Abstract
Unlike all other picornaviruses, the primary cleavage of the hepatitis A virus (HAV) polyprotein occurs at the 2A/2B junction and is carried out by the only proteinase encoded by the virus, 3Cpro. The resulting P1-2A capsid protein precursor is subsequently cleaved by 3Cpro to generate VP0, VP3, and VP1-2A, which associate as pentamers. An unidentified cellular proteinase acting at the VP1/2A junction releases the mature capsid protein VP1 from VP1-2A later in the morphogenesis process. Although these aspects of polyprotein processing are well characterized, the function of 2A is unknown. To study its role in the viral life cycle, we assessed the infectivity of synthetic, genome-length RNAs containing 11 different in-frame deletions in the 2A region. Deletions in the N-terminal 40% of 2A abolished infectivity, whereas deletions in the C-terminal 60% resulted in viruses with a small-focus replication phenotype. C-terminal deletions in 2A had no effect on RNA replication kinetics under one-step growth conditions, nor did they have an effect on capsid protein synthesis and 3Cpro-mediated processing. However, C-terminal deletions in 2A altered the VP1/2A cleavage, resulting in accumulation of uncleaved VP1-2A precursor in virions and possibly accounting for a delay in the appearance of infectious particles with these mutants, as well as a fourfold decrease in specific infectivity of the virus particles. When the capsid proteins were expressed from recombinant vaccinia viruses, the N-terminal part of 2A was required for efficient cleavage of the P1-2A precursor by 3Cpro and assembly of structural precursors into pentamers. These data indicate that the N-terminal domain of 2A must be present as a C-terminal extension of P1 for folding of the capsid protein precursor to allow efficient 3Cpro-mediated cleavages and to promote pentamer assembly, after which cleavage at the VP1/2A junction releases the mature VP1 protein, a process that appears to be necessary to produce highly infectious particles.
Hepatitis A virus (HAV) is a common cause of acute viral hepatitis in humans. Although effective vaccines are available, their use is limited, and type A hepatitis remains prevalent in many countries, especially those with poor public health infrastructures. A positive-strand RNA virus with an ∼7,500-nucleotide (-nt) genome, HAV is classified within the family Picornaviridae and shares a number of features in common with other members of the family Picornaviridae, particularly those in the genera Aphthovirus (Foot and mouth disease virus) and Cardiovirus (e.g., Encephalomyocarditis virus [EMCV]) (21). Its genomic organization is similar to that of all picornaviruses, including members of the Enterovirus (e.g., Poliovirus) and Rhinovirus genera. Among the common features in the viruses of this family, the picornavirus genome encodes a unique large polyprotein that undergoes a cleavage cascade performed by virus-encoded protease activities to yield the precursors for capsid proteins ([L]-P1-[2A]) and nonstructural proteins ([2A]2BC-P3) necessary to the viral life cycle (for a review, see reference 36). Despite these similarities, HAV has a number of distinctive features that have led to its classification within a separate genus, the genus Hepatovirus.
Unlike the polyproteins of each of the other major picornavirus genera that contain at least two, and in some cases three, distinct proteinase activities (31), the polyprotein of HAV contains only a single proteinase, 3Cpro, which acts both in cis and in trans to effect cleavage of the polyprotein. Although HAV processing events have been difficult to analyze in infected cells due to the protracted replication cycle of the virus and the failure of the virus to inhibit host cell protein synthesis, recent studies have shown that the primary cleavage event within the HAV polyprotein takes place at the 2A/2B junction. This cleavage has been precisely mapped by N-terminal sequencing of the 2B polypeptide and is carried out by the 3Cpro proteinase (20, 10). The P1-2A capsid protein precursor is subsequently cleaved by 3Cpro to generate VP0 (VP4-VP2), VP3, and VP1-2A (also termed PX). This has been shown in experiments in which purified bacterially expressed 3Cpro is incubated with P1-2A generated by cell-free translation of synthetic RNA (32), as well as with P1-2A and either P3 or 2BC-P3 as a source of 3Cpro, all expressed from recombinant vaccinia viruses (VVs) (19, 30). This primary cleavage mechanism in HAV differs significantly from that of enteroviruses and rhinoviruses, in which the chymotrypsin-like cysteine proteinase 2A is responsible for the primary cleavage of the polyprotein between VP1 and 2A (35), as well as from the cardioviruses and aphthoviruses, in which a 16-amino-acid (-aa) sequence at the C terminus of 2A promotes an unusual cotranslational cleavage between proteins 2A and 2B involving an Asn-Pro-Gly/Pro motif (8). Thus, HAV appears to be unique among the picornaviruses in terms of the absence of proteinase activity associated with the 2A polypeptide and in having the primary cleavage of the polyprotein directed by the same viral proteinase, 3Cpro, that directs most other cleavage events in the polyprotein.
A second unique feature of HAV processing concerns the VP1-2A polypeptide that is found, together with VP0 and VP3, in pentamer assemblies that represent the earliest intermediate in the morphogenesis process (5). The mature VP1 capsid protein is generated from the VP1-2A precursor at a later point in the assembly process, by an as yet incompletely defined mechanism. Two independent studies have provided lines of evidence that argue strongly against the involvement of HAV 3Cpro proteinase in this maturation process. Mutagenesis of the only possible dipeptide sequence that could serve to target the 3Cpro proteinase to the region of the polyprotein consistent with the size of the mature VP1 capsid protein neither abolished the infectivity of HAV RNA transcripts nor prevented normal maturation of VP1 in the virus rescued from the mutated RNA (19). Mapping of the carboxy-terminus of VP1 from purified virions by mass spectrometry also indicated that the mature capsid protein is not produced by a 3Cpro-mediated cleavage event (11). Thus, at present it seems likely that the VP1/2A cleavage occurs under the direction of an as-yet-unidentified cellular proteinase. This aspect of polyprotein processing is unique to HAV among picornaviruses and appears to lead to some degree of heterogeneity at the carboxy terminus of VP1 (11). The only possible parallel to this observation among other picornaviruses is found in the cardiovirus genus, where the VP1 capsid protein undergoes a postassembly, carboxy-terminal trimming of 3 aa directed by an apparent cellular proteinase (4). However, this occurs only after 3Cpro-mediated cleavage at the VP1/2A junction (29).
The function of HAV 2A remains unknown and its role in the viral life cycle is uncertain. An earlier report described an HAV mutant derived from a cell culture-adapted strain lacking 15 aa in the C-terminal part of 2A which was still replication competent in cell culture (12). More recently, using a heterologous expression system based on recombinant VVs, Probst et al. reported that the coexpression of a capsid protein precursor bearing a complete deletion of 2A and P3 as a source of 3Cpro resulted in the lack of pentamer assembly (30).
Here, we report a detailed analysis of the role of 2A in HAV replication based on the use of synthetic, genome-length RNAs containing 11 different in-frame deletions in the 2A region. We show that deletions in the N-terminal 40% of 2A abolish infectivity, whereas deletions in the C-terminal 60% result in viruses with a small focus replication phenotype. C-terminal deletions in 2A alter the VP1/2A cleavage that takes place after pentamer assembly, resulting in accumulation of uncleaved VP1-2A precursor in virions and possibly accounting for a decrease in the specific infectivity of the virus particles. To gain a better understanding of the role of the N-terminal domain of 2A, we expressed HAV polyproteins with N-terminal 2A deletions. We show that the N-terminal domain of 2A must be present as a C-terminal extension of P1 to fold the capsid protein precursor in the conformation required for efficient 3Cpro-mediated cleavages and to promote pentamer assembly.
MATERIALS AND METHODS
Cell culture.
Fetal rhesus kidney (FRhK-4) cells were used for rescue of infectious HAV following transfection with synthetic, genome-length HAV RNA transcripts (7) and for the in vivo expression of HAV polypeptides following infection with recombinant VVs. African green monkey kidney (BS-C-1) cells were used for radioimmunofocus assays (RIFA) (17) to characterize the replication phenotype of mutant HAVs and to determine the titer of HAV stocks. These cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL or Eurobio) supplemented with 5% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) (DMEM-5%) and a mixture of nonessential amino acids (Gibco BRL). Human 143B thymidine kinase-deficient (tk−) cells were used for the isolation of recombinant VVs, and monkey kidney (CV1) or human cervix carcinoma (HeLa) cells were used for propagation of these viruses. These three cell lines were maintained in DMEM-5%.
Construction of full-length, HAV cDNAs with deletions in 2A.
The parent HAV infectious molecular clone was a chimeric cDNA, p5′P2P3-18f (38) (hereinafter referred to as p18f), containing the P1 segment of a relatively low passage, cell culture-adapted variant of the HM175 strain of HAV, HAV/7 (6, 7), in the background of a rapidly replicating, cytopathic HM175 variant, 18f (18). HAV full-length, mutant cDNAs Δ2A-2 to Δ2A-11 (Fig. 1) were generated by an enzymatic inverse PCR procedure adapted from the method described by Stemmer and Morris (34) using p18f as a template and the proofreading Pwo DNA polymerase (Roche) to amplify two fragments, as follows. The upstream fragment was amplified with a forward primer complementary to HAV nt 2982 to 2999, spanning the SacI site (nt 3002), and a reverse primer complementary to HAV cDNA sequence located upstream of each deletion and designed to introduce a BsaI restriction site at the 3′ end of the fragment. The second amplimer was obtained using a forward primer complementary to HAV sequence downstream of the deletion and designed to create a BsaI site at the 5′ end of the fragment and a reverse primer complementary to HAV nt 4195 to 4217, spanning the PflMI restriction site (nt 4230). After purification by low-melting-point agarose gel electrophoresis, and hydrolysis by BsaI, which allowed generation of exact in-frame, fusion junctions, these two fragments were ligated in vitro. Each resulting, mutated fragment was purified on a silica gel membrane (QIAGEN) and hydrolyzed by restriction enzymes SacI and PflMI prior to introduction into the background of p18f. All PCR-amplified SacI-PflMI restriction fragments containing the various deletions were sequenced to exclude spurious mutations.
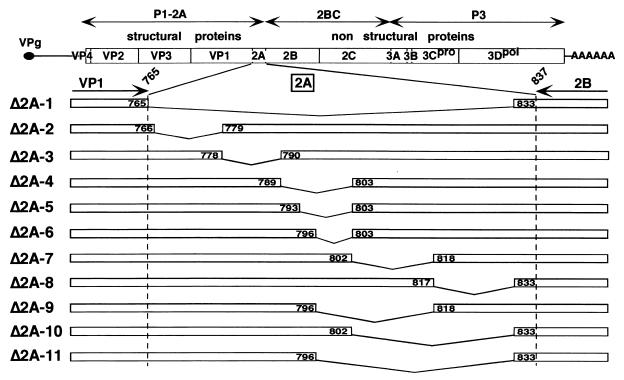
FIG. 1.
Schematic representation of HAV cDNAs with deletions in 2A. The C terminus of VP1 and N terminus of 2B are indicated at the top of the 2A enlargement, with the amino acid residues within the HAV polyprotein numbered according to p5′P2P3-18f cDNA (38). Internal 2A deletions are indicated by broken lines. The positions of amino acids framing each deletion within the HAV polyprotein are indicated in boxes.
The mutant Δ2A-1 cDNA (Fig. 1) was engineered by a PCR-based fusion strategy by using overlapping primers complementary to each cDNA region framing the deletion to introduce. A second amplification step was carried out by mixing the downstream and upstream PCR fragments thus generated with an overlap of 27 nt. The resulting SacI-PflMI fragment, carrying the Δ2A-1 deletion, was reintroduced into the p18f background as described above.
Construction of HAV expression plasmids and generation of recombinant VVs.
For expression of HAV full-length polyprotein (P1P2P3) in eukaryotic cells, we have cloned the appropriate HAV cDNA fragment from p18f into plasmid pTM1 (25) downstream of the T7 RNA polymerase promoter. This plasmid, pTM/P1P2P3, was constructed starting with plasmid pTM/P1-2A, previously described (19), by fusing the SacI-SmaI restriction fragment of p18f (nt 3002 to 7533), encompassing the HAV 2ABC-P3 encoding cDNA segment and the 3′ noncoding region, between the SacI (HAV nt 3002) and StuI (pTM1 polylinker) sites of pTM/P1-2A. The resulting pTM/P1P2P3 plasmid allowed the generation of a recombinant VV (VV-18f) by homologous DNA recombination as described previously (20). VV-18f drives the expression of the full-length HAV polyprotein (P1P2P3).
To create pTM/P1P2P3 derivatives expressing the 2A deletion polyproteins Δ2A-1, -3, and -5, the SacI-EcoRI restriction fragments from the corresponding full-length HAV clones were introduced into the pTM/P1P2P3 background. Corresponding recombinant VVs (VV-Δ2A-1, -3, and -5, respectively) were generated as described above.
Transcription and transfection of full-length HAV RNAs.
Transcription of full-length HAV RNA with SP6 RNA polymerase and liposome-mediated RNA transfection of FRhK-4 cells were carried out as described previously (19). After a 2-week incubation period, cells were harvested mechanically together with supernatant, subjected to three freeze-thaw cycles, and extracted with an equal volume of chloroform.
HAV RIFA.
Lysates of transfected cells were assayed for infectious HAV by RIFA carried out in BS-C-1 cells as described previously (17). Infected cells were maintained at 37°C for 7 days before processing.
RT-PCR of viral RNA.
Viral RNA was isolated from 200 μl of clarified virus stock by treatment with proteinase K (400 μg/ml) in 0.1 M Tris-HCl (pH 8.8)-12.5 mM EDTA-150 mM NaCl-1% sodium dodecyl sulfate (SDS) during 1 h at 55°C. RNAs were then phenol extracted and ethanol precipitated. Reverse transcription (RT) of one-fourth of the recovered viral RNAs was carried out using avian myeloblastosis virus reverse transcriptase (Promega). One-fourth of the RT reaction mixture was then used to PCR amplify the resulting cDNA in a 100-μl total volume with a 5′ primer corresponding to HAV nt 2969 to 2988 and a 3′ primer complementary to nt 3766 to 3784, using DNA Taq polymerase (Perkin-Elmer). After purification of the PCR product on a silica gel membrane and agarose gel electrophoresis to assay the quality and quantity of the product, approximately 30 ng of DNA fragment were subjected to direct sequencing on an ABI 377 instrument using a Big-Dye terminator kit (Applied Biosystems) and a reverse primer complementary to HAV nt 3529 to 3543.
Monitoring of HAV RNA replication in cell cultures.
A total of 2 × 105 FRhK-4 cells in 35-mm-diameter petri dishes were infected with parent or mutant HAVs at a multiplicity of infection (MOI) of 4 radioimmunofocus units (RFU)/cell, incubated at 37°C, and then lysed at various times postinfection (p.i.) in 0.2 ml of 50 mM Tris-HCl (pH 7.5)-150 mM NaCl-1 mM EDTA-1% NP-40-1 mM dithiothreitol-RNasin (400 U/ml). After removal of nuclei, cytoplasmic RNAs were purified by phenol-chloroform extractions, ethanol precipitated, and resuspended in 40 μl of RNase-free water. Half of each sample was mixed with 3 volumes of SSPE buffer (1× SSPE is 0.15 M NaCl, 10 mM NaH2PO4, and 1mM EDTA [pH 7.7]) and formaldehyde (7.4% final concentration), heated at 65°C for 15 min, and immobilized on a Hybond N membrane (AP Biotech) using a slot blot apparatus (Bio-Rad). Positive-strand HAV RNA was detected by hybridizing the membrane with a 32P-labeled, negative-strand RNA probe (complementary to HAV nt 2203 to 3102) and quantitated by PhosphorImager analysis of the blot.
HAV polypeptide expression assays and immunoblot detection of HAV proteins.
FRhK-4 cells (2 × 105) in 35-mm-diameter petri dishes were infected with either mutant HAVs at an MOI of 1 to 4 RFU/cell or coinfected with HAV-VV recombinants expressing polyproteins with 2A deletions (VV-2A-1, VV-2A-3, and VV-2A-5) and vTF7-3, a recombinant VV expressing T7 DNA-dependent RNA polymerase (9), each at an MOI of 5 PFU/cell. Cytoplasmic extracts were prepared at 36, 48, 72, or 96 h p.i. (hpi) (for HAV infections) or at 20 hpi (for VV infections) by lysis of cells in 0.2 ml of 50 mM Tris-Cl (pH 7.5)-150 mM NaCl-1 mM EDTA-1% Nonidet P-40-0.1% sodium deoxycholate-aprotinin (25 μg/ml). A 10- to 20-μl aliquot was subjected to SDS-10% polyacrylamide gel electrophoresis (PAGE), followed by semidry transfer onto a polyvinylidene difluoride membrane (Amersham). Nonspecific binding sites were blocked in phosphate-buffered saline containing 0.1% Tween 20 (PBST) and 5% nonfat milk for 1 h at room temperature. Membranes were incubated overnight at 4°C with either HAV anti-VP1, -VP2, or -VP3 guinea pig antipeptide antibodies (16) or HAV anti-2B rabbit antipeptide antibodies (10), diluted in PBST containing 1% bovine serum albumin (PBST-BSA). After four washes with PBST, the membrane was incubated with anti-guinea pig or anti-rabbit antibodies conjugated to horseradish peroxidase (Sigma) diluted in PBST-BSA for 1 h at room temperature. After four washes with PBST, the HAV polypeptides were visualized by chemiluminescence (ECL Plus; AP Biotech).
Fractionation of morphogenesis intermediates on sucrose gradients.
Cytoplasmic extracts from HAV-infected cells or recombinant HAV-VV-infected cells (6 × 105 cells) were layered on top of 5 to 45% (wt/wt) sucrose gradients in 100 mM NaCl-10 mM Tris (pH 7.4). Gradients were centrifuged at 4°C in a Beckman SW41 rotor for 4 h at 40,000 rpm. Fractions of 0.7 ml were collected from the bottom of each tube, and proteins were precipitated from each fraction by adding 0.05% (wt/vol) of BSA and 9 volumes of methanol. After an overnight incubation at −20°C, proteins were pelleted by centrifugation at 3,000 rpm at 4°C and resuspended in 40 μl of Laemmli buffer. Half of each fraction was subjected to electrophoresis on an SDS-12% polyacrylamide gel containing 3.5 M urea. HAV-specific proteins were then detected by Western blotting using a mixture of HAV anti-VP1 and anti-VP2 antibodies, as described in the preceding section.
Determination of the specific infectivity of virus particles.
Virion-containing fractions 4 from sucrose gradients, derived from parent HAV or mutant vΔ2A-11-infected cells, were treated for 30 min at 16°C with micrococcal nuclease (150 U/ml) in the presence of 0.3 mM of CaCl2, to eliminate traces of nonencapsidated RNAs. Nuclease was then inactivated by addition of 2 mM EGTA. Particles were then treated with proteinase K for 60 min at 56°C, and the resulting RNAs were phenol extracted and ethanol precipitated. The amount of total virus particles present in these fractions was determined by quantitating the encapsidated genome content using slot blot hybridization with a 32P-labeled RNA probe as described above, while the amount of infectious particles was determined by RIFA. A ratio of genome copy number to infectious particles was determined for both mutant vΔ2A-11 and parent v18f.
RESULTS
Construction of full-length HAV cDNAs with deletions in 2A.
In an attempt to identify the role of the 2A protein of HAV in the virus life cycle, 10 partial in-frame deletions distributed along the 2A sequence (Δ2A-2 to -11 [Fig. 1]) or a total in-frame deletion of the 2A sequence (Δ2A-1 [Fig. 1]) was introduced into the full-length infectious cDNA of a rapidly replicating, cell culture-adapted HAV variant (p5′P2P2-18f, herein referred to as p18f [see references 18 and 38]). We considered the 2A polypeptide to be comprised of 71 aa with Met766 being the N-terminal residue, since residue 765 was the most abundant C-terminal residue identified in previous efforts to sequence the heterogeneous terminus of the preceding VP1 capsid protein (11).
The deletion of the complete 2A sequence (Δ2A-1) was designed to preserve the C-terminal four residues of the 2A sequence, in order to allow proper 3Cpro cleavage at the newly engineered VP1/2B junction. From the analysis of HAV 3Cpro processing of synthetic peptides (15) and by analogy with poliovirus 3Cpro requirements (27), we considered it likely that the four residues upstream of the 2A/2B cleavage site, along with the downstream 2B residues, would be sufficient for proper recognition by 3Cpro. This was subsequently found to be the case when an heterologous sequence was fused at its C terminus to these 4 aa of HAV 2A and inserted at the 2A/2B junction (2).
Partial deletions (Δ2A-2 to -11 [Fig. 1]) were created throughout the 2A sequence, with lengths ranging from 6 to 36 aa. Two distinct series of deletions were engineered, the first designed to scan the 2A sequence with contiguous 11- to 15-aa deletion windows (Δ2A-2, -3, -4, -7, and -8), and a second designed to more precisely map the regions that were either necessary or dispensable for virus infectivity (Δ2A-5, -6, -9, -10, and -11). Since a previous report demonstrated that a deletion of residues 803 to 817 in another HAV cDNA background resulted in a viable virus (12), one of the mutants (Δ2A-7) was a replica of this construct in a p18f background.
Infectivity of RNA transcripts with deletions in the 2A sequence.
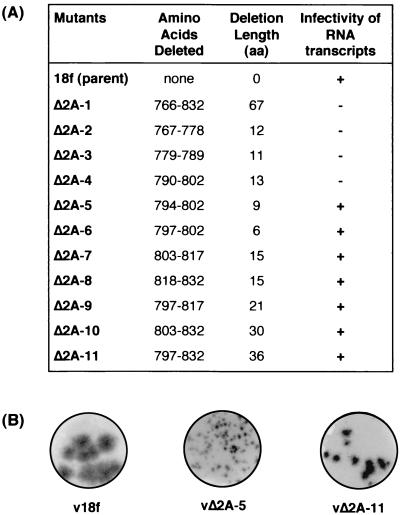
To assess the effect of 2A deletions on the infectivity of synthetic HAV RNA, FRhK-4 cells were transfected with in vitro RNA transcripts derived from each cDNA. Cell lysates were prepared 2 weeks after transfection, and the presence of virus was determined by RIFA in BS-C-1 cells (as described in Materials and Methods). No virus was recovered from cells transfected with any of the RNA transcripts carrying deletions that extended into the N-terminal two-fifths of the 2A sequence (Δ2A-1 to -4 [Fig. 2A ]).
FIG. 2.
Infectivity of full-length HAV RNA transcripts with deletions in 2A. (A) For each genome-length RNA transcript, the extent of the deletion is indicated, both by the positions within the polyprotein of the corresponding deleted amino acids and by the number of amino acid residues deleted. The infectivity of deleted transcripts is indicated (+, infectious; −, lethal), as determined by RIFA in BS-C-1 cells inoculated with FRhK-4 cell lysates harvested 2 weeks posttransfection of RNA. (B) RIFA autoradiograms for selected progeny viruses.
In contrast, RNA transcripts carrying deletions in the C-terminal three-fifths of the 2A sequence (Δ2A-5 to -11), i.e., downstream of His-793, resulted in viable progeny viruses (Fig. 2A). However, the replication phenotype of these viruses was impaired, as shown by production of smaller foci in RIFA (Fig. 2B) and by slightly lower virus titer yields (∼0.5 log lower) than those of the parent virus v18f. Variants vΔ2A-6, -7, -8, -9, and -10 produced foci (data not shown) of a size comparable to those from the vΔ2A-11 variant, shown in Fig. 2B. It is noteworthy that the vΔ2A-5 variant, carrying the most-extreme N-terminal 2A deletion still capable of generating a viable virus (aa 794 to 802), produced minute RIFA foci (Fig. 2B) and replicated very inefficiently, giving virus yields approximately 3 to 4 logs lower than those of the parent virus v18f.
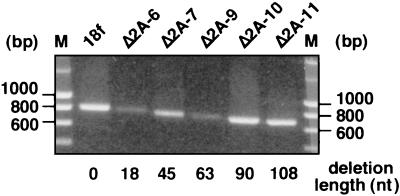
To confirm that the 2A deletions were retained in the genomes of the rescued viruses, the 2A coding region (nt 2951 to 3748) of each progeny virus after one passage in cell culture was amplified by RT-PCR using appropriate oligonucleotide primers, as described in Materials and Methods. The size of the resulting DNA fragments was assessed by agarose gel electrophoresis and found to correspond to that expected for the appropriate deletions (Fig. 3 and data not shown). Furthermore, each PCR product was subjected to direct DNA sequencing, and these results demonstrated that each of the rescued HAV variants retained the original 2A deletion in their genomes. Similar attempts to amplify viral genomes directly from cells transfected with RNAs containing lethal mutations, as well as with the infectious mutant Δ2A-5 RNA, failed to produce any detectable fragment. This suggests that transfection efficiency was low and prevented us from determining whether RNAs that failed to give rise to virus remained capable of replication.
FIG. 3.
Retention of engineered deletions in the genomes of viable 2A mutants. The 2A coding region of the mutant viral RNA isolated from passage 1 virions was amplified by RT-PCR, and the resulting fragments were run on a 1% agarose gel. Positions of the DNA molecular weight markers are indicated on both sides of the gel. The length of the corresponding 2A deletion, in terms of the number of nucleotides deleted, is indicated for each mutant below the lanes.
We conclude from these results that deletions in the N-terminal 40% of 2A (upstream of Ile-794) abolish infectivity, whereas deletions in the C-terminal 60% result in a virus with a small-focus replication phenotype.
Polyprotein synthesis and processing of 2A deletion variants.
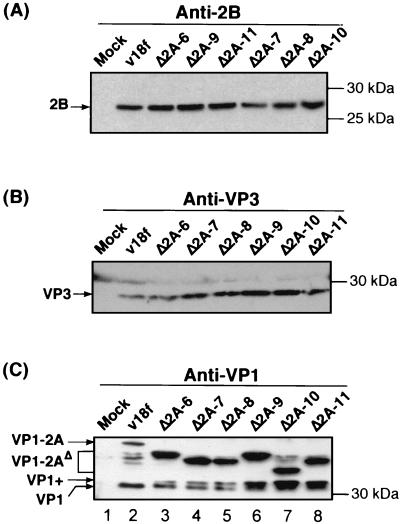
To identify the step in the virus growth cycle that was involved in the impaired replication phenotype of the viable C-terminal 2A-deletion mutants, we first undertook a determination of whether these deletions affected the ability of the polyprotein to undergo proteolytic processing, particularly at cleavage sites located in the vicinity of the deletion (VP1/2A and 2A/2B junctions). For that purpose, FRhK-4 cells were infected with deletion mutants at an MOI of 1 RFU/cell, and the viral proteins were characterized at 72 hpi. The presence of polypeptides 2B, VP3, and VP1-2A, which result from 3Cpro-mediated cleavages, as well as of protein VP1, which results from a different processing mechanism most likely directed by a cellular proteinase, was determined by immunoblotting with specific antisera (Fig. 4). As indicated by the relative abundance and size of the 2B and VP3 proteins (Fig. 4A and B), the 2A deletion mutants appeared to be capable of normal protein synthesis and 3Cpro-mediated processing at the 2A/2B, VP0/VP3, and VP3/VP1 junctions, at least at this relatively late point in the virus cycle.
FIG. 4.
Polyprotein synthesis and 3Cpro processing of 2A deletion mutants. FRhK-4 cells were mock infected (Mock) or infected with the parent virus (v18f) or the indicated mutant at an MOI of 1 RFU/cell. Proteins from cytoplasmic extracts prepared at 72 hpi were separated by SDS-10% PAGE and identified by immunoblot with anti-2B (A), anti-VP3 (B), or anti-VP1 (C) antibodies. HAV polypeptides and molecular mass markers, are indicated on the left and right sides of each panel, respectively.
Normally, VP1 maturation occurs late in the virus cycle, and the VP1-2A precursor (Fig. 4C, lane 2), as well as an intermediate product, referred to as VP1+ (see Fig. 6A and 8B), are often observed in v18f-infected cells. At 72 hpi, however, the mature VP1 capsid protein is the most-abundant product, compared to the precursors VP1-2A and VP1+ (Fig. 4C, lane 2). In the case of the 2A deletion mutants, the mature VP1 proteins were of normal size (Fig. 4C, compare lanes 3 through 8 to lane 2), suggesting that the VP1-2A precursors are processed faithfully at the usual VP1/2A junction. However, the VP1/2A cleavage appeared to be far less efficient in all of the mutants, since we observed an abnormally high level of accumulation of the VP1-2AΔ and VP1+ precursors (Fig. 4C, compare lanes 3 through 8 to lane 2).
FIG. 6.
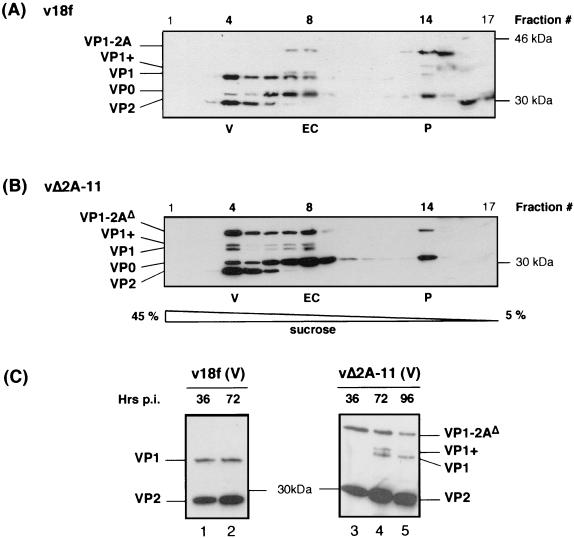
vΔ2A-11 capsid protein assembly. FRhK-4 cells were infected with the parent virus (v18f) (A) or the vΔ2A-11 mutant (B) at an MOI of 1 RFU/cell. Morphogenesis intermediates present in cytoplasmic cell extracts harvested at 72 hpi were fractionated on 5 to 45% sucrose gradients. Each fraction was loaded on an SDS-3.5 M urea-12% polyacrylamide gel. The HAV polypeptide content of each fraction was determined by immunoblotting using a mixture of anti-VP1 and anti-VP2 antibodies. (C) FRhK-4 cells were infected with the parent virus (v18f) or the vΔ2A-11 mutant at an MOI of 4 RFU/cell, and cytoplasmic cell extracts were harvested at the indicated times p.i. (36, 72, or 96 hpi). Morphogenesis intermediates were separated on a 5 to 45% sucrose gradient, and the polypeptide content of fraction 4, containing virus particles (V), was analyzed by immunoblotting with anti-VP1 and anti-VP2 antibodies. HAV polypeptides, as well as molecular mass markers, are indicated on each side of the panels.
FIG. 8.
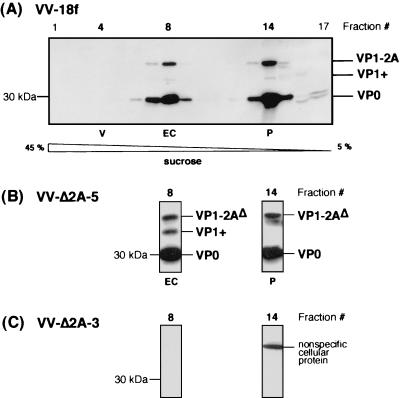
Effect of N-terminal 2A deletions on assembly of capsid proteins expressed by recombinant VVs. FRhK-4 cells were coinfected with vTF7-3 and virus expressing either the parent polyprotein, VV-18f (A), or the indicated mutant polyproteins, VV-Δ2A-5 (B) or VV-Δ2A-3 (C), each at an MOI of 5 PFU/cell. Morphogenesis intermediates were fractionated on 5 to 45% sucrose gradients and loaded on an SDS-3.5 M urea-12% polyacrylamide gel, and their polypeptide composition was determined by immunoblot analysis using a mixture of anti-VP1 and anti-VP2 antibodies. All fractions are shown for the gradient containing the parent VV-18f (A), whereas only fractions in which pentamers (fraction 14 [P]) and empty capsids (fraction 8 [EC]) were expected are shown for the mutant viruses (B and C).
As expected, the VP1-2AΔ polypeptides of all deletion mutants exhibited an increased mobility compared with the parent VP1-2A. However, surprisingly, the changes in the electrophoretic mobilities of the deleted precursors on an SDS-10% polyacrylamide gel did not correspond to the size of the deletions. For example, the VP1-2AΔ polypeptide produced by mutant vΔ2A-11, lacking 36 aa, ran slower than that produced by mutant vΔ2A-10, which contains only a 30-aa deletion (Fig. 4C, compare lanes 7 and 8). Since in all cases the accurate mature VP1 and 2B proteins were present (Fig. 4C and A, respectively), defects in 3Cpro-mediated cleavages at the VP3/VP1 or 2A/2B junction, or in cellular proteinase-mediated cleavage at the VP1/2A junction, could not account for these abnormalities. We conclude that the unexpected electrophoretic mobility characteristics of these polypeptides are likely to be due to their specific amino acid compositions. Consistent with this hypothesis, the relative electrophoretic mobilities were different when polypeptides were separated on an SDS-polyacrylamide gel containing 3.5 M urea (data not shown). Consistent with this interpretation, we have previously observed that a single amino acid substitution, replacing a Glu-764 with either a Gln or an Arg, substantially modifies the electrophoretic mobility of the VP1-2A and VP1 polypeptides (19).
Taken together, these results show that the viable 2A deletion variants are capable of accurate 3Cpro-mediated processing of the polyprotein but demonstrate incomplete VP1 maturation.
Effect of C-terminal deletions in 2A on kinetics of HAV RNA replication and infectious particle synthesis.
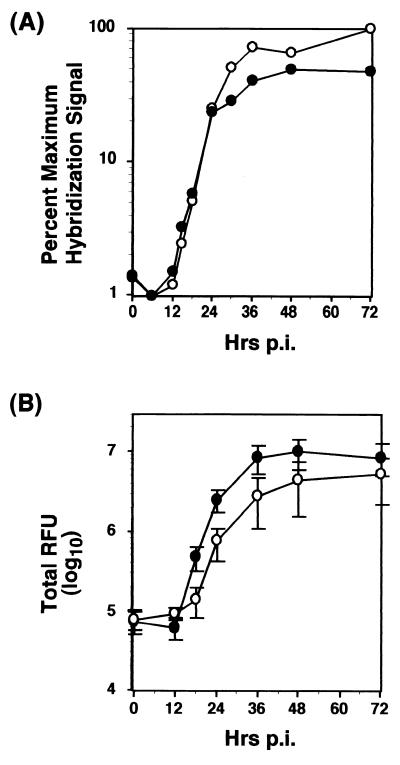
We next investigated whether the defective VP1/2A cleavage exhibited by the 2A deletion mutants affected the kinetics of RNA replication or infectious particle production. Under one-step growth conditions, cells were infected with vΔ2A-11, which contains the most-extensive C-terminal deletion in 2A and for the purposes of this analysis served as the prototype of the mutants with C- terminal deletions of 2A. FRhK-4 cell monolayers were infected with vΔ2A-11 or v18f at an MOI of 4 RFU/cell, and virus adsorption was carried out for an hour before thoroughly washing the cells to ensure proper elimination of input virus. At various times thereafter, cells and supernatant media were harvested and assayed for infectious virus titers by RIFA as described in Materials and Methods. The accumulation of intracellular HAV-specific, positive-strand RNA was quantified using a 32P-labeled negative-strand RNA probe. All measurements were made in duplicate, and the experiment was repeated three times.
These results demonstrated that there was no apparent defect in the RNA replication kinetics of the mutant vΔ2A-11 at early time points (< 30 hpi), as indicated by quantification of the blots using a PhosphorImager (Fig. 5A). However, accumulation of vΔ2A-11 RNA appeared to be reproducibly higher at late times p.i. compared with the parent virus (Fig. 5A, from 30 hpi). In contrast, there was a slight delay in the production of infectious particles in the case of vΔ2A-11 (Fig. 5B). Furthermore, despite greater RNA abundance, virus yields were consistently two to four times lower with vΔ2A-11, and the appearance of the characteristic HAV cytopathic effect in the infected cell monolayers, as monitored by light microscopy, was somewhat delayed with the mutant.
FIG. 5.
Single-cycle growth and RNA replication kinetics of vΔ2A-11 variant. FRhK-4 cells were infected at an MOI of 4 RFU/cell, either with the virus carrying the most-extensive deletion (vΔ2A-11 [open circles]) or with the parent virus (v18f [closed circles]). (A) Cytoplasmic RNAs were harvested at the indicated times p.i., denatured, immobilized onto a nylon membrane, and hybridized to an HAV-specific, 32P-labeled, negative-strand riboprobe. HAV-specific positive-strand RNA was quantified by PhosphorImager analysis. Results of one experiment are represented and expressed as the relative percentage of the maximum hybridization signal obtained in the experiment. (B) Virus present in the supernatant and within the cells was harvested at the indicated times p.i. and titrated by RIFA. Each point represents the mean of three independent experiments. Error bars indicate the standard deviation.
Effect of C-terminal deletions of 2A on capsid assembly.
The delay in infectious-particle synthesis relative to RNA replication in vΔ2A-11 (Fig. 5) could reflect a defect in virus assembly. In addition, the C-terminal deletion of 2A sequences clearly alters the VP1/2A processing, resulting in the persistence of unusually large amounts of unprocessed VP1-2A precursor in infected cells (Fig. 4). We considered the possibility that this could impair proper assembly and maturation of morphogenesis products. To determine the impact of the 2A deletion on virus morphogenesis, we analyzed the nature of the assembly intermediates in vΔ2A-11-infected cells, in comparison to the species found in v18f-infected cells. To isolate morphogenesis intermediates, FRhK-4 cells were infected either with vΔ2A-11 or the parent v18f at an MOI of 1 RFU/cell, and cytoplasmic extracts prepared at 72 hpi were fractionated on a 5 to 45% sucrose rate-zone gradient. The polypeptide composition of each fraction was determined by Western blotting with a mixture of anti-VP1 and anti-VP2 antibodies.
In the case of v18f-infected cells, we identified three types of morphogenesis intermediates: (i) pentamers, comprised of capsid protein precursors VP1-2A and VP0, as well as of mature capsid protein VP3; (ii) empty capsids, comprised of mostly mature VP1 and some yet-uncleaved VP1-2A precursor, as well as VP0 and VP3; and (iii) virions, comprised of mature capsid proteins VP1, VP2, and VP3, as well as occasional small amount of uncleaved VP0 precursor, and viral RNA (Fig. 6A, fractions 14, 8, and 4, respectively). Whether VP4, derived from the late cleavage of precursor VP0, is associated with the HAV virion has never been determined. VP4 has indeed never been found in virus particles, but this may simply be due to its small size (21 or 23 aa) and technical difficulties in its detection (21).
As shown in Fig. 6B, the most-striking feature of the vΔ2A-11 assembly intermediates, compared with those of v18f (Fig. 6A), was the presence in virus particles of large amounts of the uncleaved precursor VP1-2AΔ and the other VP1-reactive precursor, VP1+, in addition to mature VP1 (Fig. 6B, fraction 4). This also proved to be the case for empty capsids (Fig. 6B, fraction 8). In the case of the parent virus, the VP1+ polypeptide was only detectable in empty capsids (Fig. 6A, fraction 8) and was not present in virions (Fig. 6A, fraction 4). Despite the presence of this 2A extension on the vΔ2A-11 particles, their sedimentation coefficient in sucrose gradients was virtually unchanged compared to that of parent particles. A similar pattern was observed in the polypeptide composition of the morphogenesis intermediates of all other mutants carrying C-terminal deletions in 2A (vΔ2A-6 to -10 [data not shown]). The surprisingly high amount of uncleaved VP0 precursor found in the virion fractions from both parent and mutant viruses is likely to reflect the presence of provirions due to the use of a relatively low MOI (1 RFU/cell) in this experiment. Alternatively, we cannot rule out potential contamination of fraction 4 by empty-capsid-containing fractions. With respect to the presence of VP0-containing material in this experiment, however, the mutant vΔ2A-11 virus did not appear to differ significantly from the parent virus.
We next examined the kinetics of virion morphogenesis. Cells infected at an MOI of 4 RFU/cell were harvested at different times p.i., followed by fractionation of cell lysates on sucrose gradients. The polypeptide content of fraction 4, which contains virus particles, is shown for both parent and mutant viruses, at each time point, in Fig. 6C. Even at early times p.i. (36 hpi), v18f parent virus particles contained only mature VP1 (Fig. 6C, lane 1). In contrast, at 36 hpi, vΔ2A-11 virus particles contained only the VP1-2AΔ precursor (Fig. 6, lane 3). At 72 hpi, uncleaved VP1-2A and VP1+ precursors remained present, along with mature VP1 in the case of vΔ2A-11 (Fig. 6C, lane 4) but, as before, were not present in the v18f lysates (Fig. 6C, lane 2). Even at 96 hpi, the VP1/2A cleavage did not reach completion in the mutant with C-terminal deletion of 2A (Fig. 6C, lane 5). No remaining VP0 precursor was detected in these fractions.
We next undertook a determination of whether the persistence of uncleaved VP1-2A precursor would affect the infectivity of vΔ2A-11 particles. For that purpose, we ascertained the relative abundance of infectious particles versus the total particle-associated RNA present in fraction 4 of these gradients (Fig. 6C), which contains virus particles. In the case of vΔ2A-11, the specific infectivity of the RNA was approximately fivefold lower than that of the parent virus v18f at 36 hpi and approximately fourfold lower at 72 hpi (Table 1). Therefore, whether there is only uncleaved VP1-2A precursor in the mutant (at 36 hpi [Fig. 6C]) or some mature VP1 coexisting with VP1-2A and VP1+ precursors (at 72 hpi [Fig. 6C]), the infectivity of the mutant particles is decreased. This suggests that the most-extensive C-terminal deletions in 2A that impair the VP1/2A cleavage and lead to retention of 2A sequences on the virus particle also reduce particle infectivity. This seems likely to account for the delay in infectious-particle production (Fig. 5B), as well as the small focus replication phenotype (Fig. 2) of vΔ2A-11.
TABLE 1.
Specific infectivity of the vΔ2A-11 mutant particles
| Time (hpi) | Genome copy number/RFUa
|
Relative particle specific infectivity (v18f/v2A-11) | |
|---|---|---|---|
| v18f | v2A-11 | ||
| 36 | 53 | 278 | 5.2 |
| 72 | 50 | 217 | 4.3 |
To determine the genome copy number present in fraction 4 after fractionation of the infected cell extract on a sucrose gradient, virus particles were treated first with nuclease and then with proteinase K, and the resulting encapsidated RNAs were blotted and quantified after hybridization with a 32P-labeled, negative-strand RNA probe, using a PhosphorImager. Comparison with a standard prepared from HAV RNA transcripts of known concentration allowed determination of the genome copy number present in the fraction. The number of infectious particles (RFU) present in the same fraction was determined by RIFA.
Effect of lethal N-terminal 2A deletions on processing and assembly of capsid proteins.
To determine whether the lethal effect of N-terminal 2A deletions described in this work (Fig. 1 and 2) was also due to impaired capsid protein assembly, we used a heterologous expression system based on recombinant VVs. We have previously demonstrated that the full-length v18f polyprotein, when expressed from the recombinant VV-18f, is appropriately processed in FRhK-4 cells (Fig. 7) and leads to the assembly of pentamers and empty capsids that can be isolated on a sucrose gradient (Fig. 8A). In the absence of the full-length HAV genome, no virus particles are expected to be produced in this system.
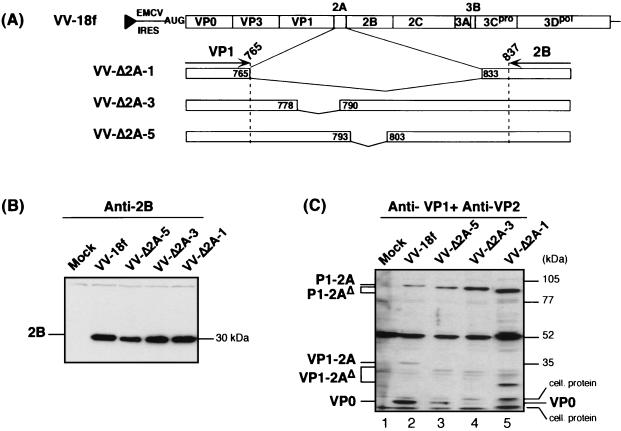
FIG. 7.
Effect of N-terminal 2A deletions on 3Cpro-mediated processing of the capsid protein precursors expressed by recombinant VVs. (A) Schematic showing HAV polyprotein expressed by the recombinant VV containing the open reading frame, as well as the 3′ nontranslated region, of the v18f genome downstream of the EMCV internal ribosome entry site and the T7 RNA polymerase promoter (black triangle). Enlargements are shown for the 2A deletions introduced into the polyproteins of the indicated mutants. The positions within the HAV polyprotein of amino acids framing each deletion are indicated in boxes. FRhK-4 cells were infected with vTF7-3 (Mock) or coinfected with vTF7-3 and either the parent virus (VV-18f) or the indicated mutant virus (VV-Δ2A-1, -3, or -5), each at an MOI of 5 PFU/cell. HAV proteins from cytoplasmic extracts prepared at 20 hpi were separated by SDS-10% PAGE and identified by immunoblotting using anti-2B antibodies (B) or a mixture of anti-VP1 and anti-VP2 antibodies (C). HAV polypeptides, as well as molecular mass markers, are indicated on each side of the panels.
We studied the behavior of HAV polyproteins containing either the full-length 2A deletion (Δ2A-1) or the lethal N-terminal deletion Δ2A-3, expressed by recombinant VVs (Fig. 7A). An HAV polyprotein containing the Δ2A-5 deletion in the central region of 2A, which resulted in a viable, but poorly replicating, virus phenotype (Fig. 2), was also expressed by a recombinant VV. HAV protein expression in recombinant VV-infected cells was induced by the coexpression of T7 RNA polymerase following coinfection with the appropriate recombinant VV (vTF7-3) (see Materials and Methods and reference 19).
As shown in immunoblots using anti-2B antibodies (Fig. 7B), the cleavage at the 2A/2B junction, which releases the P1-2A precursor, was not affected by any 2A deletion. In contrast, using a mixture of anti-VP1 and anti-VP2 antibodies, it was apparent that uncleaved P1-2AΔ precursors accumulated with all three mutants (VV-Δ2A-1, -3, or -5), when compared to the parent P1-2A (VV-18f) (Fig. 7C, compare lanes 3 through 5 to lane 2). In addition, VP0, one of the capsid products normally released by 3Cpro cleavage (Fig. 7C, lane 2), could hardly be detected with the lethal deletions Δ2A-1 (full length) and Δ2A-3 (N terminal) (Fig. 7C, lanes 5 and 4, respectively). In the case of the Δ2A-5 deletion, which does not abolish HAV genome infectivity but is very detrimental to virus replication, a reduced amount of cleaved VP0 product was observed (Fig. 7C, lane 3). Cleavage at the VP3/VP1 junction did not seem to be affected, as indicated by the comparable relative abundance of VP1-reactive products (Fig. 7C, compare for example lanes 2 and 5, VP1-2A or VP1-2AΔ versus P1-2A or P1-2AΔ in the case of VV-Δ2A-1 versus VV-18f). These results suggest the production of an unusual VP0-VP3 product in the case of N-terminal 2A deletions due to impaired cleavage at the VP0/VP3 junction. However, this particular precursor polypeptide is likely to comigrate with the nonspecific cellular product present in the assay around 55 kDa (Fig. 7C). Taken together, these data reveal that deletions in the N-terminal one-third of 2A may alter 3Cpro-mediated processing of the P1-2A precursor.
Upon fractionation of these VV-infected cell extracts on sucrose gradients, both pentamers and empty capsids were readily identified with the parent polyprotein (VV-18f) (Fig. 8A), as well as from the mutant polyprotein Δ2A-5 (Fig. 8B). In contrast, with the mutants VV-Δ2A-3 (Fig. 8C) and VV-Δ2A-1 (data not shown) no pentamers appeared to be assembled. These results indicate the requirement of the N-terminal domain of 2A (aa 766 to 793) for efficient 3Cpro-mediated processing of the P1-2A capsid protein precursor and, either secondarily or as an independent direct effect, for pentamer assembly.
DISCUSSION
One of the major differences in the organization of the genomes of the various genera within the Picornaviridae family relates to the size and the function of the 2A protein. In enteroviruses and rhinoviruses, 2A is a chymotrypsin-like cysteine proteinase that is responsible for the cis-active primary cleavage of the polyprotein at its N terminus and host protein synthesis shutoff (for a review, see reference 31). In addition, for these viruses, 2A has been suggested to play a role in RNA replication (24). In aphthoviruses and cardioviruses, 2A possesses cis-active protease activity responsible for the cotranslational cleavage of the polyprotein at its C terminus (8). In cardioviruses, 2A has another as yet incompletely defined function, as it is indirectly involved in 3Cpro-mediated processing of the full-length L-P1-2A capsid protein precursor (23, 39) and possibly in translation or RNA synthesis (39). The data described in this paper shed light on the role of the hepatovirus 2A polypeptide, a protein that is 71 aa in length and of previously unknown function.
Harmon et al. reported that an in-frame deletion of 15 aa in the C-terminal half of the HAV 2A protein (residues 803 to 817) within the background of a cell culture-adapted virus strain (HM175-p35) did not abolish virus replication in cell culture, nor did it impair virus replication and pathogenicity in marmosets when placed within the background of a wild-type, pathogenic strain of HAV (12). Our data significantly extend this finding. Using the background of a fast-replicating, cytolytic variant of HAV (v18f), we demonstrated that it is possible to introduce in-frame deletions within sequence encoding the C-terminal half of 2A (aa 797 to 832) without adversely affecting replication of the genome in cell culture. Mutants with a partial deletion (vΔ2A-6 to -10) or a total deletion (vΔ2A-11) of the C-terminal half of 2A had similar replication phenotypes, including a two- to threefold-reduced replication focus size (Fig. 2) and two- to fourfold-reduced virus yields (Fig. 5) compared to the parent virus.
No defect in the kinetics and efficiency of RNA synthesis, protein synthesis, or 3Cpro-mediated processing was observed that could account for the small-focus phenotype of those mutants. Results presented in Fig. 5 indicate that the early steps in the virus life cycle, i.e., virus penetration into the cell and genome uncoating, are likely to be unaltered and instead suggest a defect in late stages of the virus life cycle, such as particle assembly. Nonetheless, all morphogenesis intermediates were present in cells infected with the C-terminally 2A-truncated mutants and in amounts similar to v18f. Although the hepatoviruses comprise the only genus within the Picornaviridae family for which no three-dimensional structure of the virion is available, we can reasonably speculate that HAV particles, like all picornavirus particles, possess an icosahedral symmetry and are composed of 60 copies of each capsid protein (see reference 14). The incomplete VP1/2A cleavage that was observed with the mutants was reflected in a large abundance of uncleaved VP1-2A precursor and of an intermediate product (VP1+), along with mature VP1, in gradient fractions containing the 2A deletion mutant virions. These virus particles containing uncleaved VP1-2A precursor exhibited an approximately fourfold-decreased specific infectivity (Table 1). Provirions, containing VP0 but otherwise mature capsid proteins, have a decreased specific infectivity (3). However, the reduced infectivity of the C-terminally 2A-truncated mutant could not be accounted for by the presence of abnormal amounts of provirions, as VP0 was found to be cleaved to completion with the mature capsid protein VP2 present in the mutant virus particles (Fig. 6C).
The vΔ2A-5 mutant is particularly interesting in that it replicated very poorly in cell culture. It was not possible to increase vΔ2A-5 yields by passaging this virus in cell culture (data not shown). We therefore speculate that the deletion introduced in this mutant either results in a defect in virion assembly or yields virus particles with dramatically decreased infectivity that cannot be overcome by simple compensatory mutation elsewhere. Whether this defect relates to the presence of excessive amounts of VP1-2A precursors could not be investigated due to very low virus titers. In addition, the heterologous expression system based on recombinant VVs could not be used to address this question, as the VP1/2A cleavage does not take place in this system (Fig. 8). Since the mechanism of action of the cellular proteinase which is thought to be involved in the VP1/2A maturation is unknown, it is not possible to design experiments aimed at knocking out the VP1/2A cleavage in order to unequivocally address the question of the importance of the trimming of 2A from the virus particles. The only possible parallel to our results among other picornaviruses resides in the fact that the deletion of more than half of the C-terminal part of the 2A protein of Theiler's virus (a cardiovirus) resulted in a virus with normal RNA replication kinetics and production of infectious particles but that failed to form visible plaques in cultured cells (23). The authors speculated that this mutant was probably hindered in its ability to spread from cell to cell, perhaps as a result of a lack of 3Cpro-directed cleavage at the VP1/2A junction due to the large deletion in 2A. While this study demonstrated that the 2A protein of Theiler's virus is not involved in RNA replication, it pointed to a possible role of 2A in viral assembly, release, or entry. In the case of HAV, we show that while the C-terminal sequences of 2A are not necessary for virus infectivity in vitro, they appear to be important for complete maturation of VP1. The defect in the release of the 2A extension from virus particles could account for their decreased infectivity, and hence for the small-focus replication phenotype of the mutant HAVs.
Our data relative to in-frame deletions within the N-terminal 40% part of the 2A protein (aa 766 to 793) indicate the importance of these 2A sequences in determining conformation of the capsid protein precursor required for efficient 3Cpro-mediated cleavage, notably at the VP3/VP0 junction (Fig. 7C). We also have observed this defect in cells infected with recombinant VVs expressing on, one hand, full-length or C-terminally truncated versions of capsid protein precursor P1-2A and, on the other hand, 2BC-P3 as a source of 3Cpro (19; unpublished results). In this case, the defect in VP0/VP3 cleavage was found to be increased with the extent of the C-terminal deletion. Surprisingly, however, this defect was not described by Probst et al. when they coexpressed the capsid protein precursor P1 (devoid of the 2A sequence) and P3 as a source of 3Cpro, using recombinant VVs (30). Our results are reminiscent of what was found in the case of poliovirus (an enterovirus), for which it had been shown that C-terminal deletions of the P1 capsid protein precursor within VP1 prevent 3Cpro-derived cleavages at the VP0/VP3 and VP3/VP1 junctions (26, 37). In addition, exchange of the mengovirus 2A sequence with that of another cardiovirus (Theiler's virus) was also shown to result in a defect in 3Cpro-mediated cleavage of P1 (39). The processing cascade of cardiovirus L-P1-2A thus appears to occur sequentially and seems to be regulated by subsequent conformational transitions of the cleavage products after each proteolytic event. In agreement with these observations, it has recently been reported that structural epitopes are differentially accessible on various processing intermediates of an enterovirus P1 capsid protein precursor, demonstrating conformational changes during proteolytic processing of picornavirus capsid proteins (33). Thus, it is not surprising that modifying the integrity of the HAV P1-2A structural precursor by deletions in its C terminus could affect the accessibility of even distant cleavage sites.
We found that a deletion within the N-terminal 40% of HAV 2A (the first 25 to 28 aa) resulted in a complete failure of morphogenesis, as no pentamers were assembled (Fig. 7 to 8). Similarly, we have also found that a recombinant P1 precursor protein, P1776, which contains only the first 11 aa of 2A, failed as a substrate for pentamer assembly, whereas P1791, which contains the first 26 aa of 2A, generated pentamers and empty capsids, like full-length P1-2A, when expressed in a coinfection with a recombinant VV expressing 2BC-P3 (19; unpublished results). Our results thus extend a previous report in which the complete deletion of 2A prevented pentamer formation in a coinfection experiment with recombinant VVs expressing P1 and P3 (30). Whether this assembly defect in N-terminally 2A-deleted mutants is secondary to the P1 processing defect described above or related to a direct role of the 2A sequences in recruiting the capsid protomers or the uncleaved P1-2A precursors to promote pentamer assembly is a difficult question to address. However, previous studies with poliovirus have shown that the processing and assembly of capsid proteins are intimately linked (13) and that partial defects in 3Cpro processing of capsid proteins result in failure to generate morphogenesis intermediates (1).
On the other hand, a direct role of 2A in assembly would be compatible with a previous speculative model of HAV capsid protein assembly, which involves the assembly of five copies of the uncleaved P1-2A precursors first, followed by 3Cpro cleavage to generate 14S pentamers (5, 30). Although older studies of EMCV, a cardiovirus, would argue in favor of such a model (22), more recent studies indicate that 3Cpro processing is necessary prior to pentamer assembly (28). None of our data favor the existence of HAV pentamers containing uncleaved P1-2A, as we did not observe pentamers containing uncleaved P1-2A precursors in sucrose gradients. Even with the central deletion of 2A (Δ2A-5) generating increasing amounts of uncleaved P1-2AΔ (Fig. 7), we did not observe pentamers containing P1-2AΔ (data not shown).
In conclusion, our study has shed light on the role of 2A as part of the HAV capsid protein precursor. This polypeptide appears to have two distinct domains. The N-terminal domain may act in a chaperone-like fashion directing the folding of the capsid protein precursor for proper 3Cpro processing. As a secondary effect of the former defect, or possibly also directly, the presence in cis of this 2A domain is necessary for pentamer assembly. The C-terminal domain is required for efficient, non-3Cpro-mediated processing of the VP1-2A precursor that generates the mature VP1 protein, a process that appears to be necessary to optimize the infectivity of virus particles. These findings suggest that the role of the 2A protein of HAV is primarily in 3Cpro-mediated processing of the capsid protein precursor and in virion morphogenesis, although we cannot at present rule out a potential, additional role of the N-terminal domain of 2A in RNA replication. A model of the HAV capsid structure with and without the 2A extension would be helpful in gaining a better understanding of the structural implication of the 2A sequence.
Acknowledgments
We are grateful to Stanley M. Lemon for his interest in this work and critical review of the manuscript and to Sylvie van der Werf for her continuous support. We thank Manfred Weitz and David Sangar for providing antibodies to HAV proteins.
This work was supported by the Pasteur Institute and the Centre National de la Recherche Scientifique (CNRS).
REFERENCES
- 1.Ansardi, D. C., and C. D. Morrow. 1993. Poliovirus capsid proteins derived from P1 precursors with glutamine-valine cleavage sites have defects in assembly and RNA encapsidation. J. Virol. 67:7284-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard, M. R., L. Cohen, S. M. Lemon, and A. Martin. 2001. Characterization of recombinant hepatitis A virus genomes containing exogenous sequences at the 2A/2B junction. J. Virol. 75:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, N. E., and D. A. Anderson. 2000. Uncoating kinetics of hepatitis A virus virions and provirions. J. Virol. 74:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boege, U., and D. G. Scraba. 1989. Mengo virus maturation is accompanied by C-terminal modification of capsid protein VP1. Virology 168:409-412. [DOI] [PubMed] [Google Scholar]
- 5.Borovec, S. V., and D. A. Anderson. 1993. Synthesis and assembly of hepatitis A virus-specific proteins in BS-C-1 cells. J. Virol. 67:3095-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J. I., B. Rosenblum, J. R. Ticehurst, R. J. Daemer, S. M. Feinstone, and R. H. Purcell. 1987. Complete nucleotide sequence of an attenuated hepatitis A virus: comparison with wild-type virus. Proc. Natl. Acad. Sci. USA 84:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I., J. R. Ticehurst, S. M. Feinstone, B. Rosenblum, and R. H. Purcell. 1987. Hepatitis A virus cDNA and its RNA transcritps are infectious in cell culture. J. Virol. 61:3035-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly, M. L., D. Gani, M. Flint, S. Monaghan, and M. D. Ryan. 1997. The cleavage activities of aphthovirus and cardiovirus 2A proteins. J. Gen. Virol. 78:13-21. [DOI] [PubMed] [Google Scholar]
- 9.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthetizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosert, R., P. Cassinotti, G. Siegl, and M. Weitz. 1996. Identification of hepatitis A virus non-structural protein 2B and its release by the major virus protease 3C. J. Gen. Virol. 77:247-255. [DOI] [PubMed] [Google Scholar]
- 11.Graff, J., O. C. Richards, K. M. Swiderek, M. T. Davis, F. Rusnak, S. A. Harmon, X. Y. Jia, D. F. Summers, and E. Ehrenfeld. 1999. Hepatitis A virus capsid protein VP1 has a heterogeneous C terminus. J. Virol. 73:6015-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmon, S. A., S. U. Emerson, Y. K. Huang, D. F. Summers, and E. Ehrenfeld. 1995. Hepatitis A viruses with deletions in the 2A gene are infectious in cultured cells and marmosets. J. Virol. 69:5576-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellen, C. U., and E. Wimmer. 1992. Maturation of poliovirus capsid proteins. Virology 187:391-397. [DOI] [PubMed] [Google Scholar]
- 14.Hogle, J. M., M. Chow, and D. J. Filman. 1985. Three-dimensional structure of poliovirus at 2.9 Å resolution. Science 229:1358-1365. [DOI] [PubMed] [Google Scholar]
- 15.Jewell, D. A., W. Swietnicki, B. M. Dunn, and B. A. Malcom. 1992. Hepatitis A virus 3C proteinase substrate specificity. Biochemistry 31:7862-7869. [DOI] [PubMed] [Google Scholar]
- 16.Lemon, S. M., E. Amphlett, and D. Sangar. 1991. Protease digestion of hepatitis A virus: disparate effects on capsid proteins, antigenicity, and infectivity. J. Virol. 65:5636-5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemon, S. M., L. N. Binn, and R. H. Marchwicki. 1983. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J. Clin. Microbiol. 17:834-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemon, S. M., P. C. Murphy, P. A. Shields, L. H. Ping, S. M. Feinstone, T. Cromeans, and R. W. Jansen. 1991. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: evidence for genetic recombination. J. Virol. 65:2056-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, A., B. D., S. F. Chao, L. M. Cohen, and S. M. Lemon. 1999. Maturation of the hepatitis A virus capsid protein VP1 is not dependent on processing by the 3Cpro proteinase. J. Virol. 73:6220-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, A., N. Escriou, S. F. Chao, M. Girard, S. M. Lemon, and C. Wychowski. 1995. Identification and site-directed mutagenesis of the primary (2A/2B) cleavage site of the hepatitis A virus polyprotein: functional impact on the infectivity of HAV RNA transcripts. Virology 213:213-222. [DOI] [PubMed] [Google Scholar]
- 21.Martin, A., and S. M. Lemon. 2002. The molecular biology of hepatitis A virus, p. 23-50. In J.-H. J. Ou (ed.), Hepatitis viruses. Kluwer Academic Publishers, Norwell, Mass.
- 22.McGregor, S. 1975. Evidence for the existence of protomers in the assembly of encephalomyocarditis virus. J. Virol. 15:1107-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michiels, T., V. Dejong, R. Rodrigus, and C. Shaw-Jackson. 1997. Protein 2A is not required for Theiler's virus replication. J. Virol. 71:9549-9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molla, A., A. V. Paul, M. Schmid, S. K. Jang, and E. Wimmer. 1993. Studies on dicistronic polioviruses implicate viral proteinase 2Apro in RNA replication. Virology 196:739-747. [DOI] [PubMed] [Google Scholar]
- 25.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 26.Nicklin, M. J., H. G. Krausslich, H. Toyoda, J. J. Dunn, and E. Wimmer. 1987. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc. Natl. Acad. Sci. USA 84:4002-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallai, P. V., F. Burkhardt, M. Skoog, K. Schreiner, P. Bax, K. A. Cohen, G. Hansen, D. E. Palladino, K. S. Harris, M. J. Nicklin, and et al. 1989. Cleavage of synthetic peptides by purified poliovirus 3C proteinase. J. Biol. Chem. 264:9738-9741. [PubMed] [Google Scholar]
- 28.Palmenberg, A. C. 1982. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J. Virol. 44:900-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmenberg, A. C., G. D. Parks, D. J. Hall, R. H. Ingraham, T. W. Seng, and P. V. Pallai. 1992. Proteolytic processing of the cardioviral P2 region: primary 2A/2B cleavage in clone-derived precursors. Virology 190:754-762. [DOI] [PubMed] [Google Scholar]
- 30.Probst, C., M. Jecht, and V. Gauss-Muller. 1999. Intrinsic signals for the assembly of hepatitis A virus particles. Role of structural proteins VP4 and 2A. J. Biol. Chem. 274:4527-4531. [DOI] [PubMed] [Google Scholar]
- 31.Ryan, M. D., and M. Flint. 1997. Virus-encoded proteinases of the picornavirus super-group. J. Gen. Virol. 78:699-723. [DOI] [PubMed] [Google Scholar]
- 32.Schultheiss, T., W. Sommergruber, Y. Kusov, and V. Gauss-Muller. 1995. Cleavage specificity of purified recombinant hepatitis A virus 3C proteinase on natural substrates. J. Virol. 69:1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth, M. S., A. Trudgett, J. H. Martin, E. M. Hoey, and S. J. Martin. 2000. Conformational changes during proteolytic processing of a picornavirus capsid proteins. Arch. Virol. 145:1473-1479. [DOI] [PubMed] [Google Scholar]
- 34.Stemmer, W. P. C., and S. K. Morris. 1992. Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site-directed mutagenesis. BioTechniques 13:215-220. [PubMed] [Google Scholar]
- 35.Toyoda, H., M. J. H. Nicklin, M. G. Murray, C. W. Anderson, J. J. Dunn, F. W. Studier, and E. Wimmer. 1986. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell 45:761-770. [DOI] [PubMed] [Google Scholar]
- 36.Wimmer, E., C. U. Hellen, and X. Cao. 1993. Genetics of poliovirus. Annu. Rev. Genet. 27:353-436. [DOI] [PubMed] [Google Scholar]
- 37.Ypma-Wong, M. F., and B. L. Semler. 1987. Processing determinants required for in vitro cleavage of the poliovirus P1 precursor to capsid proteins. J. Virol. 61:3181-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, H., S.-F. Chao, L.-M. Ping, K. Grace, B. Clarke, and S. M. Lemon. 1995. An infectious cDNA clone of a cytopathic hepatitis A virus: genomic regions associated with rapid replication and cytopathic effect. Virology 212:686-697. [DOI] [PubMed] [Google Scholar]
- 39.Zoll, J., F. J. van Kuppeveld, J. M. Galama, and W. J. Melchers. 1998. Genetic analysis of mengovirus protein 2A: its function in polyprotein processing and virus reproduction. J. Gen. Virol. 79:17-25. [DOI] [PubMed] [Google Scholar]