Abstract
We investigated the role of the immune system in protecting against virus-induced demyelination by generating lines of transgenic B10 (H-2b) congenic mice expressing three independent contiguous coding regions of the Theiler's murine encephalomyelitis virus (TMEV) under the control of a class I major histocompatibility complex (MHC) promoter. TMEV infection of normally resistant B10 mice results in virus clearance and development of inflammatory demyelination in the spinal cord. Transgenic expression of the viral capsid genes resulted in inactivation of virus-specific CD8+ T lymphocytes (class I MHC immune function) directed against the relevant peptides, but it did not affect production of virus capsid-specific antibodies or lymphocyte proliferation to the virus antigen (class II MHC immune functions). Following intracerebral infection with TMEV, all three lines of mice survived the acute encephalitis but transgenic mice expressing VP1 (or the cluster of virus capsid proteins [VP4, VP2, and VP3] mapping to the left of VP1 in the TMEV genome) developed virus persistence and subsequent demyelination in spinal cord white matter. Transgenic mice expressing noncapsid proteins mapping to the right of VP1 (2A, 2B, 2C, 3A, 3B, 3C, and 3D) cleared the virus and did not develop demyelination. These results are consistent with the hypothesis that virus capsid gene products of TMEV stimulate class I-restricted CD8+ T-cell immune responses, which are important for virus clearance and for protection against myelin destruction. Presented within the context of self-antigens, inactivation of these cells by ubiquitous expression of relevant virus capsid peptides partially inhibited resistance to virus-induced demyelination.
Theiler's murine encephalomyelitis virus (TMEV), a positive-stranded RNA virus belonging to the family Picornaviridae, induces a central nervous system (CNS) demyelinating disease that serves as an excellent model for human multiple sclerosis (13). In TMEV-induced disease, the immune system is critically important for resistance to virus infection and subsequent resistance to demyelination (1, 4, 18, 19, 23). Infection of B10 (H-2b) mice, the prototypic resistant strain, results in acute encephalitis, but the virus is cleared within 21 days without the development of chronic demyelination or persistent neurological deficits. However, following infection with TMEV in these mice, immunosuppression with sublethal gamma irradiation or depletion of CD4+ or CD8+ T lymphocytes with monoclonal antibodies results in demyelination (25). Treatment of TMEV-infected resistant mice with anti-μ immunoglobulin G (IgG), which depletes B lymphocytes from birth, or infection of B lymphocyte-deficient mice (xid mutation, or μ-knockout mice) has no effect on the late demyelinating phase, even though some mice die from early encephalitis (21). Similarly, depletion of natural killer (NK) cells with monoclonal antibodies during the early phase of disease enhanced the frequency of lethal encephalitis but did not predispose to demyelination (15). These results indicate that the T-cell immune response, but not B-cell or NK function, plays the major role in protecting normally resistant genotype mice from chronic virus-induced demyelination and prevents virus persistence in glial cells of spinal cord white matter.
There is also evidence that the cellular immune response contributes to demyelination and neurologic deficits in susceptible strains of mice. In SJL/J mice, the prototypic susceptible strain, which develops virus persistence and demyelination, treatment with various immunosuppressive agents such as cyclophosphamide (12), anti-lymphocyte serum (29), cyclosporine (27), or monoclonal antibodies to T-lymphocyte subsets (28, 31) or immune response gene products (22) results in less severe demyelinating disease. Further evidence for the role of the host immune response in altering pathogenesis of TMEV disease comes from experiments utilizing severe combined immunodeficiency (SCID) mice (26). These mice develop severe encephalitis and die within 14 to 17 days following infection with TMEV, but without demyelination. Adoptive transfer of splenocytes (T and B lymphocytes) from immunocompetent BALB/c mice (parental strain of the C.B-17 mouse) into SCID mice 4 h prior to infection allowed recipient mice to survive acute infection and develop demyelination by day 21 following infection. In contrast, adoptive transfer of splenocytes from immunodeficient SCID mice, which contain functional macrophages and NK lymphocytes but no CD4 or CD8+ T cells, did not protect infected SCID mice from encephalitis. Transfer of either CD4-depleted or CD8-depleted T lymphocytes from BALB/c mice into infected SCID mice also resulted in demyelination. These results indicate that both CD4 and CD8+ T cells independently contribute to TMEV-induced pathogenesis.
An important unresolved question in TMEV pathogenesis is the specificity of the antigens recognized by T cells which contribute to resistance. To determine the regions of the virus genome responsible for generating the protective immune responses, studies have focused on identifying TMEV epitopes involved in T-lymphocyte activation and antibody production (2, 3, 5-9, 33). VP2121-130 is the immunodominant epitope recognized by 55 to 75% of class I-restricted CD8+ T cells infiltrating the CNSs of resistant H-2b mice (8). An epitope located in VP1 (VP1233-244) was identified as the main T-lymphocyte antigen (class II restricted) in demyelinated lesions of SJL/J mice (33). Preimmunization with VP1 or VP2 protein protects susceptible mice from development of demyelination, whereas immunization with VP3 does not (32), suggesting that an immune response to VP1 and VP2 proteins is beneficial. In susceptible mice, antibody epitopes have been shown to be located in the VP1 protein (VP112-25, VP1146-160, and VP1262-276), VP2 protein (VP22-16 and VP2165-179), and VP3 protein (VP324-37) (7, 9). In summary, the data provide support for the hypothesis that the immune responses to capsid antigens of TMEV contribute to the pathogenesis of TMEV infection.
Our laboratory has been interested in determining in vivo the identity of the genes encoded by TMEV that are critical for inducing the protective immune response in resistant strains of mice. We surmised that an in vivo transgenic strategy was the most definitive way to establish the role of TMEV capsid genes in virus-induced disease. To begin to address this assumption, we generated transgenic mice expressing three contiguous coding regions encompassing the entire TMEV genome under control of a class I major histocompatibility complex (MHC) promoter. Transgenes were designed to specifically test the role of VP1 in TMEV pathogenesis. Thus, we generated three transgenes, the first encompassing the TMEV genes mapping to the left of VP1 (LP, VP4, VP2, and VP3) in the TMEV genome, the second comprising the VP1 coding block, and the third encompassing the TMEV genes mapping to the right of VP1 (2A, 2B, 2C, 3A, 3B, 3C, and 3D). By challenging mice expressing the TMEV transgenes with infectious TMEV, we determined whether ubiquitous expression of the coding region of TMEV inhibited resistance to virus persistence and demyelinating disease.
MATERIALS AND METHODS
Construction of TMEV transgenes.
We divided the TMEV genome region into three regions to specifically test the hypothesis that VP1 plays the crucial role in the immune response to virus infection. Region I is the coding sequence (L, VP4, VP2, and VP3) mapping 5′ of VP1, designated LP (left side of VP1) (Fig. 1); region II is the entire VP1 coding block; and region III is the coding sequence (2A, 2B, 2C, 3A, 3C, and 3D) mapping 3′ of VP1, designated RP (right side of VP1). Region I, II, and III sequences were isolated by PCR from full-length cDNAs of the TMEV genome (kindly provided by Raymond Roos, University of Chicago [30]) and cloned directly into vector pTKgPtF1s. To express these coding sequences, the constructs utilized the promoter and enhancer of H-2Kb and the 3′ untranslated fragment of H-2Ld coming from plasmid 5A7 (Fig. 1) (16). The promoter region, signal peptide, and first intron of class I MHC were retained at the 5′ end of the construct. For each transgene, region I, II, or III was added as a single exon. The termination codon, 3′ noncoding region, and poly(A) signal of the class I gene were fused to the end that meets the TMEV coding region (Fig. 1). The three fragments were ligated 3′ to the first intron of H-2Kb. The recombinant constructs were amplified in Escherichia coli and then sequenced.
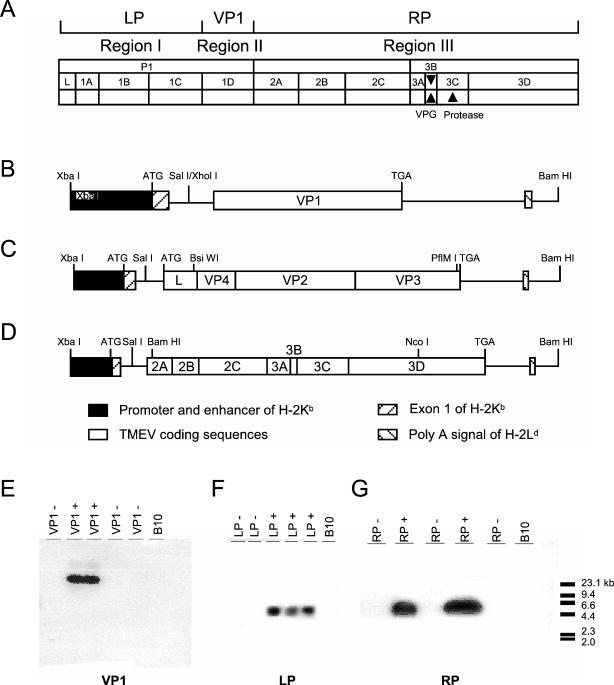
FIG. 1.
Construction of TMEV transgenes and detection of mice containing transgenes. (A) The TMEV genome was divided into three regions. Region I (LP) is the coding sequence mapping 5′ of VP1 (L, VP4, VP2, and VP3). Region II (VP1) is the entire VP1 coding block. Region III (RP) is the coding sequence mapping 3′ of VP1 (2A, 2B, 2C, 3A, 3C, and 3D). Region I (B), region II (C), and region III (D) nucleotide sequences were isolated by PCR from full-length cDNAs of the TMEV genome and cloned into vector pTKgPtF1s, adding a promoter and enhancer of H-2Kb and a 3′ untranslated fragment of H-2Ld, which came from plasmid 5A7. The promoter region, signal peptide, and first intron of class I MHC were retained at the 5′ end of the construct. Each transgene was added as a single exon. The termination codon, 3′ noncoding region, and poly(A) signal of the class I gene were fused to the end that meets the TMEV coding region. The recombinant constructs were amplified in E. coli and sequenced, and their ability to be transcribed was confirmed by the transfection of C57SV cells. The transgenes were injected into CBA × B10.M mouse embryos, and transgene-positive founders were backcrossed to achieve a resistant B10 genotype. Southern blots from two mice containing VP1 (E), three mice containing LP (F), and two mice containing RP (G) transgenes are shown.
We showed previously that these TMEV transgenes function as targets for cytotoxic T-lymphocyte (CTL) response. H-2Kb and H-2Db cells were transfected with region I, II, and III transgenes to function as targets for cytotoxic lymphocyte assays (10, 11). We demonstrated virus-specific cytotoxicity of CNS-infiltrating lymphocytes (CNS-ILs) to capsid proteins encoded by LP and VP1, but not RP, which were exclusively H-2Db restricted. Within the LP region, virus-specific cytotoxicity in H-2b mice was directed exclusively to VP2, which also proved to be H-2Db restricted. Cell depletion experiments demonstrated that cell cytotoxicity was mediated exclusively by CD8 T cells. These constructs, which proved to express functional antigens in transfected target cells for cytotoxicity assays, were used for the generation of TMEV transgenic mice.
Expression of TMEV transgenes.
Reverse transcription-PCR (RT-PCR) was used to detect expression of TMEV fragments in RP+ transgenic mice. The strategy was designed to detect PCR products from spliced RNA (documenting RNA expression) but not from chimeric constructs. To increase expression of TMEV transgenes under the control of a class I MHC promoter, transgenic and nontransgenic mice were systemically treated with gamma interferon. Using the urea-LiCl method, RNA was isolated from splenocytes of young mice. One microgram of RNA was used as a template for RT reaction with the First Strand cDNA synthesis kit (Pharmacia, Piscataway, N.J.). One microliter of reaction mixture was used as a template for PCR for a total of 30 cycles at 94°C for 1 min, 60°C for 0.5 min, and 72°C for 1.5 min. The primers were upstream primer Kb (5′GACAGGATCCATGGTACCGTGCACGCTGCTCCT 3′) and downstream primer RP3 for region III (3′ CCTAGGGTCCGAAGCCAG 5′). Twenty-microliter volumes of PCR products were run in a 2.5% agarose gel, transferred to nylon film, and hybridized with [γ-32P]dATP-labeled oligonucleotide probe KbEx1RP (5′ GCAGGATTGCCCGCGCGGG3′). Underlined bases are TMEV sequences and two extra base extension GCs for correcting the reading frame after splicing the first intron. Nonunderlined bases are sequences of the first exon of the Kb gene. These probes were designed to hybridize to PCR products from spliced RNA but not to chimeric constructs from Daniel's strain of TMEV (DA).
To detect expression of the Kb-VP1 and Kb-L transgenes in CNS tissue, brains and spinal cords from mice perfused with Trumps fixative (100 mM phosphate buffer, 4% formaldehyde, 1.5% glutaraldehyde, pH 7.2) were embedded in paraffin blocks. Five-micrometer-thick sections cut from these blocks were adhered to slides. In situ hybridization was performed as described on page 136 of the Roche Nonradioactive In Situ Hybridization Application manual (http://www.roche-applied-science.com/prod_inf/manuals/InSitu/InSi_toc.htm). Briefly, slides were deparaffinized with xylol, rehydrated with graded ethanol rinses, fixed with 4% paraformaldehyde, denatured with 0.2 M HCl, and soaked in 20 μg of protease K/ml at 37°C. Slides were then treated with 0.5% acetic anhydride and dehydrated with graded ethanol rinses, followed by one rinse of chloroform. Slides were then placed in a humid chamber at 55°C for 30 min, followed by 94°C for 3 min. Slides were then coverslipped and hybridized overnight at 55°C in the presence of 400 μg of biotinylated Kb-VP1 probe/ml in hybridization buffer (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 10% dextran sulfate, 0.01% herring sperm, 0.02% sodium dodecyl sulfate [SDS], 50% formamide). Following hybridization, slides were stringently washed with 50% formamide in 1× SSC at 55°C and incubated with blocking reagent (Roche, Basel, Switzerland) containing 10% fetal calf serum (FCS). Detection of tissue-bound biotinylated Kb-VP1 probe was performed using a Vectastain avidin biotin immunoperoxidase system (Vector Laboratories, Burlingame, Calif.). Slides were developed using Hanker-Yates (Polysciences, Warrington, Pa.) reagent overnight at 4°C.
The biotinylated Kb-VP1 probe (5′ biotin-GTTGTCGCTTCCGCCCGCGCGGGTCTG 3′) spans the junction of the Kb and VP1 sequences on mRNA expressed by the Kb-VP1 transgene. Likewise, the Kb-L probe (5′ biotin-ATGTTTGCAAGCCATGCCCGCGCGGGTCTG 3′) spans the junction of the Kb and L sequences on mRNA expressed by the Kb-L transgene. In both probes, bases underlined are those of the TMEV sequence and an extra two-base (GC) extension for correction of the reading frame after splicing the first intron. The Kb-VP1 and Kb-L probes were designed to hybridize with mRNA transcribed from the Kb-VP1 and Kb-L transgenes at 55°C and not with the integrated transgenes themselves, whose sequences contain an intron incompatible with hybridization.
Generation of virus capsid antigens.
Viral capsid proteins were generated using the pET system (Novagen Inc., Madison, Wis.). This system used cloned genes under control of bacteriophage T7 transcription to generate proteins of interest with HisTag labels that can be purified by metal ion-chelating columns. Capsid cDNAs for VP1 and VP2 were cloned from pDAFL3 (30) and ligated into the pET30 vector. Plasmids were cloned in DH5α cells (Life Technologies Inc., Frederick, Md.) lacking the T7 RNA polymerase. Isolated plasmids were then transformed into the expression host cell BL21(DE3) (Novagen), and colonies were used to inoculate 250 ml of Luria broth fortified with 50 μg of kanamycin/ml. Expression of the capsid proteins was under the lac UV5 promoter, and genes were induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Cultures were incubated for 3 h at 37° and then harvested by centrifugation and resuspended in phosphate-buffered saline (PBS). Next, samples were treated with Triton X-100 and sonicated to disrupt cell membranes. Cellular debris was then washed three times with PBS. Washed pellets were then solubilized in 6 M urea/PBS. The solubilized material was run through a 0.2-μm-pore-size filter to remove any nonsoluble debris. The filtrate was run over an Ni+ ion column equilibrated with 6 M urea. The bound protein was washed with a 6× volume of 50 mM imidazole in 6 M urea to remove low-specificity binding particles. The HisTag-labeled capsid proteins were then eluted with 500 mM imidazole buffer. Fractions were collected in 3-ml aliquots and tested by SDS-polyacrylamide gel electrophoresis for purity. Pooled samples were dialyzed into PBS and used for subsequent immunologic assays.
CTL assay.
C57SV cells transfected with the genes encoding the TMEV proteins VP1 and VP2 were used as targets as previously described (11). Untransfected C57SV cells were used as a negative control. Target cells were labeled with 200 μCi of sodium chromate (51Cr; Amersham Life Sciences, Arlington Heights, Ill.), washed with RPMI medium, and resuspended to a concentration of 2 × 104/ml in RPMI medium with 5% FCS. The target cell suspensions were placed in 96-well round-bottomed microtiter plates (Nunc, Roskilde, Denmark). CNS-ILs from Theiler's virus-infected mice (day 7 postinfection) were insolated by Percoll gradient (11) and used as effector cells in this assay. The CNS-ILs were resuspended to a concentration of 2 × 106/ml in RPMI medium with 5% fetal calf serum, and twofold serial dilutions were made to provide effector-to-target ratios of between 100:1 and 6.25:1. Targets also received medium alone or with 10% Triton X-100 (Sigma Chemical, St. Louis, Mo.) to determine spontaneous release or maximum release of chromium from targets, respectively. Plates were incubated for 5 h at 37°C in 5% CO2. Supernatants were harvested with a Skatron supernatant collection system (Skatron Instruments, Inc., Sterling, Va.) and assayed in a gamma counter (Beckman Gamma 5500; Beckman Instruments, Irvine, Calif.) for determination of the amount of radioactivity. Mean values were calculated from triplicate wells, and results were expressed as percent specific lysis according to the formula [(experimental counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100%.
ELISA for TMEV-specific IgG.
TMEV-specific IgGs were determined by enzyme-linked immunosorbent assay (ELISA). Mice were bled by cardiac puncture at the time of sacrifice, blood was allowed to clot, and serum was aliquoted and stored at −70°C. Polystyrene microtiter plates (Corning Inc., Corning, N.Y.) were coated overnight with either purified TMEV antigens (0.5 μg/well) or purified virus capsid antigens at 4°C. Plates were blocked with PBS containing 1% bovine serum albumin for one hour at room temperature, and sera from individual mice were diluted fourfold (1:100 to 1:25,600) in PBS containing 0.2% bovine serum albumin and incubated on the coated plates for 2 h at 37°C. Bound IgGs were detected by incubating plates with biotinylated goat anti-mouse IgG (Zymed Laboratories, South San Francisco, Calif.) at 37°C for 2 h, followed by amplification with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Color development was observed by adding p-nitrophenylphosphate substrate, and absorbance was read at 405 nm.
Western blotting.
Purified VP1 and VP2 proteins were processed using SDS-10% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were cut into strips, and individual strips were stained with immune serum from infected VP1+ and LP+ transgenic mice. Transgene-negative mice were used as controls. Rabbit anti-TMEV and rabbit anti-HisTag (Qiagen, Santa Clara, Calif.) were used as positive controls for the recombinant capsid proteins. Biotinylated rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) and biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.) were used as secondary antibodies. Alkaline phosphatase-labeled streptavidin (Jackson ImmunoResearch Laboratories, Inc.) was used to amplify the signal, and immunoprecipitated antigens were detected by the substrate solution containing nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate p-toluidine (Life Technologies Inc.).
T-cell proliferative assay.
VP1+ and VP1− mice were immunized with 2 μg of purified VP1 protein in complete Freund's adjuvant. Mice were injected with the protein-adjuvant mixture on day 0 and reinjected on days 10 and 17. On day 21, mice were sacrificed and spleens were harvested for lymphocytes. Cells were challenged in vitro with serial dilutions of VP1 with 100 ng of concanavalin A (ConA) at the zero time point and pulsed with 1 μCi of [3H]thymidine at 48 h. Cells were harvested at 72 h by transfer to filter plates and were quantitated using tritium-labeled DNA. Data were expressed as the ratio of VP1 or ConA stimulation to media control (stimulation index).
Virus.
The DAV was used for all in vivo experiments. The virus was grown in BHK-21 cells, and the titers of the virus were determined by plaque assay in L2 cells as described previously. Purified virus was prepared from infected BHK-21 cells by ultracentrifugation on sucrose and cesium chloride gradient as described previously.
Infection, harvesting, and morphology of the CNS.
At 4 to 6 weeks of age, transgenic and littermate nontransgenic mice were infected intracerebrally with 2 × 105 PFU of TMEV in a total volume of 10 μl. Forty-five days after infection, mice were perfused with Trump's fixative via intracardiac puncture. The 45-day time point was chosen because it has been shown previously to allow assays to distinguish between mice susceptible and resistant to TMEV-induced demyelination (22, 26, 28). Spinal cords were dissected and cut into 1-mm blocks. Every third block was embedded in glycol methacrylate and stained with a modified erichrome stain with a cresyl violet counterstain (15a) to detect inflammation and demyelination. The rest of the spinal cord blocks were embedded in Araldite plastic for electron microscopy or in paraffin for in situ hybridization for virus RNA. Thin sections for electron microscopy were counterstained with uranyl acetate and lead citrate and viewed for ultrastructural morphology.
Immunochemistry for viral antigen.
For immunochemical staining of TMEV antigen, brain sections were embedded in paraffin (14). Slides were deparaffinized in xylol and then rehydrated through a series of ethanol rinses (absolute, 95%, 70%, and 50%) prior to the addition of primary antibody. Slides with brain slices were then incubated with a polyclonal rabbit antiserum to purified Daniel's strain TMEV that specifically reacts with all structural proteins of TMEV (24). Slides were incubated with a biotinylated secondary antibody, and detection was performed using the avidin biotin immunoperoxidase system (Vector Laboratories).
Brain pathology.
Brain pathology was assessed at day 45 postinfection. Following perfusion with Trump's fixative, two coronal cuts were made in the intact brain at the time of removal from the skull (one section through the optic chiasm and a second section through the infundibulum). This allowed for systematic analysis of the pathology of the cortex, corpus callosum, hippocampus, brain stem, striatum, and cerebellum. The resulting slides were then stained with hematoxylin and eosin. Pathological scores were assigned without knowledge of the identity of the experimental group for the following areas of the brain: cortex, corpus callosum, hippocampus, brain stem, striatum, and cerebellum. Each area of the brain was graded on a scale of 0 to 4 as follows: 0, no pathology; 1, no tissue destruction but only minimal inflammation; 2, early tissue destruction (loss of architecture) and moderate inflammation; 3, definite tissue destruction (demyelination, parenchymal damage, cell death, neurophagia, and neuronal vacuolation); 4, necrosis (complete loss of all tissue elements with associated cellular debris). Meningeal inflammation was assessed and graded as follows: 0, no inflammation; 1, one cell layer of inflammation; 2, two cell layers of inflammation; 3, three cell layers of inflammation; 4, four or more cell layers of inflammation. The area with maximal extent of tissue damage was used for assessment of each brain region.
In situ hybridization for virus.
In situ hybridization was performed to detect virus genome in the CNSs of TMEV-infected transgenic and nontransgenic mice as described previously (26). Briefly, sections of paraffin-embedded spinal cord were hybridized overnight with a 35S-labeled probe complementary to the coding region of VP1. After extensive washing, slides were exposed to autoradiography for 48 h, developed, and counterstained with hematoxylin.
RESULTS
Generation and confirmation of TMEV transgenic mice.
Embryos of CBA × B10.M mice were injected with transgenes, and the resulting mice were screened for founders. After screening of 77 mice, 2 or 3 founder mice independently expressing region I, II, or III were identified. Transgene-positive mice were backcrossed to the resistant B10 genotype for 12 generations to minimize any possible influence of unknown background genes on pathogenesis. Southern DNA blotting with VP1-, VP2-, or RP-specific probes confirmed that region I, II, and III transgenes were incorporated in the genomes of transgenic mice (Fig. 1E, F, and G). In addition, C57SV cell lines were transfected with LP, VP1, or RP. Transgenic expression of LP, VP1, or RP in transfected C57SV cell lines was below the level necessary for fluorescence-activated cell sorter or Western blotting detection. However, these transfected cells served as excellent targets for cytotoxic lymphocyte responses (10, 11). We conclude that even though the levels of expression by these transgenes were low, they were sufficient for the transgenes to serve as excellent targets for CD8+ T cells.
Expression of RP, LP, and VP1 in transgenic mice.
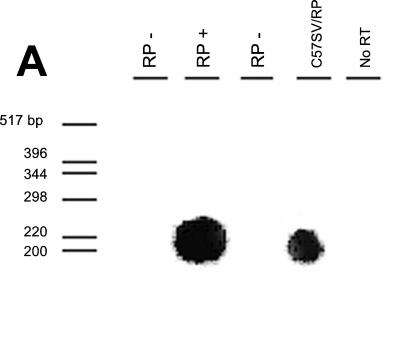
To determine levels of expression of the RP transgene, we used RT-PCR, followed by Southern hybridization using probes designed to hybridize to PCR products from spliced RNA but not to TMEV chimeric constructs. As shown in Fig. 2A, RP trans genes were expressed in splenocytes after intraperitoneal injection of gamma interferon resulted in upregulation of transgenes under the control of an MHC class I promoter. However, expression of RP was below the level necessary for Western blotting or fluorescence-activated cell sorter detection using polyclonal rabbit antiserum directed against purified TMEV particles.
FIG. 2.
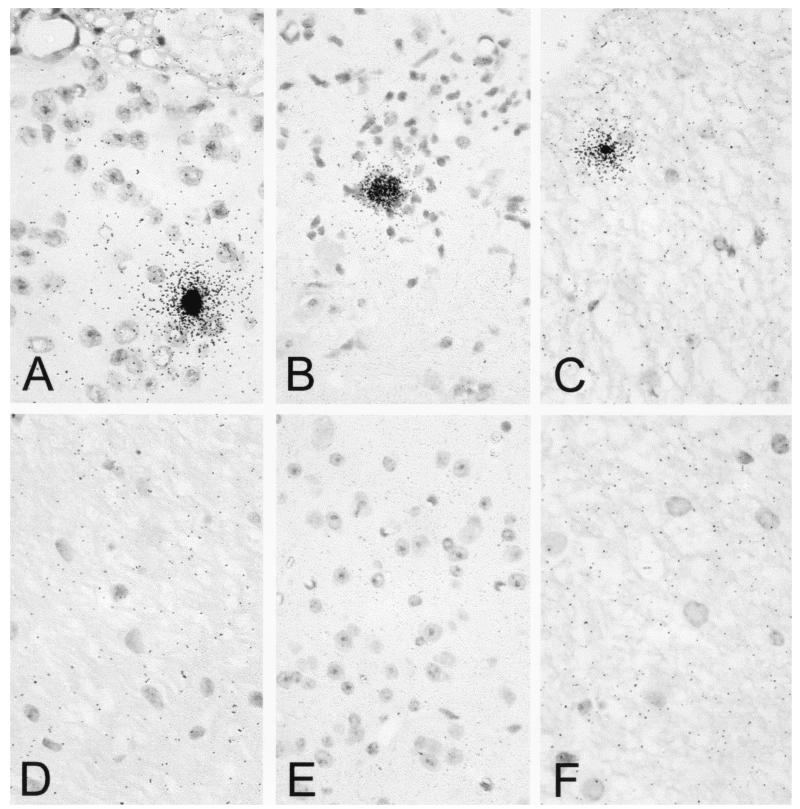
TMEV coding region mRNA expression in transgenic mice. (A) Expression of RP transgene after stimulation with gamma interferon, as detected by RT-PCR followed by Southern blotting, was low but detectable. Examples of expression for the RP transgene are shown for both a transgene-positive B10 mouse (second lane from left) and transfected C57SV cells (fourth lane from left). (B and C) In situ hybridization to detect Kb-LP mRNA was performed on spinal cords of LP+ transgenic mice (B) and spinal cords of LP− littermate control mice (C). (D and E) In situ hybridization to detect Kb-VP1 mRNA was performed on spinal cords of VP1+ transgenic mice (D) and spinal cords of VP1− littermate control mice (E). To demonstrate the stringency by which the Kb-VP1 probe hybridizes to mRNA expressed by the Kb-VP1 transgene alone, the in situ experiment was repeated on the brains of 7-day-infected nontransgenic C57BL/10 mice. (E and F) Using anti-TMEV immunostaining, the hippocampus of 7-day-infected C57BL/10 mice stained positive for detection of TMEV infection (F) but negative for in situ detection of Kb-VP1 mRNA (E). Note that, as shown in panel E, the Kb-VP1 probe used to detect Kb-VP1 mRNA did not hybridize with Kb or TMEV mRNA.
To determine whether LP and VP1 transgenes were expressed in the CNS of LP+ and VP1+ transgenic mice, in situ hybridization using a biotinylated DNA probe specific for Kb-LP and Kb-VP1 mRNA was performed. These probes were designed to hybridize with Kb-LP and Kb-VP1 mRNA expressed by the integrated Kb-VP1 transgene but not with Kb, LP, or VP1 mRNA alone (see Materials and Methods). Results of in situ hybridization with the Kb-VP1 probe are shown in Fig. 2B to G. Expression of the Kb-LP and Kb-VP1 transgenes is detectable in the spinal cords of 45-day-TMEV-infected LP+ and VP1+ transgenic animals (Fig. 2B and D) but not in those of the infected LP and VP1 littermate controls (Fig. 2C and E). Like class I molecules, expression of the Kb-LP and Kb-VP1 transgenes in LP+ and VP1+ transgenic mice is localized to glial cells in the spinal cord white matter of infected animals. To ensure that the Kb-VP1-specific probe was not hybridizing to mRNAs encoding Kb or VP1, we performed in situ hybridization on sections of brain from 7-day-TMEV-infected C57BL/10 (Db, Kb) mice. Seven-day-infected C57BL/10 mice have extensive virus infection of the hippocampus, as detected by anti-TMEV immunostaining (Fig. 2F). However, the hippocampus in a serial section did not hybridize with the Kb-VP1 probe (Fig. 2G), demonstrating that the Kb-VP1 probe did not hybridize with viral VP1 or Kb mRNA. These experiments demonstrate conclusively that Kb-RP mRNA is expressed in splenocytes from RP+ transgenic mice and that mRNA expressed from Kb-VP1 and Kb-LP transgenes can be detected in the CNSs of LP+ and VP1+ transgenic mice.
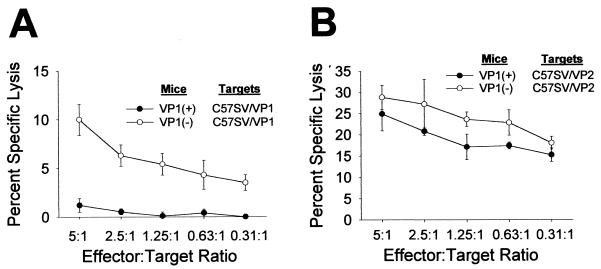
TMEV-infected VP1+ transgenic mice do not develop cytotoxic responses against VP1+-transfected targets but do mount cytotoxic responses to VP2+-transfected targets.
We evaluated whether transgenic expression of the Theiler's virus genome would interfere with the generation in the CNS of virus-specific CTLs specific to the corresponding transgene. We focused our immunologic experiments on VP1 transgenic mice, because the design of region I, II, and III transgenes specifically addressed the role of VP1 in TMEV pathogenesis. VP1+ transgenic mice (H-2b) and VP1− littermate controls were infected intracerebrally with TMEV. Seven days later, CNS-infiltrating cells were isolated by Percoll gradient and a standard cytotoxicity assay was performed against VP1+- and VP2+-transfected C57SV (H-2b) targets. VP1 and VP2 were used as targets because previous data indicated that the cytotoxic response in H-2b mice is exclusively directed against these two target antigens (11). As expected, both VP1+ and VP1− mice showed a CTL response against VP2-transfected targets, which are the immunodominant epitope (Fig. 3B). In contrast, VP1+ mice failed to respond to VP1+ targets whereas VP1− mice showed a clear cytotoxic response against VP1+ targets (Fig. 3A). The absence of VP1-specific killing in the VP1+ transgenic mice supports our conclusion that expression of TMEV antigens under the control of the class I MHC resulted in nonresponsiveness to CTL immune responses.
FIG. 3.
VP1- and VP2-specific CTL assay. VP1+ transgenic and littermate nontransgenic mice were infected intracerebrally with TMEV and CNS-ILs harvested after 7 days for the CTL assay. The CNS-ILs were incubated with 51Cr-labeled C57SV cells transfected with either the VP1 or VP2 transgene, and lysis was determined by the level of chromium release. (A) CNS-ILs from TMEV-infected VP1+ transgenic mice did not lyse VP1-transfected C57SV cells, whereas those from nontransgenic littermates did. (B) In contrast, CNS-ILs from both VP1 transgenic and VP1 nontransgenic littermate mice infected with TMEV lysed VP2-transfected cells. Data shown are means of triplicate samples using pooled lymphocytes from the CNSs of four or five mice per experimental group.
T lymphocytes from VP1+ transgenic mice proliferate to VP1 peptide.
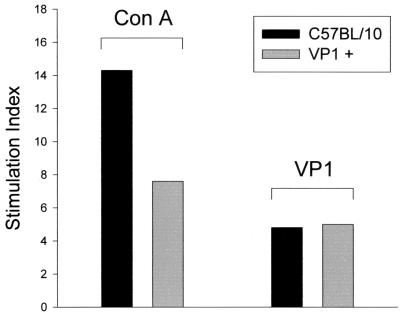
To determine whether TMEV transgenic mice were tolerant to virus antigens in the context of lymphocyte proliferation (MHC class II response), VP1+ transgenic and nontransgenic mice were immunized with recombinant VP1 protein in combination with complete Freund's adjuvant. Twenty-one days after immunization, splenocytes were collected and stimulated in vitro with either VP1 peptide or ConA. Another group of VP1+ mice were injected with PBS mixed with adjuvant to serve as a specificity control. Both VP1+ transgenic mice and nontransgenic mice showed lymphocyte proliferation in the presence of ConA (Fig. 4). In general, more proliferation in the presence of ConA was observed in nontransgenic mice than in VP1+ mice. Lymphocytes from VP1+ transgenic mice proliferated in the presence of purified VP1 protein to an extent similar to that of lymphocytes from nontransgenic mice, indicating that the MHC class II pathways were not affected by viral transgene expression.
FIG. 4.
Lymphocyte proliferation assay following in vitro stimulation with ConA or VP1. Splenocytes were obtained from VP1+ transgenic mice and from control mice previously immunized with recombinant VP1 protein in combination with complete Freund's adjuvant. Splenocytes were stimulated in vitro with ConA or recombinant VP1 peptide, and stimulation indices were determined using a [3H]thymidine uptake assay. Splenocytes from VP1+ and VP1− mice incubated with ConA (100 ng/well) proliferated 8- to 14-fold above the response with media alone. VP1+ and VP1− mice showed similar proliferative responses against recombinant VP1 protein (5 ng/well).
Transgenic mice expressing TMEV capsid proteins generate Ig responses to virus antigens following virus infection.
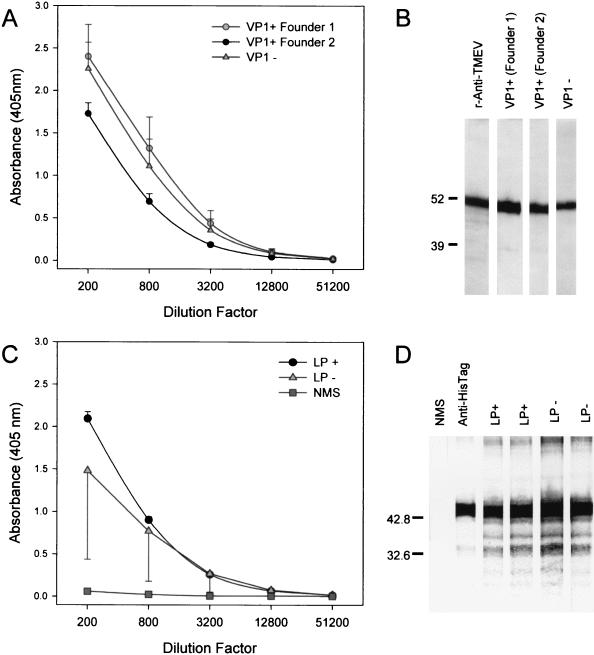
To further evaluate class II MHC immune effector functions in TMEV transgenic mice, we intracerebrally infected VP1+ and LP+ transgenic mice and littermate control nontransgenic mice with TMEV and collected serum 45 days later. ELISA (Fig. 5A) and Western blotting (Fig. 5B) using recombinant VP1 protein as the antigen showed that VP1+ transgenic mice generated antibodies to the protein. The antibody response in transgenic mice following virus infection was similar to that observed in infected littermate nontransgenic mice. Similarly, LP+ transgenic mice mounted strong humoral immune responses to VP2 (Fig. 5C and D) which were similar to those observed in infected littermate control LP− mice. These results indicate that the B-cell immune response to TMEV infection was not altered by transgene expression.
FIG. 5.
Detection of VP1- and VP2-specific IgG in transgenic mice infected with TMEV. LP+ transgenic, VP1+ transgenic, and littermate control nontransgenic mice were infected intracerebrally with 2 × 106 PFU of TMEV, and sera were collected 45 days later for antibody analysis. VP1- and VP2-specifc IgG levels were determined by ELISA (A and C) or Western blotting (B and D) using recombinant virus capsid antigens. Diluted sera from individual mice were added to the coated plates or blots, and bound IgGs were detected using an alkaline phosphatase system. TMEV-infected VP1+ transgenic mice generated antibodies against the VP1 protein (A and B). Similarly, TMEV-infected LP+ transgenic mice generated antibodies against the VP2 protein, which is encompassed within the LP region of the genome (C and D).
Transgenic expression of virus coding regions (VP1 or LP) results in demyelination and virus persistence.
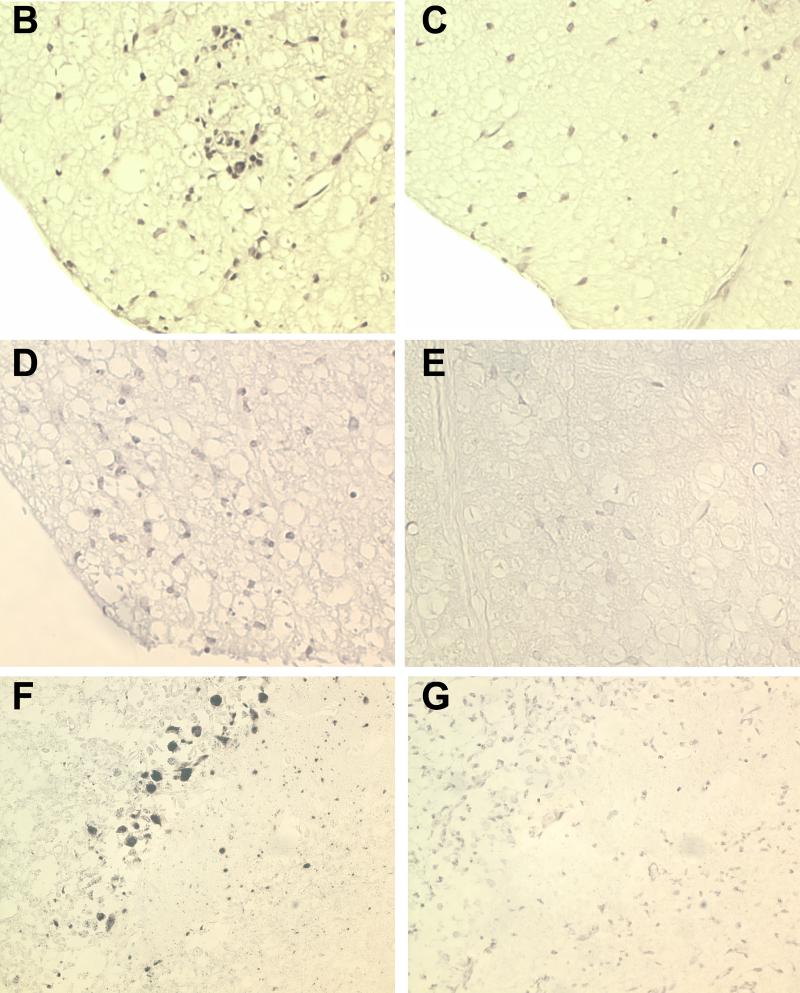
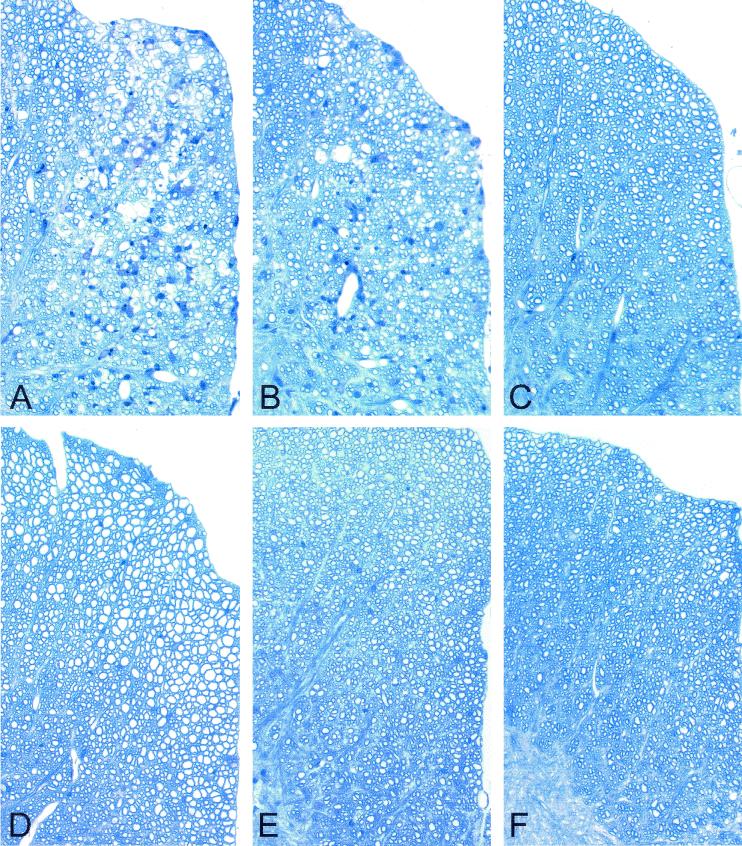
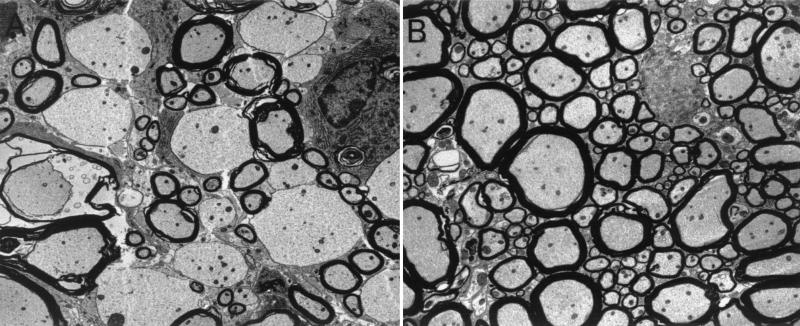
Transgene positive and transgene negative littermate control mice expressing LP, VP1, or RP were infected intracerebrally with TMEV. Forty-five days later, mice were sacrificed and brains and spinal cords were processed for morphological analysis of inflammation, demyelination, and in situ hybridization for virus RNA. The 45-day time point was chosen because this time point has been shown to allow researchers to consistently distinguish strains of mice resistant to virus persistence and subsequent demyelination from susceptible strains. Transgene-positive LP and VP1 mice showed small areas of clear demyelination (Fig. 6A and B). In contrast, littermate transgene-negative infected mice showed no or minimal demyelination (Fig. 6D and E). More specifically, 8 of 9 VP1+ and LP+ TMEV capsid transgenic mice showed demyelination whereas only 2 of 17 littermate nontransgenic mice showed any sign of demyelination (P < 0.001 by Fisher's exact test). Quantitation demonstrated that for the VP1+ transgenic mice, 7.0 ± 2.0% of spinal cord quadrants displayed focal demyelination. This is in stark contrast to the complete lack of demyelination in spinal cord quadrants of VP1− littermate controls (P < 0.001 by rank sum test). The spinal cords of the LP+ transgenic mice exhibited 5.8 ± 2.5% demyelination compared to the 0.8 ± 0.5% demyelination observed in the spinal cords of LP− littermate controls (P = 0.021). Using electron microscopy, primary demyelination with relative presentation of axons was observed in the spinal cord sections of VP1+ and LP+ transgenic mice (Fig. 7). The extent of inflammation was slight and did not approach the extent of inflammation usually observed in normally susceptible mice (SJL or B10.Q). All lesions observed were small and focal within the spinal cord white matter. Neurologic deficits were not observed in any of the mice showing focal demyelination. No demyelination was observed in transgene-positive RP+ transgenic mice (Fig. 6C) or RP− nontransgenic mice (Fig. 6F).
FIG. 6.
Demyelination in the spinal cord white matter of transgenic mice following TMEV infection. VP1+ (A), LP+ (B), RP+ (C), VP1− (D), LP− (E), and RP− (F) mice were infected with TMEV for 45 days, and spinal cord blocks were then embedded in glycol-methacrylate plastic. Sections were stained with erichrome-cresyl violet stain to detect demyelination and inflammation. Focal areas of demyelination are shown for LP1+ (A) and LP+ (B) transgenic mice. No demyelination was observed for RP+ (C), VP1− (D), LP− (E), or RP− (F) mice.
FIG. 7.
(A) Electron microscopy results, showing multiple demyelinated axons in the spinal cord of a VP1+ transgenic mouse which had been infected with TMEV for 45 days. Axons are well preserved and without ultrastructural abnormalities. (B) No demyelination was observed for a VP1− littermate control mouse infected with TMEV.
The presence of virus RNA was demonstrated by in situ hybridization in the spinal cord sections of VP1+, LP+, and RP+ transgenic mice (Fig. 8A to C). Virus was present within lesions, exclusively in white-matter cells. No virus RNA-positive cells were present in nontransgenic mice (panels D to F). We concluded that expression of VP1, LP, and RP under the control of class I MHC abolished a protective immune response in resistant B10 mice.
FIG. 8.
Detection of TMEV RNA persistence in spinal cord white matter of TMEV transgenic mice. Using paraffin, spinal cord sections were prepared from mice intracerebrally infected with TMEV for 45 days. TMEV RNA was localized by hybridization with a 35S-labeled probe corresponding to the VP1 region of TMEV. Viral RNA was detected in VP1+ (A), LP+ (B), and RP+ (C) transgenic mice but not in VP1− (D), LP− (E), and RP− (F) nontransgenic littermate controls. Sections were counterstained with Mayer's hematoxylin. Magnification, ×600.
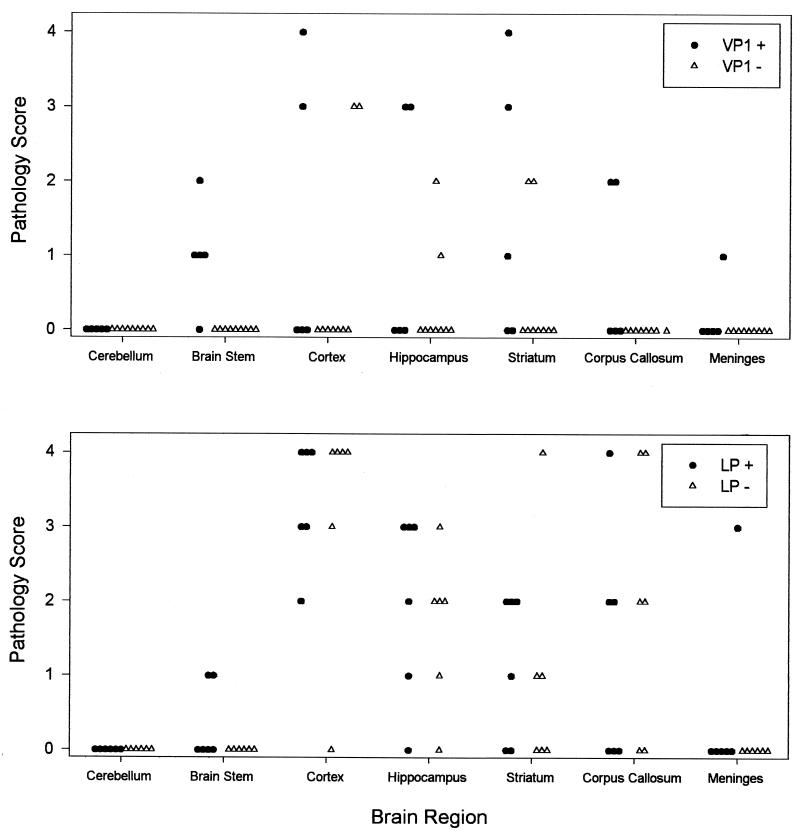
Pathological analysis of the brains of VP1+, LP+, and littermate controls at 45 days following infection demonstrated no significant differences in the severity and distribution of lesions in the brain (Fig. 9). All groups of mice developed virus-induced lesions in the cortex, hippocampus, and striatum, indicating that there were no differences in the ability of TMEV to induce early neuronal injury from virus infection in vulnerable areas of the brain gray matter. Of interest, following TMEV infection, focal areas of inflammation and demyelination were more common in VP1+ and LP+ than in littermate control VP1− or LP− mice. No lesions were observed at this time point in 15 nontransgenic mice. In contrast, four of five VP1+ mice and two of six LP1+ mice showed lesions in the brain stem. All of the lesions were in the white matter of the brain stem, which is consistent with the observation of focal demyelination in the spinal cords of VP1+ and LP+ mice.
FIG. 9.
Severity of brain disease in VP1+, VP1−, LP+, and LP− mice infected with TMEV for 45 days. Each symbol represents an individual mouse, graded in a blinded fashion for each area of the brain according to the scale detailed in Materials and Methods.
DISCUSSION
The goal of these experiments was to determine whether transgenic expression of viral coding sequences under the control of the class I MHC promoter would allow viral peptides to be expressed as self-antigens during thymic lymphocyte selection, thus resulting in tolerance. The ability to compare mice that express virus transgenes to transgene-negative littermates provides a powerful genetic system for evaluation of the immune tolerizing effect of introducing virus proteins expressed as self-antigens. We hypothesized that nonresponsiveness would occur in the context of CD8+ class I-restricted responses, whereas antibody and class II-restricted responses would be relatively unaffected. Therefore, we surmised that if specific TMEV peptides are important for the induction of protective immune responses to prevent virus persistence and demyelination in normally resistant mice, then transgenic expression of these peptides should render mice susceptible to persistent infection because the mice would not mount an effective cytotoxic immune response. Of importance in these experiments was whether tolerance to one virus coding region would result in emergence of other immunodominant viral peptides capable of stimulating a protective immune response. The data show that VP1+ transgenic mice were tolerized against this virus-coding region, because they could not lyse VP1-transfected C57SV (H-2b) cells in a class I-restricted cytotoxicity assay. However, these VP1+ transgenic mice continued to mount normal VP2-specific CTL responses following TMEV infection. Despite this response, VP1+ transgenic mice developed virus persistence and small areas of demyelination. Demyelination and/or virus persistence was also observed following TMEV infection of LP+ and RP+ transgenic mice. This would support the hypothesis that cytotoxic lymphocyte responses to peptides derived from VP1, LP, and RP independently contribute to the prevention of virus persistence and demyelination. Whether transgenic expression of the entire TMEV genome by the intercrossing of mice expressing the three regions of the TMEV genome would result in death following TMEV infection is unknown, since this would be expected to result in immune nonresponsiveness to all proteins of TMEV in the context of CD8+ T cells.
Our finding that in vitro lymphocyte proliferation and production of antibodies directed against the polypeptides encompassed by the transgenes (functions attributable to the class II MHC immune response) confirmed that expressing the transgenes under the control of class I MHC permitted nonresponsiveness which was restricted to CD8+ T-cell and class I functions. The three independent lines of transgenic mice carrying contiguous TMEV coding regions were not susceptible to lethal acute TMEV-induced encephalitis following inoculation with infectious virus, which suggested that none of the three regions is independently responsible for resistance against acute fatal encephalitis as observed in Rag−/− or SCID mice. Possibly the intact class II-restricted T-cell immune response to the virus and normal virus-specific antibody repertoire protected mice against lethal encephalitis. There is strong evidence that both CD4+ T cells and antibody participate in limiting virus infection following TMEV infection (21, 27, 32). The humoral response during TMEV infection was not significantly impaired in VP1+, LP+, and RP+ mice, since we observed strong antibody responses toward virus proteins that were actively being expressed as self-proteins (Fig. 5). This implies that, unlike those for class I-mediated responses, there is a requirement for virus proteins to be both extracellular and accompanied by danger signals characteristic of virus infection for an effective humoral response to be generated.
The most important finding from this study was that VP1+ and LP+ transgenic mice from a resistant H-2b background were partially susceptible to TMEV-induced demyelination whereas RP transgenic mice were not, indicating that coding regions in the capsid proteins of TMEV are important for protection against demyelination. The likely candidate on LP is VP2, based on the immune dominance of VP2121-130 in the generation of virus-specific CD8 T-cell responses in the CNSs of H-2b mice (8). The generation of transgenic mice exclusively expressing VP2 is under way in our laboratory to test this hypothesis. Because VP1+ and LP+ transgenic mice developed focal demyelination, the implication is that CD8+ T-cell responses directed against these epitopes are not absolutely required for myelin injury. The results of the present study are consistent with other data which indicate that CD8+ T cells are not absolutely required for demyelination because mice lacking class I MHC and CD8+ T lymphocytes can develop TMEV-induced demyelination (4, 20). Of interest, mice lacking class I MHC or CD8+ T-cell function which show TMEV-induced demyelination develop less-severe neurologic deficits because of preservation of axons, compensatory spontaneous remyelination, and upregulation of sodium channels (17). This suggests that CD8+ T lymphocytes may be one of the factors responsible for damaging axonal surfaces to prevent myelin repair.
Thus far, VP1 and LP transgenic mice with demyelination have failed to develop neurologic deficits. However, the extent of demyelination observed in these mice is small and the length of infection is insufficient to make a definitive conclusion. One possible explanation for the relative lack of inflammation and neurologic injury in the VP1+ and LP+ transgenic mice following TMEV infection is that CD8+ T cells directed against the epitopes not only contributed to resistance but also contributed to immune-mediated injury directed against virus-infected glial cells. The generation of TMEV transgenic mice with double- and triple-knockouts encompassing region I, II, or III will be of particular interest for dissecting the codependent contributions of these virus coding regions to immune pathogenesis. In addition, crossing VP1+ and LP+ transgenic mice to normally susceptible B10.Q mice may address the contribution of virus-specific CD8 T cells to injury in the context of a susceptible genotype. These future experiments will provide a unique opportunity to directly assess the contribution of virus-specific class I-restricted immune responses in vivo to demyelination and neurologic deficits.
Acknowledgments
Xiaoqi Lin and M. Kariuki Njenga contributed equally to the generation of the manuscript.
We thank Kathy Sanborn, Michael Coenen, Laurie Zoecklein, Mabel Pierce, and Jeff Gamez for their technical assistance and the staff of Mayo Transgenic Mice Facilities for their help.
This research was supported by National Institutes of Health grants R01-NS 32129 and P01-NS 38468.
REFERENCES
- 1.Azoulay, A., M. Brahic, and J. F. Bureau. 1994. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler's virus. J. Virol. 68:4049-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borson, N. D., C. Paul, X. Lin, W. K. Nevala, M. A. Strausbauch, M. Rodriguez, and P. J. Wettstein. 1997. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J. Virol. 71:5244-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detlefs, S., N. Escriou, M. Brahic, S. van der Werf, and E. L. Larsson-Sciard. 1997. Theiler's virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J. Virol. 71:5361-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiette, L., C. Aubert, M. Brahic, and C. P. Rossi. 1993. Theiler's virus infection of β2-microglobulin-deficient mice. J. Virol. 67:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerety, S. J., R. J. Clatch, H. L. Lipton, R. G. Goswami, M. K. Rundell, and S. D. Miller. 1991. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. IV. Identification of an immunodominant T cell determinant on the N-terminal end of the VP2 capsid protein in susceptible SJL/J mice. J. Immunol. 146:2401-2408. [PubMed] [Google Scholar]
- 6.Gerety, S. J., M. K. Rundell, M. C. Dal Canto, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J. Immunol. 152:919-929. [PubMed] [Google Scholar]
- 7.Inoue, A., Y.-K. Choe, and K. S. Kim. 1994. Analysis of antibody responses to predominant linear epitopes on Theiler's murine encephalomyelitis virus. J. Virol. 68:3324-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, A. J., M. K. Njenga, M. J. Hansen, S. T. Kuhns, L. Chen, M. Rodriguez, and L. R. Pease. 1999. Prevalent class I-restricted T-cell response to the Theiler's virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J. Virol. 73:3702-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, B. S., Y.-K. Choe, M. A. Crane, and C. R. Jue. 1992. Identification and localization of a limited number of predominant conformation-independent antibody epitopes of Theiler's murine encephalomyelitis virus. Immunol. Lett. 31:199-205. [DOI] [PubMed] [Google Scholar]
- 10.Lin, X., L. P. Pease, and M. Rodriguez. 1997. Differential generation of class I H-2D versus H-2K restricted cytotoxicity against a demyelinating virus following central nervous system infection. Eur. J. Immunol. 27:963-970. [DOI] [PubMed] [Google Scholar]
- 11.Lin, X., N. R. Thiemann, L. R. Pease, and M. Rodriguez. 1995. VP1 and VP2 capsid proteins of Theiler's virus are targets of H-2D-restricted cytotoxic lymphocytes in the central nervous system of B10 mice. Virology 214:91-99. [DOI] [PubMed] [Google Scholar]
- 12.Lipton, H. L., and M. C. Dal Canto. 1976. Theiler's virus-induced demyelination: prevention by immunosuppression. Science 192:62-64. [DOI] [PubMed] [Google Scholar]
- 13.Lucchinetti, C. F., and M. Rodriguez. 1997. The controversy surrounding the pathogenesis of the multiple sclerosis lesion. Mayo Clin. Proc. 72:665-678. [DOI] [PubMed] [Google Scholar]
- 14.Njenga, M. K., L. R. Pease, P. Wettstein, T. Mak, and M. Rodriguez. 1997. Interferon alpha/beta mediates early virus-induced expression of H-2D and H-2K in the central nervous system. Lab. Investig. 77:71-84. [PubMed] [Google Scholar]
- 15.Paya, C. V., A. K. Patick, P. J. Leibson, and M. Rodriguez. 1989. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J. Immunol. 143:95-102. [PubMed] [Google Scholar]
- 15a.Pierce, M., and M. Rodriguez. 1989. Erichrome stain for myelin on osmicated tissue embedded in glycol methacrylate plastic. J. Histochem. 12:35-36.
- 16.Pullen, J. K., H. D. Hunt, R. M. Horton, and L. P. Pease. 1989. The functional significance of two amino acid polymorphisms in the antigen-presenting domain of class I MHC molecules. Molecular dissection of Kbm3. J. Immunol. 143:1674-1679. [PubMed] [Google Scholar]
- 17.Rivera-Quinones, C., D. McGavern, J. D. Schmelzer, S. F. Hunter, P. A. Low, and M. Rodriguez. 1998. Absence of neurologic deficits following extensive demyelination in class I-deficient murine model of multiple sclerosis. Nat. Med. 4:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez, M., and C. S. David. 1985. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J. Immunol. 135:2145-2148. [PubMed] [Google Scholar]
- 19.Rodriguez, M., and C. S. David. 1995. H-2Dd transgene suppresses Theiler's virus-induced demyelination in susceptible strains of mice. J. Neurovirol. 1:111-117. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez, M., A. J. Dunkel, R. L. Thiemann, J. Leibowitz, M. Zijlstra, and R. Jaenisch. 1993. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in β2-microglobulin. J. Immunol. 151:266-276. [PubMed] [Google Scholar]
- 21.Rodriguez, M., J. J. Kenny, R. L. Thiemann, and G. E. Woloschak. 1990. Theiler's virus-induced demyelination in mice immunosuppressed with anti-IgM and in mice expressing the xid gene. Microb. Pathog. 8:23-35. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez, M., W. P. Lafuse, J. Leibowitz, and C. S. David. 1986. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology 36:964-970. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez, M., J. Leibowitz, and C. S. David. 1986. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J. Exp. Med. 163:620-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez, M., J. L. Leibowitz, and P. W. Lampert. 1983. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann. Neurol. 13:426-433. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez, M., A. K. Patick, and L. R. Pease. 1990. Abrogation of resistance to Theiler's virus-induced demyelination in C57BL mice by total body irradiation. J. Neuroimmunol. 26:189-199. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez, M., K. D. Pavelko, M. K. Njenga, W. C. Logan, and P. J. Wettstein. 1996. The balance between persistent virus infection and immune cells determines demyelination. J. Immunol. 157:5699-5708. [PubMed] [Google Scholar]
- 27.Rodriguez, M., and J. Quddus. 1986. Effect of cyclosporin A, silica quartz dust, and protease inhibitors on virus-induced demyelination. J. Neuroimmunol. 13:159-174. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez, M., and S. Sriram. 1988. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J. Immunol. 140:2950-2955. [PubMed] [Google Scholar]
- 29.Roos, R. P., S. Firestone, R. Wollmann, D. Variakojis, and B. G. Arnason. 1982. The effect of short-term and chronic immunosuppression on Theiler's virus demyelination. J. Neuroimmunol. 2:223-234. [DOI] [PubMed] [Google Scholar]
- 30.Roos, R. P., S. Stein, Y. Ohara, J. L. Fu, and B. L. Semler. 1989. Infectious cDNA clone of the DA strain of Theiler's murine encephalomyelitis virus. J. Virol. 63:5492-5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welsh, C. J., P. Tonks, A. A. Nash, and W. F. Blakemore. 1987. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J. Gen. Virol. 68:1659-1667. [DOI] [PubMed] [Google Scholar]
- 32.Yahikozawa, H., A. Inoue, C.-S. Koh, Y.-K. Choe, and B. S. Kim. 1997. Major linear antibody epitopes and capsid proteins differentially induce protective immunity against Theiler's virus-induced demyelinating disease. J. Virol. 71:3105-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yauch, R. L., and B. S. Kim. 1994. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler's virus is located within VP1 (233-244). J. Immunol. 153:4508-4519. [PubMed] [Google Scholar]