of boosting with VSV glycoprotein exchange vectors versus completely heterologous viral vectors.
We previously reported that high-level primary responses were elicited to foreign viral proteins expressed in VSV (16). Here, using MHC-I tetramer staining and intracellular cytokine staining, we quantitated long-term memory and recall responses elicited in response to Gag and Env expressed in rVSVs by boosting with recombinant vaccinia viruses (rVVs) expressing these HIV proteins, as well as long-term memory to Env and Gag after boosting. We also compared the order of priming and boosting with rVSVs and rVVs.
MATERIALS AND METHODS
Virus preparation and analysis.
rVSVs expressing HIV-1 Env and Gag (VSV-Gag, VSV-Env, and VSV-GagEnv) have been described previously (15, 16, 21, 22), as has the corresponding recovered wild-type VSV (VSV-rwt) control (27). Stocks of VSV and recombinants were grown on BHK-21 cells. Viral titers were determined by plaque assay on BHK-21 cells. Stocks of virus were stored at −70°C.
Vaccinia virus (VV) recombinants expressing HIV-1 IIIb Env (vPE16) (12) and HXB2 Gag (vVK1) (24) under the control of early-late p7.5 promoter were obtained from the NIH AIDS Research and Reference Reagent Program (Rockville, Md.). Stocks of the VV recombinants were grown on HeLa cells in 15-cm-diameter tissue culture dishes in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (DMEM-10). Two days after infection, the infected cells were scraped from the culture dishes, pelleted by centrifugation at 500 × g for 10 min, and resuspended in 2 ml of phosphate-buffered saline. VV virions were harvested from infected cells by sonication and three to five cycles of freezing and thawing. Viral titers were determined by plaque assay on BHK cells. Stocks of rVV were stored at −70°C.
To test the ability of glycoprotein exchange VSVs to elicit recall responses, an rVSV that expressed HIV-1 Gag and the VSV (Chandipura) G was generated. Briefly, the gpIIa gene (30), which is the HIV-1 gag gene with an inactivating mutation in the overlapping protease gene, was excised from pBSgpIIa (30) by digestion with XhoI and XbaI. The resultant ∼2-kb DNA fragment was purified and ligated into the vector pVSV(GCh)-XN-1 (43). Infectious virus recombinants were recovered using published protocols (27, 44).
Metabolic labeling of proteins.
Briefly, ∼5 × 105 BHK cells in 35-mm-diameter tissue culture dishes were infected with VSV recombinants at a multiplicity of infection of 10 to 20. Four hours later, the culture medium was replaced with methionine-free Dulbecco's modified Eagle medium (DMEM) including 100 μCi of [35S]methionine (Easy tag EXPRESS protein labeling mix; NEN Life Sciences, Boston, Mass.), and the cells were incubated for 1 h. Cells were lysed on ice for 15 min in 500 μl of lysis buffer (1% Nonidet P-40, 0.4% deoxycholate, 66 mM EDTA, and 10 mM Tris-Cl, pH 7.4), and the nuclei were removed by centrifugation for 2 min at room temperature in an Eppendorf centrifuge. Protein extracts (10 μl of each sample) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide) (SDS-10% PAGE), and proteins were visualized on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Animals, inoculation, and isolation of splenocytes.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Yale University. Female 5- to 7-week-old BALB/c mice obtained from Charles River Laboratories were kept for at least 1 week before inoculation with rVSV or rVV expressing HIV-1 Gag and Env. Animals were maintained in microisolator cages in a biosafety level-2-equipped animal facility and given food and water ad libitum. Viral stocks were diluted in an appropriate volume of serum-free DMEM before inoculation of mice. For intraperitoneal vaccinations, mice were inoculated with 5 × 106 PFU of priming virus or 5 × 106 PFU of boosting virus in a 100- to 300-μl volume. At appropriate times, animals were euthanized with CO2, and their spleens were isolated. Bulk splenocytes were isolated by forcing the spleen through a metal strainer with ∼1-mm2 holes. Erythrocytes were lysed in buffer (pH 7.4) containing 0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA.
Peptides and MHC-I tetramers.
The Gag immunodominant peptide (N-AMQMLKETI-C) (29) was obtained from Research Genetics (Huntsville, Ala.). A phycoerythrin (PE)-conjugated MHC-I H-2Kd tetramer was synthesized using this peptide by the National Institute of Allergy and Infectious Diseases (NIAID) MHC Tetramer Core Facility (Atlanta, Ga.). The p18-I10 peptide (N-RGPGRAFVTI-C) (46) used in these experiments was also obtained from Research Genetics. A PE-conjugated MHC-I H-2Dd tetramer was synthesized using this peptide by the NIAID Tetramer Core Facility.
Tetramer staining and flow cytometric analysis.
Bulk splenocytes were resuspended in flow cytometry sample buffer (SB) (phosphate-buffered saline containing 0.5% bovine serum albumin and 0.02% NaN3), and ∼5 × 106 cells were added to wells of a V-bottom 96-well plate for staining. Cells were first blocked with unconjugated streptavidin (Molecular Probes, Eugene, Oreg.) and Fc block (Pharmingen, San Diego, Calif.) at 4°C for 15 min and pelleted at 500 × g for 5 min. Subsequently, the cells were stained with PE-conjugated tetramers, a fluorescein isothiocyanate (FITC)-conjugated anti-CD62L antibody (Pharmingen), and an allophycocyanin (APC)-conjugated anti-CD8 antibody (Pharmingen) at predetermined optimal dilutions for 30 min on ice. In experiments to determine the proportion of memory CD8+ cells, an FITC-conjugated anti-mouse CD44 antibody (Pharmingen) was used in place of the anti-CD62L antibody. The cells were then washed three times in SB and transferred to sample tubes for flow cytometric analysis. Just before analysis, 1 μl of propidium iodide staining solution (50 μg/ml; Pharmingen) was added to each sample to identify dead cells. Data were gathered using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) and analyzed using FlowJo analysis software (Tree Star, Inc., San Carlos, Calif.). In the analysis, lymphocytes were selected from forward and side scatter characteristics, propidium iodide-stained cells were gated out, and CD8+ cells were selected. The data for CD8+ cells are plotted as tetramer (PE) versus CD62L (FITC) or CD44 (FITC), as indicated.
Intracellular cytokine staining.
Intracellular cytokine staining using the Golgi Plug Kit (Pharmingen) was performed essentially as recommended by the manufacturer. Briefly, bulk splenocytes were harvested as described above, and 5 × 106 cells in 1 ml of DMEM-10 were added to 2 wells of a 24-well plate. One well served as an unstimulated control. Cells in the other well were stimulated with 10−5 M peptide (p18-I10 peptide or Gag immunodominant peptide). The cells were incubated at 37°C with 5% CO2 for 2 h, 1 μl of brefeldin A was added to each well, and the cells were incubated for an additional 3 h. The cells were harvested by centrifuging the 24-well plates at 500 × g for 5 min at 4°C, aspirating the medium, and resuspending the cells in SB. The cells from each well were transferred to wells of a 96-well V-bottom plate and blocked with Fc block for 15 min at 4°C and pelleted at 500 × g at 4°C. The cells were then stained with an APC-conjugated anti-CD8 antibody (Pharmingen) for 30 min at 4°C, washed three times with SB, and fixed in 100 μl of Cytofix/Cytoperm solution (Pharmingen) for 10 to 20 min on ice. After washing twice in 1× Perm/Wash solution (Pharmingen), intracellular cytokine was stained with FITC-conjugated rat anti-mouse gamma interferon (IFN-γ) or anti-mouse tumor necrosis factor (TNF-α) antibodies, or a FITC-conjugated rat isotype control immunoglobulin (Pharmingen) to identify background staining. The cells were analyzed by flow cytometry and are plotted as cytokine (FITC) intensity versus CD8 (APC) intensity. The percentage of CD8+ cells producing cytokine is reported in the upper right quadrant of flow cytometry plots.
RESULTS
VV and VSV recombinants expressing HIV-1 Gag and Env.
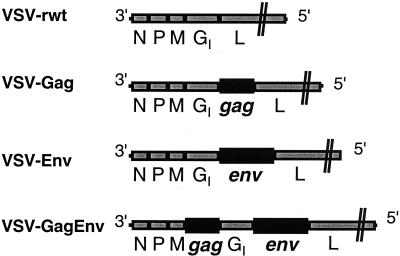
The rVSVs used in these experiments express HIV-1 Gag and Env separately (VSV-Gag and VSV-Env, respectively) or together in one virus (VSV-GagEnv). The genomes of these rVSVs encode the HIV genes at the positions indicated in Fig. 1. We used as a control VSV-rwt, which is an analogous wild-type VSV that was also recovered from plasmid DNA (27). These recombinants express high levels of the appropriate HIV-1 proteins (15, 21, 22).
FIG. 1.
VSV/HIV recombinant used in vaccination experiments. The genomes of VSV-rwt and the three VSV/HIV recombinants are diagrammed in a 3′-to-5′ orientation on the negative-stranded viral RNA. Additional genes are expressed from new transcription units generated by duplicating the conserved VSV transcription start and stop signals (44). VSV-Gag expresses the uncleaved Gag precursor pr55gag, and VSV-Env expresses the env gene of the IIIb strain. VSV-GagEnv expresses both genes from different positions in the genome. The expression of all proteins in cells infected with these recombinants has been confirmed previously (15, 21, 22).
The rVVs that were used to elicit recall responses in rVSV-vaccinated mice express the HIV-1 IIIb Env (vPE16) and HXB2 Gag (vVK1) proteins under the control of the VV early-late p7.5 promoter and have been previously characterized (12, 24). A VV expressing the T7 RNA polymerase under the p7.5 promoter was used as a control (14).
Memory response to HIV-1 Env in rVSV-vaccinated mice.
The HIV-1 IIIb Env protein contains the p18-I10 immunodominant peptide (45, 46). The p18-I10 peptide is presented on several different MHC-I alleles, but it is most efficiently presented on the H-2Dd class I molecule. Using H-2Dd MHC-I tetramers containing the p18-I10 peptide (MHC-I Env tetramers), we previously showed that vaccination of mice with VSV-Env results in a strong primary response to Env that peaks 5 to 7 days after vaccination (16). The primary response rapidly declines to about one quarter of its peak level by 15 days after vaccination (16). The magnitude of the peak primary response prompted us to determine if long-term memory cells specific for the p18-I10 peptide could be detected. To do this, we vaccinated BALB/c mice with 5 × 106 PFU of VSV-Env and isolated bulk splenocytes 220 days after vaccination. Splenocytes were then stained with the PE-conjugated MHC-I Env tetramers, APC-conjugated antibodies to CD8, and FITC-conjugated antibodies to CD62L or CD44. CD62L is a lymph node homing receptor that is downregulated upon activation of CTL (3, 4, 23, 32). CD44 is a surface protein required for lymphocyte extravasation to inflammatory sites, and its upregulation is a marker for memory CTL (9, 10). The stained splenocytes were analyzed by flow cytometry, and only CD8+ cells are shown in the analyses.
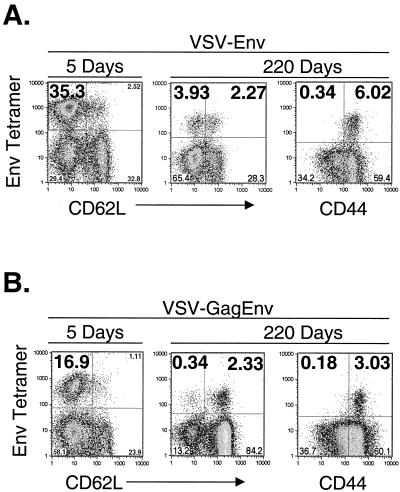
Figure 2A shows an example of the typical response after vaccination with VSV-Env. The plot on the left shows the response to Env at the peak of the primary response, at which time ∼35% of CD8+ cells were MHC-I Env tetramer positive and CD62LLo. The center and right plots show the profiles of the CD8+ memory cells stained for CD62L and CD44, respectively, at 220 days after vaccination. At this time, ∼4% of CD8+ cells were MHC-I Env tetramer positive and CD62LLo and ∼2% were tetramer positive and CD62LHi. Staining for CD44 revealed that ∼6% of CD8+ cells were MHC-I Env tetramer positive and CD44Hi. Background staining with the MHC-I Env tetramer was ≤0.1% in mice primed with VSV-rwt and boosted with vTF7-3 (data not shown).
FIG. 2.
Quantitation of Env-specific memory CTL after vaccination with rVSVs. (A) BALB/c mice were vaccinated with 5 × 106 PFU of VSV-Env, and bulk splenocytes were isolated 5 days (left plot) or 220 days (center and right plots) after vaccination (n = 2 per time point). Splenocytes were stained with MHC-I Env tetramers and antibodies to CD8. Additionally, subsets of activated and memory splenocytes were identified by staining with antibodies to CD62L (left and center plots) and CD44 (right plot). The flow cytometry results are plotted as MHC-I Env tetramer versus CD62L or CD44. Only CD8+ cells are included in the plots. (B) BALB/c mice were vaccinated with 5 × 106 PFU of VSV-GagEnv, and bulk splenocytes were isolated 5 days (left plot) or 220 days (center and right plot) after vaccination (n = 2 per time point). The cells were stained, analyzed, and plotted as described in panel A.
We also quantitated the CD8+ memory response to Env in mice vaccinated with 5 × 106 PFU of VSV-GagEnv, which expresses both Gag and Env proteins (Fig. 2B). For comparison, the plot on the left shows the response to Env 5 days after vaccination. Approximately 17% of CD8+ CTL were MHC-I Env tetramer positive and CD62LLo at this time. When splenocytes were stained with MHC-I Env tetramers and CD62L antibody 220 days after vaccination (Fig. 2B, center plot), ∼0.3% of CD8+ cells were MHC-I Env tetramer positive and CD62LLo and ∼2.3% were tetramer positive and CD62LHi. Approximately 3% of CD8+ cells were tetramer positive and CD44Hi 220 days after vaccination.
Recall responses to Env expressed in rVSVs elicited with rVVs.
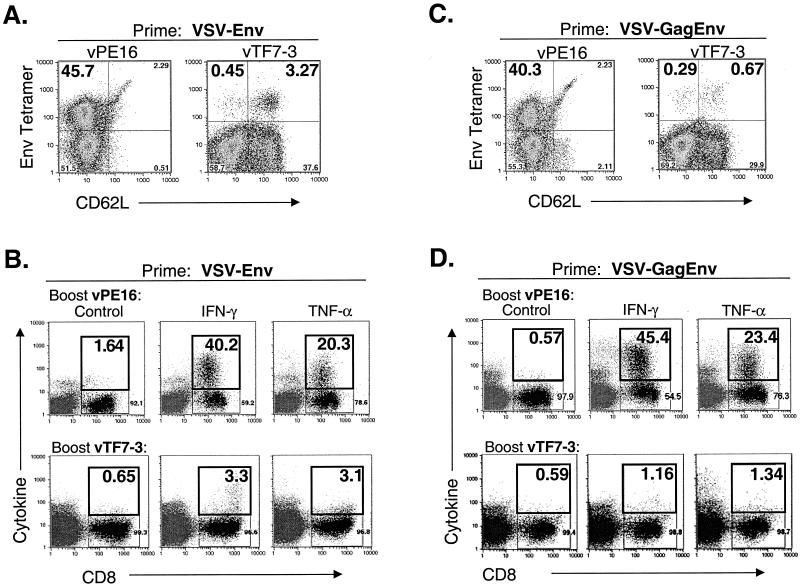
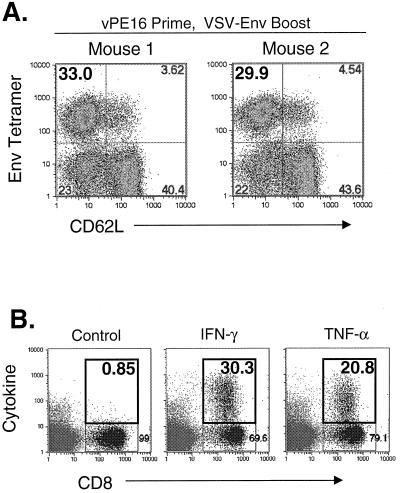
We previously showed that the primary response to Env after VSV-Env vaccination declines to ∼10% Env tetramer-positive, CD62LLo CD8+ cells by 15 days after vaccination (16). This response further declines to 6% Env tetramer-positive cells by 220 days after vaccination. To quantitate the recall response to Env in VSV-Env vaccinated mice within this time period, we vaccinated five mice with 5 × 106 PFU of VSV-Env. Sixty days later when the Env-specific CD8+ T-lymphocytes were in memory, we elicited a recall response by boosting three mice with 5 × 106 PFU of vPE16, a VV recombinant encoding Env (12). Two controls were mock boosted with 5 × 106 PFU of vTF7-3, a VV recombinant encoding T7 RNA polymerase (14). Five days after the boost, bulk splenocytes were isolated and stained with MHC-I Env tetramers. Figures 3A and B show an example of the typical recall response to Env after priming with VSV-Env. Approximately 46% of CD8+ cells were MHC-I Env tetramer positive and CD62LLo after the mice were boosted with vPE16 (Fig. 3A, left plot). In contrast, only ∼4% of CD8+ cells were tetramer positive after mice were boosted with vTF7-3, and the majority of these cells were CD62LHi. The tetramer-positive cells represent the memory population, which was not increased by mock boosting.
FIG. 3.
Recall responses to Env in rVSV-vaccinated mice elicited by boosting with rVVs. (A and C) BALB/c mice (n = 5) were vaccinated with 5 × 106 PFU of VSV-Env (A) or 5 × 106 PFU of VSV-GagEnv (C). Sixty days after vaccination, recall responses to Env were elicited by boosting with 5 × 106 PFU of vPE16 (n = 3), an rVV expressing HIV-1 Env (left plots), or by mock boosting with vTF7-3 (n = 2), an rVV expressing the T7 RNA polymerase (right plots). Five days after the boost, bulk splenocytes were isolated, stained with MHC-I Env tetramers and antibodies to CD8 and CD62L, and analyzed by flow cytometry. Only CD8+ cells are included in the plots. The number in the upper left quadrant is the percentage of CD8+ cells that were MHC-I Env tetramer positive and activated (CD62LLo). (B and D) In parallel with the tetramer staining described above, splenocytes from mice vaccinated with VSV-Env (B) and VSV-GagEnv (D) were analyzed for the production of intracellular cytokine after boosting with the rVVs indicated in the figure. Splenocytes were stimulated with p18-I10 peptide for 5 h in the presence of brefeldin A and stained with anti-CD8 antibodies in addition to anticytokine (IFN-γ and TNF-α) antibodies or an isotype control immunoglobulin. The results of flow cytometric analysis are plotted as cytokine staining versus CD8 staining. Numbers in the upper right quadrant indicate the percentage of CD8+ cells that are positive for cytokine staining. Background cytokine staining in unstimulated splenocytes was <1% (not shown).
We used intracellular cytokine staining to determine the proportion of Env-specific CTL that were functional. Five days after the VV boost, bulk splenocytes were isolated and incubated for 5 h in the presence of 10−5 M p18-I10 peptide and brefeldin A. The cells were then fixed and stained with antibodies to CD8 and antibodies to IFN-γ or TNF-α or an isotype control antibody. Approximately 40% of CD8+ cells secreted IFN-γ and approximately 20% secreted TNF-α after stimulation with p18-I10 peptide (Fig. 3B, upper plots). In mice that were vaccinated with VSV-Env and mock boosted with vTF7-3, intracellular cytokine staining revealed that ∼3% of CD8+ cells produced IFN-γ and ∼3% produced TNF-α after stimulation for 5 h with p18-I10 peptide. Splenocytes that were not stimulated with peptide showed background staining similar to that of the isotype control immunoglobulin (<1%; data not shown).
We also determined the recall response to Env in mice previously vaccinated with 5 × 106 PFU of VSV-GagEnv. Three mice were boosted with 5 × 106 PFU of vPE16, and two were mock boosted with 5 × 106 PFU of vTF7-3. Figures 3C and D show an example of the typical response 5 days after the boost. Similar to VSV-Env-vaccinated mice, ∼40% of CD8+ cells were MHC-I Env tetramer positive and CD62LLo after the vPE16 boost (Fig. 3C, left plot). In control mice that were mock boosted with vTF7-3, less than 1% of cells were MHC-I Env tetramer positive (Fig. 3C, right plot). Intracellular cytokine staining of cells 5 days after the boost with vPE16 showed that ∼45% of cells produced IFN-γ, and ∼23% expressed TNF-α after 5 h of stimulation with p18-I10 peptide (Fig. 3D, upper plots). In control mice, ∼1% of CD8+ cells produced IFN-γ and TNF-α after p18-I10 peptide stimulation (Fig. 3D, lower plots). After subtracting background staining, this translates to 0.6 to 0.7% of CD8+ cells producing cytokine. Less than 1% of unstimulated splenocytes stained positively for any of the cytokine antibodies (data not shown).
Long-term memory response after VSV-Env prime and vPE16 boost.
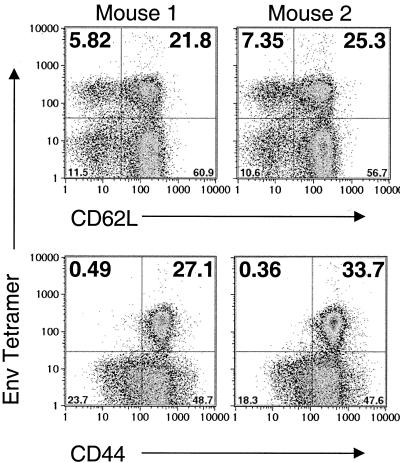
Because of the strong recall response generated by boosting, we wanted to determine the level of memory cells in spleens of mice primed with VSV-Env and boosted with vPE16. Two mice were vaccinated with 5 × 106 PFU of VSV-Env and boosted 120 days later with 5 × 106 PFU of vPE16. At the time of boosting, the level of Env-tetramer-positive CD8+ cells is expected to be between 6 and 10% (Fig. 3) (16). Splenocytes from these mice were isolated and analyzed 150 days after the boost to determine if Env tetramer-positive CD8+ cells persisted long after the boost. At the time that these splenocytes were isolated, none of the splenomegaly that was notable 5 days after boosting with rVVs was present. To quantitate Env-specific memory cells in the spleen, we stained splenocytes with MHC-I Env tetramers and antibodies to CD44 and CD62L. Figure 4 shows the results for both mice. Approximately 27 and 32% of CD8+ cells stained positively with the MHC-I Env tetramer. Approximately 5 to 7% of cells were CD62LLo, and ∼22 to 25% were CD62LHi (Fig. 4, upper plots). Nearly all MHC-I Env tetramer-positive cells were CD44Hi, indicating that they were memory cells (Fig. 4, lower plots).
FIG. 4.
Quantitation of Env-specific memory CTL after priming with VSV-Env and boosting with rVV. BALB/c mice (n = 2) were vaccinated with 5 × 106 PFU of VSV-Env and were boosted 120 days later with 5 × 106 PFU of vPE16. Bulk splenocytes were isolated 150 days after the boosting and were stained with MHC-I Env tetramers, anti-CD8 antibodies, and antibodies to CD62L (upper plots) or CD44 (lower plots) and analyzed by flow cytometry. Only CD8+ cells were included in the analysis. The results for both mice are shown.
Reversal of priming and boosting vectors for Env.
The primary response to HIV-1 Env elicited by vaccination with vPE16 is about sixfold lower than that elicited by VSV-Env and approaches background levels by 7 days after vaccination (16). To determine how reversal of the vaccination order affected the response, two mice were primed with 5 × 106 PFU of vPE16 and boosted 40 days later with 5 × 106 PFU of VSV-Env. Five days after the boost, splenocytes were isolated and stained with MHC-I Env tetramers and antibodies to CD8 and CD62L. Figure 5A shows the responses of both mice, in which ∼33 and ∼30% of CD8+ cells were MHC-I Env tetramer positive and CD62LLo. To determine if these splenocytes produced cytokine after stimulation with p18-I10 peptide, we stained for intracellular cytokine. Five days after the VSV-Env boost, ∼30% of CD8+ splenocytes produced IFN-γ and ∼21% produced TNF-α (Fig. 5B). Background staining in unstimulated splenocytes was less than 1% (data not shown). Therefore, based on both tetramer and intracellular cytokine staining, these responses are reduced about 25 to 30% compared to the response obtained when mice are vaccinated with VSV-Env and boosted with vPE16.
FIG. 5.
Recall response to Env in rVV-vaccinated mice elicited by boosting with rVSV. (A) BALB/c mice (n = 2) were vaccinated with 5 × 106 PFU of vPE16. Forty days after vaccination, recall responses to Env were elicited by boosting with 5 × 106 PFU of VSV-Env. Five days after the boost, bulk splenocytes were isolated, stained with MHC-I Env tetramers and antibodies to CD8 and CD62L, and analyzed by flow cytometry. Only CD8+ cells are included in the plots. (B) In parallel with the tetramer staining described above, splenocytes from mice vaccinated with vPE16 were analyzed for the production of intracellular cytokine after boosting with VSV-Env as described in the legend to Fig. 3B and D. The cytokine data shown are from mouse 2. Background staining in unstimulated cells was <1% (data not shown).
Recall responses to Gag expressed in rVSV and elicited with rVVs.
The Gag protein of HIV-1 contains an H-2Kd-restricted immunodominant peptide, AMQMLKETI (29). We used MHC-I tetramers complexed with this peptide (MHC-I Gag tetramers) to determine the recall response to Gag in rVSV-vaccinated mice. We did not quantitate MHC-I Gag tetramer-positive cells long after the primary vaccination (e.g., 220 days) as we did for VSV-Env-vaccinated mice, because the proportion of Gag tetramer-positive cells approaches background staining only 11 days after vaccination (16).
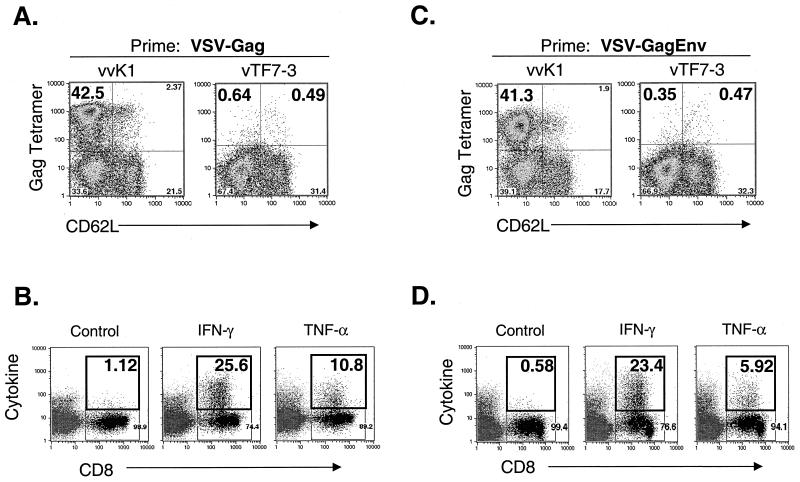
Five mice were vaccinated with 5 × 106 PFU of VSV-Gag. Sixty days later, three mice were boosted with 5 × 106 PFU of vvK1, an rVV-expressing HIV-1 Gag. Additionally, two mice were mock boosted with vTF7-3 as a control. Five days after the boost, bulk splenocytes were isolated and stained with MHC-I Gag tetramers and antibodies to CD8 and CD62L. Figures 6A and B show an example of the typical recall response to Gag after priming with VSV-Gag. Five days after the boost with vvK1, ∼42% of CD8+ splenocytes were MHC-I Gag tetramer positive and CD62LLo (Fig. 6A, left plot). In contrast, less than 1% of CD8+ cells were tetramer positive after the mock boosting with vTF7-3 (Fig. 6A, right plot). Background MHC-I Gag tetramer staining was ∼0.5% in mice primed with VSV-rwt and boosted with vTF7-3 (data not shown).
FIG. 6.
Recall response to Gag in rVSV-vaccinated mice elicited by boosting with rVVs. (A and C) BALB/c mice (n = 5) were vaccinated with 5 × 106 PFU of VSV-Gag (A) or 5 × 106 PFU of VSV-GagEnv (C). Sixty days after vaccination, recall responses to Env were elicited by boosting with 5 × 106 PFU of vvK1 (n = 3), an rVV expressing HIV-1 Gag (left plots), or by mock boosting with vTF7-3 (n = 2), an rVV expressing the T7 RNA polymerase (right plots). Five days after the boost, bulk splenocytes were isolated, stained with MHC-I Gag tetramers and antibodies to CD8 and CD62L, and analyzed by flow cytometry. Only CD8+ cells are included in the plots. The number in the upper left quadrant is the percentage of CD8+ cells that were MHC-I Gag tetramer positive and activated (CD62LLo). (B and D) In parallel with tetramer staining described above, splenocytes from mice vaccinated with VSV-Gag (B) and VSV-GagEnv (D) were analyzed for the production of intracellular cytokine after boosting with vvK1. Splenocytes were stimulated with the Gag immunodominant peptide (AMQMLKETI) for 5 h in the presence of brefeldin A and stained and analyzed as described in the legend to Fig. 3B and D. Cytokine staining in unstimulated splenocytes and in splenocytes from vTF7-3-boosted mice were similar to background levels (<1%; data not shown).
We used intracellular cytokine staining to determine if functional CTL recognizing the Gag immunodominant peptide were present during the recall response. Five days after the boost with vvK1 or vTF7-3, bulk splenocytes were isolated and stimulated with 10−5 M Gag immunodominant peptide in the presence of brefeldin A for 5 h. As shown in Fig. 6B, ∼25% of CD8+ cells produced IFN-γ and ∼10% produced TNF-α after stimulation with the Gag peptide. Stimulation of splenocytes from VSV-Gag-vaccinated mice that were boosted with vTF7-3 showed only background levels of intracellular cytokine staining (data not shown).
We also determined the recall response to Gag in VSV-GagEnv-vaccinated mice (Fig. 6C and D). Five mice were vaccinated with 5 × 106 PFU of VSV-GagEnv and were boosted 60 days later with either 5 × 106 PFU of vvK1 (three mice) or vTF7-3 (two mice). Five days after the boost, splenocytes were stained with MHC-I Gag tetramers and antibodies to CD8 and CD62L. Approximately 41% of CD8+ cells were MHC-I Gag tetramer positive and CD62LLo after the vvK1 boost (Fig. 6C, left plot). On the other hand, less than 1% of cells were tetramer positive after the vTF7-3 boost (Fig. 6C, right plot). We also determined the proportion of functional cytokine-secreting CD8+ cells after boosting (Fig. 6D). After stimulation in vitro with Gag immunodominant peptide for 5 h, ∼23% of CD8+ cells secreted IFN-γ and ∼6% secreted TNF-α. Less than 1% of unstimulated splenocytes stained for cytokine (data not shown).
Reversal of priming and boosting vectors for Gag.
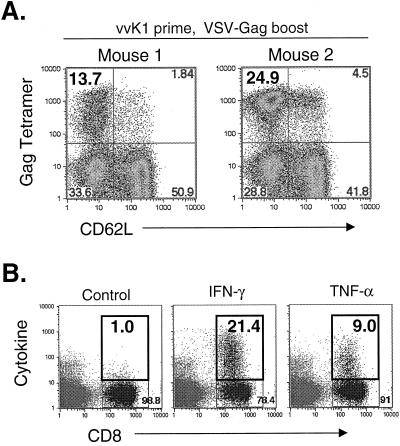
The primary response to Gag after vvK1 vaccination is about eightfold lower than after VSV-Gag vaccination (16). To determine how reversal of the priming and boosting vector order affected the responses to Gag, two mice were vaccinated with 5 × 106 PFU of vvK1 and boosted 40 days later with 5 × 106 PFU of VSV-Gag. Five days after the boost, splenocytes were isolated and stained with MHC-I Gag tetramers and antibodies to CD62L and CD8. Figure 7A shows the results of staining with MHC-I Gag tetramer for both mice. Approximately 14 and 25% of CD8+ cells were MHC-I Gag tetramer positive and CD62LLo after the boost. To determine if these cells were functional, we stained for intracellular cytokine after stimulating cells with the Gag immunodominant peptide for 5 h (Fig. 7B). The response shown in Fig. 7B was from the mouse that showed ∼25% tetramer staining. In this mouse, ∼21% of CD8+ cells produced IFN-γ, and 9% produced TNF-α after stimulation with the Gag immunodominant peptide. On average, this response was about 50% lower than that obtained when VSV-Gag was used to prime and vvK1 was used to boost.
FIG. 7.
Recall response to Gag in rVV-vaccinated mice elicited by boosting with rVSV. (A) BALB/c mice (n = 2) were vaccinated with 5 × 106 PFU of vvK1. Forty days after vaccination, recall responses to Gag were elicited by boosting with 5 × 106 PFU of VSV-Gag. Five days after the boost, bulk splenocytes were isolated and stained with MHC-I Gag tetramers and antibodies to CD8 and CD62L and analyzed by flow cytometry. The results for both mice are shown. (B) In parallel with the tetramer staining described above, splenocytes from mice vaccinated with vvK1 were analyzed for the production of intracellular cytokine after boosting with VSV-Gag. Splenocytes were stimulated with the Gag immunodominant peptide (AMQMLKETI) for 5 h in the presence of brefeldin A and stained as described in the legend to Fig. 3B and D. The cytokine data shown are from mouse 2. Background staining in unstimulated cells was <1% (data not shown).
Recall responses to Gag elicited with glycoprotein exchange rVSVs.
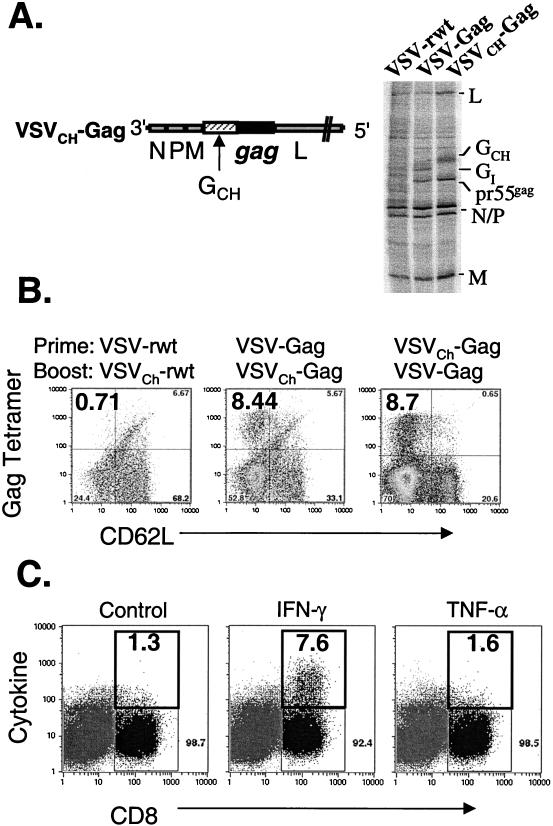
In previous studies, our laboratory used rVSVs containing the G of different VSV serotypes to boost the immune responses to foreign viral proteins (42, 43). The rVSVs characterized thus far in this study encode the Indiana serotype G (Fig. 1). To directly measure the ability of such glycoprotein exchange rVSVs to boost the cellular immune response to foreign viral proteins, we generated VSVCh-Gag, a glycoprotein exchange rVSV that expresses HIV Gag and contains the VSV G of the Chandipura serotype (Fig. 8A). We were unable to generate an rVSV expressing GCh and HIV-1 Env. To confirm the expression of VSV and HIV proteins from VSVCh-Gag, we infected cells for 4 h with VSV-rwt, VSV-Gag, and VSVCh-Gag. Proteins in infected cells were metabolically labeled for 1 h with [35S]methionine and analyzed by SDS-PAGE. A 55-kDa protein corresponding to the uncleaved HIV-1 Gag precursor pr55gag is apparent in both VSV-Gag- and VSVCh-Gag-infected cells. The GCh in VSVCh-Gag-infected cells is easily recognized by its migration, which is characteristically slower than that of the Indiana serotype G.
FIG. 8.
Recall response to Gag elicited by VSV glycoprotein exchange vectors. (A) The genome of the VSV glycoprotein exchange vector VSVCh-Gag is diagrammed in a 3′-to-5′ orientation on the negative-stranded viral RNA (left). The HIV-1 gag gene is expressed between the G and L genes, and the VSV Indiana serotype G (GI) was replaced with that of the Chandipura serotype (GCh). To confirm expression of Gag and GCh, BHK cells were infected with VSV-rwt, VSV-Gag, and VSVCh-Gag for 4 h at a multiplicity of infection of 10 to 20. Proteins were then labeled with [35S]methionine for 1 h. The cells were lysed, and the extracts were fractionated by SDS-PAGE. The uncleaved Gag precursor (pr55gag) was present in both VSV-Gag and VSVCh-Gag. The Chandipura serotype G is recognizable by its characteristically slower migration. (B) To determine the relative efficiency of boosting CTL responses with VSV glycoprotein exchange vectors, BALB/c mice were primed and boosted 40 days later with the indicated viruses (n = 2 per prime-boost condition). Five days after the boost, bulk splenocytes were isolated and stained with MHC-I Gag tetramers and antibodies to CD8 and CD62L. (C) Intracellular cytokine production was analyzed in parallel with the tetramer staining described above. Splenocytes isolated 5 days after boosting were incubated for 5 h with the Gag immunodominant peptide (AMQMLKETI) in the presence of brefeldin A and stained as described in the legend to Fig. 3B and D. The representative cytokine staining shown is from a mouse vaccinated with VSVCh-Gag and boosted with VSVI-Gag.
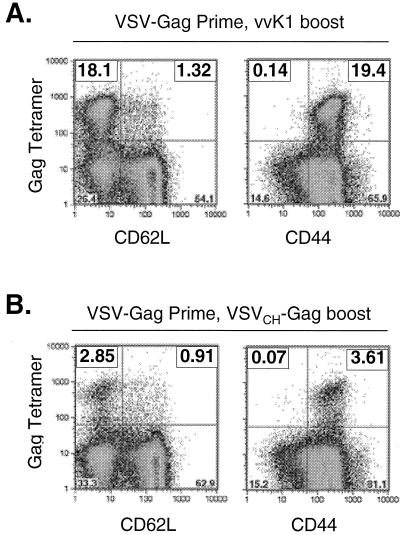
To compare the recall response generated with VV vectors with that generated by a VSV glycoprotein exchange vector, VSVCh-Gag was used to elicit recall response in mice vaccinated with VSV-Gag. Two mice were vaccinated with 5 × 106 PFU of VSV-Gag and boosted 40 days later with 5 × 106 PFU of VSVCh-Gag. Five days after the boost, splenocytes were isolated and stained with MHC-I Gag tetramers and antibody to CD62L (Fig. 8B, center plot). Approximately 8% of CD8+ cells were MHC-I Gag tetramer positive and CD62LLo. The reciprocal experiment was also conducted, in which 5 × 106 PFU of VSV-Gag was used to boost for two mice vaccinated with 5 × 106 PFU of VSVCh-Gag (Fig. 8B, left plot). Five days after boosting with VSV-Gag, ∼8% of CD8+ cells were MHC-I Gag tetramer positive and CD62LLo. Splenocytes from this mouse were also used to stain for intracellular cytokine production (Fig. 8C). Approximately 6 to 7% of cells produced IFN-γ after 5 h of stimulation with 10−5 M Gag immunodominant peptide. Production of TNF-α was near background levels in this experiment.
Long-term memory response after VSV-Gag prime and vvK1 or VSVCh-Gag boost.
We showed above that there were quantitative differences in the recall responses to Gag elicited by VSVCh-Gag and vvK1. We also quantitated Gag-specific memory CD8+ T-lymphocytes in spleens long after boosting with these rVV and glycoprotein exchange rVSVs to directly determine if the resultant memory population also differed. Two mice were vaccinated with 5 × 106 PFU of VSV-Gag and were boosted 30 days later with 5 × 106 PFU of vvK1. Splenocytes from these mice were isolated and analyzed 30 days after the boost. At the time that these splenocytes were isolated, none of the splenomegaly that was notable 5 days after rVV boosting was present. We then quantitated Gag-specific memory cells in the spleen by staining with MHC-I Gag tetramers and antibodies to CD44 and CD62L. Figure 9A shows the representative results for one mouse. About 19% of cells remained MHC-I Gag tetramer positive 30 days after the boost, and most of these cells were CD44Hi, indicating that they were memory cells (Fig. 9A, right plot). Approximately 18% of cells were CD62LLo, and ∼1% were CD62LHi (Fig. 9A, left plot).
FIG. 9.
Quantitation of Gag-specific memory CTL after priming with VSV-Env and boosting with rVV or glycoprotein exchange VSV-Gag. BALB/c mice were vaccinated with 5 × 106 PFU of VSV-Env and boosted 30 days later with 5 × 106 PFU of vvK1 (A) or 5 × 106 PFU of VSVCh-Gag (B) (n = 2 per prime-boost condition). Bulk splenocytes were isolated 30 days after boosting and stained with MHC-I Gag tetramers, anti-CD8 antibodies, and antibodies to CD62L (left plots) or CD44 (right plots) and analyzed by flow cytometry. Only CD8+ cells were included in the analysis.
We conducted a similar analysis after boosting with VSVCh-Gag. Two mice were vaccinated with 5 × 106 PFU of VSV-Gag and boosted 30 days later with 5 × 106 PFU of VSVCh-Gag. Thirty days after the boost, ∼3.6% of splenocytes remained MHC-I Gag tetramer positive and expressed CD44 (Fig. 9B, right plot). About 2.8% of cells were CD62LLo, and ∼1% were CD62LHi (Fig. 9B, left plot).
DISCUSSION
We describe here high-level memory and recall responses elicited in mice vaccinated with rVSVs expressing HIV-1 Gag and Env separately or together. Mice serve as an important model in vaccine studies to inform experiments in the more relevant primate model. We used rVVs expressing Gag and Env as boosting vectors in rVSV-vaccinated mice and to elicit recall responses. Boosting with rVVs has been shown to be a reliable method for determining recall responses without concern for cytokine-mediated bystander effects (33).
Since the primary CTL response to Env in mice vaccinated with VSV-Env was particularly strong (up to 45% of cells are MHC-I Env tetramer positive), we quantitated long-term memory CTL in these mice 220 days after a single vaccination with 5 × 106 PFU of each virus by staining with MHC-I Env tetramers and antibodies to CD62L and CD44 (Fig. 2). CD62L is a lymph node homing receptor, and its downregulation is associated with lymphocyte activation (3, 4, 23, 32). CD44 is required for extravasation of activated CTL into inflammatory sites, and its upregulation is a marker of the memory CTL phenotype (9, 10). The combined expression pattern of CD62L and CD44 defines distinct CTL memory populations capable of either immediate effector activity or effector activity after restimulation with antigen (35). The long-term memory population of Env tetramer-positive and CD44Hi cells constituted about 6% of the total CD8+ splenocytes (Fig. 2A). Staining in parallel for CD62L revealed that ∼4% of CD8+ splenocytes were MHC-I Env tetramer positive and CD62LLo and ∼2% were CD62LHi. This suggests that ∼4% of cells are of the memory class capable of effector function after restimulation with antigen and ∼2% are capable of immediate effector function (35). The number of MHC-I Env tetramer-positive, CD44Hi memory cells in VSV-GagEnv-vaccinated mice was about half that of VSV-Env-vaccinated mice (∼3%). A comparison of the primary and memory responses to VSV-Env and VSV-GagEnv reveals that the number of memory cells is proportional to the number of activated cells at the peak of the primary response (Fig. 2) (16). This result is consistent with the observations of others that the level of the primary response predicts the level of the memory response (13, 20, 48).
The recall response in VSV-Env-vaccinated mice elicited by boosting with vPE16, an rVV expressing HIV-1 Env, showed that ∼45% of CD8+ splenocytes were MHC-I Env tetramer positive and CD62LLo 5 days after boosting, and intracellular cytokine staining for IFN-γ revealed that the majority of these cells were functional. About half of these cells produced TNF-α, which is likely a reflection of the fact that activated T cells are divided between IFN-γ+ TNF-α+ and IFN-γ+ TNF-α− populations (18, 19, 47). Mock boosting of VSV-Env-vaccinated mice with vTF7-3, an rVV expressing the T7 RNA polymerase, revealed that a total of ∼3.7% of CD8+ splenocytes were Env tetramer positive, and these cells produced IFN-γ upon stimulation with p18-I10 peptide (Fig. 3A and B). This result closely mirrors that obtained the direct identification of CD44Hi memory cells described above for mice that were not boosted. The reason that a slightly lower total percent of MHC-I Env tetramer-positive cells was identified after a vTF7-3 boost (∼3.7%; Fig. 3A and B) than after no boost (∼6%; Fig. 2) is that an increased proportion of CD8+ cells would be expected to be recruited to recognize VV epitopes, thus lowering the relative proportion of MHC-I Env tetramer-positive cells.
To determine how boosting with rVVs after initial vaccination with rVSV affected the population of long-term memory cells, we vaccinated two mice with 5 × 106 PFU of VSV-Env, boosted with 5 × 106 PFU of vPE16 120 days later, and stained for markers of memory cells 150 days after the boost (Fig. 4). Remarkably, 27 to 33% of CD8+ splenocytes remained Env tetramer positive and CD44Hi at a time when CTL were in memory. Parallel staining for CD62L showed that the majority of CD44Hi cells (∼80%) were also CD62LHi, marking them as memory cells capable of responding immediately to antigen. Therefore, boosting with vPE16 induced an increase of fivefold or more in the percentage of long-term memory cells recognizing the immunodominant p18-I10 peptide, although the initial recall response was no greater than that induced by the VSV-Env vector in the primary response. This result is consistent with the observations of others, showing that the memory CTL response after boosting is approximately 50% of the recall response (28).
At the peak of the primary response to Env after vaccination with vPE16, ∼6% of CD8+ cells stained with MHC-I Env tetramers, compared to ∼40% for VSV-Env-vaccinated mice (16). However, when these mice were boosted with VSV-Env, the recall response was then increased to ∼30% tetramer-positive cells. This response is about 30% lower than that observed when mice were primed with rVSV and boosted with rVV. Interestingly, the recall response elicited by VSV-Env is somewhat lower than the primary response induced by VSV-Env. This lower secondary response could be due to competition due to the large number of VV antigens produced in the primary infection.
Boosting with vvK1, an rVV expressing HIV-1 Gag, in VSV-Gag-vaccinated mice also elicited high levels of MHC-I Gag tetramer-positive cells (Fig. 6). For both VSV-Gag and VSV-GagEnv-vaccinated mice, ∼41% of CD8+ cells were tetramer positive and CD62LLo. However, a consistent discrepancy emerged in these experiments between the percentage of tetramer-positive CD8+ cells (∼41%) and the percentage of IFN-γ-producing cells (∼25%). This discrepancy may reveal an overproliferation of Gag-specific cells before they are able to develop functional properties. Another interesting possibility is that the Gag tetramer-positive cells that do not express cytokine might have become anergic. These issues will be explored in greater detail in future studies. When the priming and boosting vectors were reversed, 13 to 25% of CD8+ cells stained with MHC-I Gag tetramers, and similar percentages produced IFN-γ. Similar to the prime-boost reversal for recall to Env, the response to Gag in vvK1-vaccinated mice boosted with VSV-Gag was strong, although slightly lower overall than that when VSV-Gag was used to prime. This is likely due to the limitation of secondary responses caused by responses to epitopes in the ∼200 VV proteins.
A boosting method recently developed in our laboratory is based on changing the serotype of the glycoprotein of rVSVs to eliminate antibody neutralization of the vector upon boosting (43). Boosting with the same vector was not effective, but sequential vaccination with three rVSV glycoprotein exchange vectors generated greatly increased antibody titers in both mice and monkeys (42, 43). However, boosting with vBD3, an rVV expressing 89.6 Env, after three sequential glycoprotein exchange vector inoculations generates only a moderate increase (20%) in antibody titer to Env as seen by enzyme-linked immunosorbent assay (43). Here, we used a glycoprotein exchange vector VSVCh-Gag to elicit a recall response in VSV-Gag-vaccinated mice. Boosting with a glycoprotein exchange VSV elicited ∼8% tetramer-positive, CD62LLo CTL, which were functional in their release of IFN-γ. This is in contrast to the ∼40% tetramer-positive CTL induced after boosting with vvK1. Furthermore, staining for CD44 long after boosting with vvK1 and VSVCh-Gag also showed that the vvK1 boost elicited a proportionally higher percentage of memory cells to Gag. These results indicate that a completely heterologous vector may be superior in boosting CTL responses. When boosts are performed with VSV vectors, it is likely that CTL responses are less focused on Gag because all of the internal VSV proteins (N, P, M, and L) are shared between the priming and boosting vectors. Additionally, this difference might have been the reason that we observed little if any TNF-α staining after boosting with a glycoprotein exchange VSV vector. Antigenic competition might retard the development of recall responses and, therefore, the development of TNF-α-expressing T cells (47). However, when boosting is performed using the completely heterologous VV-Gag vector, all of the secondary responses are focused on Gag alone. In this regard, our results are similar to those of Robinson et al. (2, 41), who have also found that prime-boost regimens based on DNA vectors followed by modified vaccinia virus Ankara are superior to those based on either vector alone. Although the VSV vaccine vector system alone has been highly effective in the rhesus macaque AIDS model and involves different routes of vaccination, our results with mice suggest that even greater boosting of CTL responses might be achieved by boosting with completely heterologous vectors encoding Env and Gag.
Acknowledgments
We thank members of the Rose and Pamer laboratories for helpful comments and suggestions during the preparation of the manuscript, JoAnn Falato for providing valuable administrative assistance, and Matthias Schnell for helpful advice. We are grateful to the NIAID Tetramer Core Facility for providing the MHC-I Env and Gag tetramers. We also thank Gouzel Tokmoulina for helpful advice and assistance with flow cytometry and the Howard Hughes Medical Institute at Yale University for use of the flow cytometer.
K.H. was supported by a fellowship from the Bayer Corporation. This study was supported by NIH grants AI40357 and AI45510 to J.K.R. and AI42135 to E.P.
REFERENCES
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, E. C., J. P. Christensen, O. Marker, and A. R. Thomsen. 1994. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J. Immunol. 152:1237-1245. [PubMed] [Google Scholar]
- 4.Andersson, E. C., J. P. Christensen, A. Scheynius, O. Marker, and A. R. Thomsen. 1995. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand. J. Immunol. 42:110-118. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., M. Barner, A. Viola, and M. Kopf. 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29:291-299. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann, M. F., D. E. Speiser, and P. S. Ohashi. 1997. Functional management of an antiviral cytotoxic T-cell response. J. Virol. 71:5764-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budd, R. C., J. C. Cerottini, C. Horvath, C. Bron, T. Pedrazzini, R. C. Howe, and H. R. MacDonald. 1987. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 138:3120-3129. [PubMed] [Google Scholar]
- 10.DeGrendele, H. C., P. Estess, and M. H. Siegelman. 1997. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 278:672-675. [DOI] [PubMed] [Google Scholar]
- 11.Doherty, P. C., S. Hou, and R. A. Tripp. 1994. CD8+ T-cell memory to viruses. Curr. Opin. Immunol. 6:545-552. [DOI] [PubMed] [Google Scholar]
- 12.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, K. J., J. M. Riberdy, J. P. Christensen, J. D. Altman, and P. C. Doherty. 1999. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 96:8597-8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haglund, K., J. Forman, H. G. Krausslich, and J. K. Rose. 2000. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology 268:112-121. [DOI] [PubMed] [Google Scholar]
- 16.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8+ T-cell response to human immunodeficiency virus type 1 Gag and Env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrer, T., E. Harrer, S. A. Kalams, T. Elbeik, S. I. Staprans, M. B. Feinberg, Y. Cao, D. D. Ho, T. Yilma, A. M. Caliendo, R. P. Johnson, S. P. Buchbinder, and B. D. Walker. 1996. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retrovir. 12:585-592. [DOI] [PubMed] [Google Scholar]
- 18.Hassett, D. E., M. K. Slifka, J. Zhang, and J. L. Whitton. 2000. Direct ex vivo kinetic and phenotypic analyses of CD8+ T-cell responses induced by DNA immunization. J. Virol. 74:8286-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homann, D., L. Teyton, and M. B. A. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 20.Hou, S., L. Hyland, K. W. Ryan, A. Portner, and P. C. Doherty. 1994. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369:652-654. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, J. E., W. Rodgers, and J. K. Rose. 1998. A plasma membrane localization signal in the HIV-1 envelope cytoplasmic domain prevents localization at sites of vesicular stomatitis virus budding and incorporation into VSV virions. Virology 251:244-252. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, J. E., M. J. Schnell, L. Buonocore, and J. K. Rose. 1997. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J. Virol. 71:5065-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung, T. M., W. M. Gallatin, I. L. Weissman, and M. O. Dailey. 1988. Down-regulation of homing receptors after T cell activation. J. Immunol. 141:4110-4117. [PubMed] [Google Scholar]
- 24.Karacostas, V., K. Nagashima, M. A. Gonda, and B. Moss. 1989. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc. Natl. Acad. Sci. USA 86:8964-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedl, R. M., and M. F. Mescher. 1998. Qualitative differences between naive and memory T cells make a major contribution to the more rapid and efficient memory CD8+ T cell response. J. Immunol. 161:674-683. [PubMed] [Google Scholar]
- 26.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masopust, D., J. Jiang, H. Shen, and L. Lefrancois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 29.Mata, M., P. J. Travers, Q. Liu, F. R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985-2993. [PubMed] [Google Scholar]
- 30.Mergener, K., M. Facke, R. Welker, V. Brinkmann, H. R. Gelderblom, and H. Krausslich. 1992. Analysis of HIV particle formation using transient expression of subviral constructs in mammalian cells. Virology 186:25-39. [DOI] [PubMed] [Google Scholar]
- 31.Montefiori, D. C., J. T. Safrit, S. L. Lydy, A. P. Barry, M. Bilska, H. T. Vo, M. Klein, J. Tartaglia, H. L. Robinson, and B. Rovinski. 2001. Induction of neutralizing antibodies and Gag-specific cellular immune responses to an R5 primary isolate of human immunodeficiency virus type 1 in rhesus macaques. J. Virol. 75:5879-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller, C., H. K. Gershenfeld, C. G. Lobe, C. Y. Okada, R. C. Bleackley, and I. L. Weissman. 1988. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J. Exp. Med. 167:1124-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 34.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 35.Oehen, S., and K. Brduscha-Riem. 1998. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 161:5338-5346. [PubMed] [Google Scholar]
- 36.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 37.Opferman, J. T., B. T. Ober, and P. G. Ashton-Rickardt. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283:1745-1748. [DOI] [PubMed] [Google Scholar]
- 38.Razvi, E. S., and R. M. Welsh. 1995. Apoptosis in viral infections. Adv. Virus Res. 45:1-60. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 42.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 43.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirai, M., S. Kozlowski, D. H. Margulies, and J. A. Berzofsky. 1997. Degenerate MHC restriction reveals the contribution of class I MHC molecules in determining the fine specificity of CTL recognition of an immunodominant determinant of HIV-1 gp160 V3 loop. J. Immunol. 158:3181-3188. [PubMed] [Google Scholar]
- 46.Shirai, M., C. D. Pendleton, and J. A. Berzofsky. 1992. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J. Immunol. 148:1657-1667. [PubMed] [Google Scholar]
- 47.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 48.Vijh, S., and E. G. Pamer. 1997. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J. Immunol. 158:3366-3371. [PubMed] [Google Scholar]
- 49.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel, R. M., M. F. Bachmann, T. M. Kundig, S. Oehen, H. Pirchet, and H. Hengartner. 1996. On immunological memory. Annu. Rev. Immunol. 14:333-367. [DOI] [PubMed] [Google Scholar]