Abstract
Potent and safe vaccinia virus vectors inducing cell-mediated immunity are needed for clinical use. Replicating vaccinia viruses generally induce strong cell-mediated immunity; however, they may have severe adverse effects. As a vector for clinical use, we assessed the defective vaccinia virus system, in which deletion of an essential gene blocks viral replication, resulting in an infectious virus that does not multiply in the host. The vaccinia virus Lister/Elstree strain, used during worldwide smallpox eradication, was chosen as the parental virus. The immunogenicity and safety of the defective vaccinia virus Lister were evaluated without and with the inserted human p53 gene as a model and compared to parallel constructs based on modified vaccinia virus Ankara (MVA), the present “gold standard” of recombinant vaccinia viruses in clinical development. The defective viruses induced an efficient Th1-type immune response. Antibody and cytotoxic-T-cell responses were comparable to those induced by MVA. Safety of the defective Lister constructs could be demonstrated in vitro in cell culture as well as in vivo in immunodeficient SCID mice. Similar to MVA, the defective viruses were tolerated at doses four orders of magnitude higher than those of the wild-type Lister strain. While current nonreplicating vectors are produced mainly in primary chicken cells, defective vaccinia virus is produced in a permanent safety-tested cell line. Vaccines based on this system have the additional advantage of enhanced product safety. Therefore, a vector system was made which promises to be a valuable tool not only for immunotherapy for diseases such as cancer, human immunodeficiency virus infection, or malaria but also as a basis for a safer smallpox vaccine.
A major challenge in the development of live vaccines and immunotherapy vectors is the generation of safe delivery systems for clinical use that induce efficient cell-mediated immune responses. The safety of live vaccines is a major concern in times of widespread immunosuppression due to human immunodeficiency virus (HIV) infection. The induction of cell-mediated immune effector mechanisms by active immunotherapy eliminates cells infected with intracellular pathogens, and also tumor cells and several tumor-specific antigens which are accessible to cell-mediated immune responses could be identified (for a review, see reference 23). In patients suffering from the AIDS, a critical role of cell-mediated immunity in the down-regulation of viremia after acute HIV infection was observed (18), and HIV-specific cytotoxic T lymphocytes (CTLs) are present in the blood of individuals who are frequently exposed to HIV but remain uninfected (46). Clinical data on hepatitis B virus-infected individuals indicate that cell-mediated immunity plays an essential part during clearance of an established viral infection (26, 30). Also in malaria, an association between the major histocompatibility complex class I allele HLA-B53 and resistance to malaria indicates a protective role for the cellular immune response (12).
Live viral vectors are promising in fighting these diseases. However, before live viruses can be used as gene delivery vectors for immunotherapy in humans, a number of key issues have to be addressed, such as safety, immunogenicity, and vaccine preparation by state-of-the-art production methods. Although the vector should be able to efficiently infect the target cell, the virus should not replicate and spread in the host or cause adverse effects. Further, a strong and specific immune response targeted mainly against the inserted antigen should be induced. Due to their unique immunological properties in eliciting long-term protective humoral and cell-mediated immune responses and their stability, recombinant vaccinia viruses are considered to be prime candidates for use in immunotherapy (5, 27). The vaccinia virus strains used during the World Health Organization program on the eradication of smallpox induced, mainly in immunocompromised patients, severe adverse effects, which was one of the reasons to discontinue vaccination after eradication. Today the principal candidates as gene delivery vectors for clinical use in the immunotherapy of chronic disease or cancer are vaccinia virus strains that have been classically or genetically attenuated. Reemerging smallpox due to bioterrorism is a further scenario requiring safer vaccinia virus-based vectors (10).
Several attenuated and safe vaccinia virus strains were developed for clinical use. The vaccinia virus strain NYVAC, derived from the Copenhagen strain, for instance, was genetically attenuated by deletion of many nonessential genes, including virulence and host range genes, resulting in a strain growing only in primary cells (40). The currently widely used modified vaccinia virus Ankara (MVA) strain, which has proven to be safe even in immunocompromised individuals, was classically attenuated by being passaged more than 500 times on chicken embryo fibroblasts (CEFs) (20). The growth of MVA in mammalian cells (baby hamster kidney cells) has been described (3, 7), opening the way to also produce MVA vectors in permanent cells. Passaging in mammalian cells, however, presumably also increases virulence in mammals, resulting in new MVA-like strains with unknown safety profiles in humans.
In addition to vaccinia virus vectors, the avipox vector ALVAC has potential as an immunotherapy vector (29). Although immunogenic in patients, the vectors produced in primary chicken cells do not have an optimal safety profile. There is a high risk of contamination with adventitious agents because a production batch may consist of hundreds of eggs, a flaw that cannot be corrected by procedures to inactivate viruses, as are performed during the production of subunit or inactive whole virus vaccines. State-of-the-art production of biologicals should be done today in permanent cell lines, allowing cell banking and safety testing (44). A novel approach to generate a nonreplicating vaccinia virus was the deletion of a single gene essential for viral replication, the uracil DNA glycosylase gene encoded by the D4R open reading frame (ORF). The resulting viruses grow exclusively in a complementing permanent cell line (14), excluding reversion to virulence and obviating the need for primary cells. To distinguish these entirely impaired viruses from attenuated vaccinia viruses not replicating in most permanent mammalian cell lines (nonreplicating vaccinia viruses), they are referred to as defective vaccinia viruses (dVVs). In these viruses, the replication cycle is blocked prior to late gene expression in any cell line except the complementing cells. Using a tick-borne encephalitis virus model, it was shown that recombinant dVVs based on the Western Reserve (WR) strain expressing the prME antigens were immunogenic in mice and conferred antibody-mediated protection from a lethal challenge (16). The WR strain, however, is a vaccinia virus laboratory strain passaged in mouse brain that has unfavorable properties, such as neurovirulence and gonadotropism, not suitable for clinical use (11). In this study, we describe the suitability of recombinant dVVs based on the Lister (Elstree) vaccine strain as a vector in immunotherapy. We evaluate the safety and immunogenicity of the vector system alone and with a recombinant gene insertion, using human p53 as a model antigen. In both safety and immunogenicity, the vector system proved to be comparable to or better than the corresponding constructs based on MVA.
MATERIALS AND METHODS
Viruses and cell lines.
The African green monkey kidney cell lines CV-1 (ATCC CCL-70) and Vero (ATCC CCL-81), the human fibroblast cell line MRC-5 (ATCC CCL-171), and the rabbit kidney cell line RK13 (ATCC CCL 37) were obtained from the American Type Culture Collection. The mouse fibroblast cell lines 10(3)Tx4BT87 (B27) and 10(3)273.1NT24 (NT24), the latter of which is transfected with the mutated form of the human p53 cDNA, have been described previously (33). Primary CEFs were prepared from 12-day chicken embryos. The cells were grown in tissue culture medium 199 (Gibco BRL) supplemented with 5% fetal bovine serum. The vaccinia virus strains WR (ATCC VR-1354), Lederle-Chorioallantoic (ATCC VR-118), and Lister (ATCC VR-862) were from the American Type Culture Collection. The Copenhagen strains were obtained from J. Esposito (Centers for Disease Control and Prevention, Atlanta, Ga.) and R. Drillien (Strasbourg, France). The MVA strain was the cloned isolate M4, derived from MVA II/85, which originated from MVA passage 575 (1983), and has been described previously (2). The defective virus eVAC-1, as well as the D4R gene-transformed rabbit kidney cell line RK-D4R-44.20 (RK44), were described previously (14).
Animals.
Chickens (Leghorn, 6 to 8 weeks old), guinea pigs (Dunquin Hartley, 6 to 10 weeks old), and mice (BALB/c, 6 to 8 weeks old) were purchased from Charles River. BALB/c/SCID mice (strain CBySmn.CB17-Prkdc scid/J) were purchased from Jackson Laboratories.
Construction of plasmids. (i) pDM5hp53-iLg.
The plasmid pDW (15) contains a multiple cloning site (MCS) and an adjacent unstable Escherichia coli lacZ and gpt marker cassette (flanked by direct repeats) for the generation of marker-free defective viruses. Both elements are flanked by vaccinia virus D3R and D5R gene regions for recombination (thereby deleting the essential D4R gene during recombination). To obtain the plasmid pDM5hp53-iLg, the human p53 ORF was isolated as a NcoI/SalI-fragment from the plasmid pC53-SN3 (33) and placed under the control of the vaccinia virus mH5 promoter (5′-GTT AAC AAA AAT TGA AAA TAA ATA CAA AGG TTC TTG AGG GTT GTG TTA AAT TGA AAG CGA GAA ATA ATC ATA AAT AAT TTC ATT ATC GCG ATA TCC GTT AAG TTT AGG CCT CCA TGG-3′). The mH5 promoter-p53 gene cassette was inserted into the MCS of pDW, resulting in pDM5hp53-iLg, which allows transient dominant selection of vaccinia viruses (9, 35).
(ii) pDRMa.
A stable lacZ/gpt marker gene cassette was inserted into the MCS of plasmid pER (15); this plasmid has inserts consisting of the D3R, D4R, and D5R genes, and the MCS is located in the D4/D5 intergenic region. The resulting plasmid was designated pDRMa.
(iii) pDRM5-hp53.
To obtain the plasmid pDRM5-hp53, the mH5 promoter-p53 gene cassette (see above) was inserted upstream of the lacZ/gpt marker gene cassette of pDRMa (for gene order, see Fig. 1D), resulting in pDRM5-hp53.
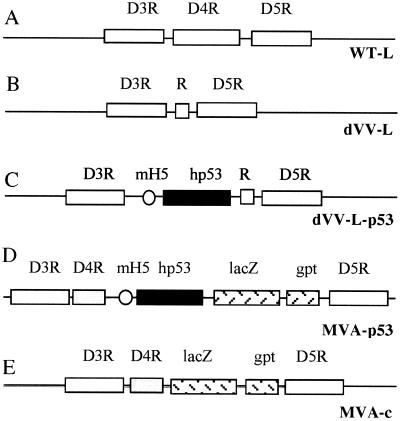
FIG. 1.
Schematic representation of the insertion sites of the viruses. The insertion site in all viruses is the region around the D4R ORF. The regions of the wild-type Lister virus (WT-L) (A), the defective Lister virus without foreign gene (dVV-L) (B), the defective Lister virus expressing the human p53 gene (dVV-L-p53) (C), and the two MVA-based viruses (MVA-p53 and MVA-c) (D and E)are shown. R, small noncoding DNA insert; mH5, strong vaccinia virus early/late promoter mH5; hp53, human wild-type p53 cDNA; lacZ and gpt, color and selection markers, respectively.
(iv) pRSET-hp53.
The human p53 gene was inserted as an NcoI/SmaI fragment from the plasmid pC53-SN3 in frame into the E. coli protein expression vector pRSET (Invitrogen. Inc.).
Plasmids were constructed by standard procedures (34); the correct assembly of all plasmids was confirmed by restriction analysis and double-stranded DNA sequencing. The sequences of all plasmids are available upon request.
Generation of defective viruses dVV-Cop, dVV-Led, dVV-L, and dVV-L-p53.
Recombinant viruses were generated using the respective vaccinia virus strain and the complementing cell line RK-D4R-44.20. For virus constructions, 5 × 106 RK-D4R-4420 cells were infected with the respective wild-type virus strain at a multiplicity of infection (MOI) of 0.5 and incubated for 2 h at 37°C in a CO2 incubator prior to transfection with 7.5 μg of pDW (to obtain the defective empty viruses) or pDM5-hp53-iLg plasmid DNA (to obtain the p53-expressing virus dVV-L-p53) using DOTAP liposomal transfection reagents (Boehringer, Mannheim, Germany). After overnight incubation, the transfected cells were overlaid for gpt selection (8). Products of single recombination events were identified by X-Gal ((5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining (4) and plaque purified on RK44.20 cells under gpt selection. Starting with the third round of plaque purification, clones were screened for selective growth in permissive RK-D4R-44.20 cells and in nonpermissive CV-1 cells. Plaque purification on RK-D4R-44.20 cells was further performed until selective growth was achieved (usually in purification rounds 6 to 8). Clones were then amplified in the absence of gpt selection to allow deletion of the marker cassette. The amplified viruses were further plaque purified in the absence of gpt selection until purely white plaques were obtained. The virus was expanded, and sucrose cushion-purified viral stocks were prepared and titrated on RK-D4R-44.20 cells. dVV-L-p53 was additionally tested for p53 protein expression.
Generation of MVA-based recombinants MVA-c and MVA-p53.
Recombinant viruses were generated using the sequenced MVA strain M4 (2) and CEFs. Chicken cells were infected with the wild-type virus at an MOI of 0.5 and incubated for 2 h in a CO2 incubator prior to transfection with 7.5 μg of pDRMa or pDRM5-hp53 plasmid DNA, respectively, as described above. After overnight incubation, the recombination was overlaid for gpt selection. Products of single recombination events were identified by X-Gal staining and purified through four to six rounds of plaque purification on CEFs under gpt selection. Positive clones were screened in Southern blots; the virus MVA-p53 was additionally checked for p53 protein expression. The viruses were expanded, and sucrose cushion-purified viral stocks were prepared and titrated on CEFs by X-Gal staining. The genomic structures of all final recombinant viruses were confirmed by Southern blot analysis (data not shown).
Generation of antisera. (i) Guinea pig anti-late vaccinia virus protein.
Sucrose gradient-purified defective virus eVAC-1 was inactivated with psoralen (AMT; Lee Biomolecular Research Inc., San Diego, Calif.) for 30 min, using 10 μg of AMT per ml under UV light at 365 nm. The equivalent of 107 functional PFU was used to immunize guinea pigs three times at monthly intervals. Characterization of the serum demonstrated specificity for late vaccinia virus proteins in Western blots and neutralizing activity in vaccinia virus neutralization tests.
(ii) Chicken anti-early vaccinia virus protein.
CEFs were infected with eVAC-1 at an MOI of 1. Cells were harvested at 3 days postinfection and homogenized by sonication, and the cell lysate was inactivated with psoralen as described above. Chickens were immunized with the equivalent of 107 infected cells in complete Freund's adjuvant, followed by a boost in incomplete Freund's adjuvant 4 weeks later. The serum showed specificity in Western blots for early vaccinia virus proteins as well as for vaccinia infection-induced cellular proteins but not for proteins of purified vaccinia virus virions.
Protein expression.
Protein expression was induced by addition of isopropylthiogalactopyranoside (0.5 mM; Sigma) to pRSET-hp53-transformed E. coli ER2566 cell cultures (New England Biolabs), and inclusion bodies of recombinant hp53 protein were purified (25). After solubilization in 4 M urea-200 mM Tris pH 8.0 and refolding dialysis against 10 mM Tris-150 mM NaCl (pH 8.0), the soluble protein was further purified on Ni-nitrilotriacetic acid agarose (Qiagen, Inc.) and finally dialyzed against 10 mM Tris-150 mM NaCl. The purity of the protein was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to be at least 80%.
Western blotting.
Samples (equivalent to 105 cells) were separated by SDS-PAGE on 12.5% gels and blotted onto nitrocellulose membranes. p53 expression was assessed using a mouse anti-p53-specific monoclonal antibody (1:500 dilution; Calbiochem Inc.). Early and late vaccinia virus protein expression and RK-D4R-44.20 cellular protein contamination were assessed using anti-vaccinia virus late protein-specific guinea pig serum (1:1,000 dilution), anti-vaccinia virus early protein-specific chicken serum, and anti-RK-D4R-44.20 cell lysate chicken serum, respectively. Detection was done using species-specific alkaline phosphatase-conjugated anti-immunoglobulin G (IgG) sera (Sigma).
IgG subclass ELISA.
Vaccinia virus and p53-specific IgG subclasses IgG1, IgG2a, IgG2b, and IgG3 in mouse sera were analyzed on day 14 after immunization by enzyme-linked immunosorbent assay (ELISA) using microtiter plates (Costar, Cambridge, Mass.) coated with human recombinant p53 protein and recombinant vaccinia virus eVAC-1, respectively. In parallel, serially diluted IgG isotype standards (PharMingen, San Diego, Calif.) were added to wells coated with goat anti-mouse IgG [F(ab)2; Accurate, Westbury, N.Y.] for standardization purposes. Alkaline phosphatase-conjugated goat anti-mouse IgG isotype-specific antibodies (Accurate) were used as secondary antibodies. A reference standard curve, obtained by plotting the absorbance versus the standard IgG concentrations, was used to calculate the specific IgG isotype concentrations in the sera.
CTL assay. (i) Preparation of spleen cell suspension.
Mice were sacrificed by cervical dislocation at day 14 after immunization. A single-cell suspension was prepared from the pooled spleens of four to six animals by forcing minced tissue through 200-mesh stainless steel sieves. Red blood cells were depleted by incubation for 5 min at room temperature with lysis buffer containing 0.15 M NH4Cl, 10 mM KHCO3, and 0.1 M Na2-EDTA, pH 7.4. The leukocytes were resuspended in complete culture medium (a 1:1 mixture of RPMI 1640 [Gibco, Paisley, Scotland] and Click's medium [Sigma, Irvine United Kingdom] supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 100 IU of penicillin-streptomycin per ml [all from Gibco] and 50 μM 2-mercaptoethanol [Bio-Rad, Hercules, Calif.]).
(ii) In vitro restimulation.
A two-step in vitro restimulation protocol was performed for the p53-specific CTL assay. First, leukocytes (5 × 106/ml) were seeded into 24-well culture plates (Costar) in the presence of p53-expressing vaccinia virus vp53 (multiplicity of infection [MOI], 0.05). After 7 days of incubation (37°C, 5% CO2), viable cells were isolated by centrifugation over Lympholyte-M (Cedarlane, Hornby, Ontario, Canada). A second round of in vitro restimulation was performed by coculturing these cells with irradiated (100 Gy from a 137Cs source) p53-positive NT24 stimulator cells (105/ml) and purified interleukin-2 (5 U/ml; Biotest, Frankfurt, Germany). Viable effector cells were harvested after a 5-day incubation period for analysis in the 51Cr or gamma interferon (IFN-γ) release assay. For the vaccinia virus-specific CTL assay, restimulation was done once using only the first step described above.
(iii) 51Cr release assay.
The ability of effector cells to lyse the appropriate target cells was tested in a standard 51Cr release assay. B87 and NT24 target cells were labeled with 14.8 MBq of Na251CrO4 (Amersham, Little Chalfont, United Kingdom) for 2 h at 37°C. When indicated, B87 target cells had been infected previously for 16 h with vdTK (a thymidine kinase-negative vaccinia virus without foreign genes) or the p53-expressing virus vp53 (MOI, 1). After extensive washing, the target cells were mixed with effector cells at the indicated ratios in V-bottom microtiter plates (Nunc, Roskilde, Denmark). After incubation for 4 h at 37°C, supernatants were harvested and radioactivity was measured in a beta counter (Microbeta; Wallac, Turku, Finland). Spontaneous and maximum 51Cr release were determined by incubating target cells in culture medium or 1% Triton X-100, respectively. Spontaneous release of the target cells was between 5 and 25%. The percent specific lysis was calculated using the formula (counts per minute for test release − counts per minute for spontaneous release)/(counts per minute for maximum release − counts per minute for spontaneous release).
IFN-γ release assay.
For analysis of IFN-γ secretion, an in situ IFN-γ ELISA as was performed (22) with slight modifications. Briefly, 96-well ELISA plates (Costar) were coated with anti-IFN-γ monoclonal antibody (PharMingen) overnight at 4°C and then blocked for 2 h with complete culture medium. Serially diluted effector cells were seeded onto the coated plates and cultured for 16 h with NT24 or B87 stimulator cells (9 × 104/well) at 37°C with 5% CO2. Serially diluted recombinant IFN-γ (PharMingen) was added to separate control wells for standardization purposes. The next day, the wells were washed and the amount of IFN bound was detected using biotinylated anti-IFN-γ monoclonal antibody (PharMingen) and alkaline phosphatase-conjugated streptavidin (Zymed, San Francisco, Calif.). A reference standard curve, obtained by plotting the absorbance versus the standard IFN-γ concentrations, was used to calculate the specific IFN-γ release of the effector cells.
T-cell proliferation assay.
Spleen cells were prepared from immunized mice as described above and were further purified into a T-cell-enriched population by depletion of B cells with magnetic beads coated with anti-mouse B220 monoclonal antibodies (Mouse pan B, B220; Dynal A.S., Oslo, Norway). Subsequently, 105 enriched T cells/well were stimulated with recombinant human p53 at concentrations of 0.3, 1, 3, and 9 μg/ml in 0.2 ml of complete culture medium. Cultures were incubated in 96-well flat-bottom microtiter plates (Costar) for 5 days (37°C, CO2 incubator), and 18.5kBq (0.5 μCi) of [3H]thymidine per well was added during the last 16 h of the culture period. The cells were then harvested using an automatic cell harvester (Tomtec; Wallac), and incorporated radioactivity was measured in a beta counter (Microbeta; Wallac). The stimulation index was calculated as the counts per minute in the stimulated sample/counts per minute in a nonstimulated control sample.
In vitro safety test.
For induction of cytopathic effects (CPE) in permissive and nonpermissive cell lines, the indicated cell line was seeded into six-well tissue culture plates and grown to approximately 80% confluency. The cells (duplicate wells) were infected with the indicated viruses. Starting at an MOI of 10, the cells lines were infected with 10-fold dilutions of virus at MOIs ranging from 10 to 10−6. Cells were monitored visually under a microscope daily for signs of CPE (such as cell rounding and cytoplasmic contraction), and the extent of CPE in a well was estimated for 7 days. To confirm the visual monitoring results, the plates were finally stained with crystal violet. For comparison of the CPE of the different viruses, the lowest MOI at which 50% or more of the cells in a well showed a CPE after 6 days was determined. Three independent experiments were performed.
In vivo safety test.
Groups of four female BALB/c/SCID mice were challenged subcutaneously with the indicated amounts of the different vaccinia virus strains or phosphate-buffered saline (PBS) in a volume of 0.5 or 1 ml. Mice were monitored for weight and symptoms of progressive vaccinia virus disease. Two independent experiments were performed.
RESULTS
The Lister strain as a backbone for the dVV technology.
The original study describing the dVV technology used the WR strain (14). However, this strain is a vaccinia virus laboratory strain with unfavorable properties which preclude its use as a clinical vector for humans. To find a more appropriate parental virus, a number of former vaccine strains were screened for their ability to grow as uracil DNA glycosylase deletion viruses in the rabbit kidney-derived complementing cell line RK-D4R-44.20. The first step was to construct defective viruses based on several former vaccine strains, such as the Lister (Elstree) strain, the Copenhagen strain, and the New York City Board of Health strain, by deleting the essential D4R ORF. For this purpose, the plasmid pDW (15) was transfected into cells that were infected with the vaccinia virus strains. This plasmid contains the D3R and D5R flanking sequences with a precise deletion of the D4R ORF. An unstable marker gene cassette consisting of a gpt/lacZ expression unit framed by a noncoding tandem repeat of 0.45 kb at each side is located between the flanking regions, thus rendering the marker genes unstable in the viral context in the absence of selection pressure (35). Marker-free defective viruses with precise deletions of D4R, growing in complementing cells but not in wild-type CV-1 cells, could be obtained from all vaccine strains after 8 to 10 rounds of plaque purification (see Materials and Methods).
In order to analyze the growth properties of the wild-type and nonreplicating strains in the complementing cell line, RK-D4R-44.20 cells were infected at an MOI of 0.01, cells were harvested after 96 h, and the cell lysates were titrated to determine the number of viruses per infected cell (Table 1). Under these standardized conditions, yields of all tested wild-type vaccine strains were significantly decreased compared to those of the WR laboratory strain. The Lister strain showed, with 54% of the WR yield, the best growth potential on RK-D4R-44.20 cells. Under the chosen conditions, the defective viruses also grew to lower titers, ranging from 46 to 7% of the respective wild-type virus levels (Table 1). The yields of the defective viruses did not significantly differ between the vaccine strains. The decreased yields seen with the defective viruses were due to delayed growth on RK-D4R-44.20 cells infected at a low MOI. Under optimal harvest conditions, defective viruses usually grow to high titers corresponding to yields of 30 to 50 PFU/cell. Due to its importance as a smallpox vaccine and its growth potential, the Lister strain was finally chosen as the parental virus for the dVV platform.
TABLE 1.
Viruses used and yields per cell (RK-D4R-44.20 cells)
| Virus (abbreviation) | Replicationa | Marker | Yield
|
|
|---|---|---|---|---|
| PFU/cell (mean ± SD) | %b | |||
| Wild-type WR | R | —c | 61.7 ± 13.1 | 100 |
| Wild-type Copenhagen | R | — | 14.5 ± 6.2 | 100 |
| Wild-type Lederle | R | — | 14.0 ± 3.3 | 100 |
| Wild-type Lister | R | — | 33.3 ± 7.0 | 100 |
| Defective WR (dVV-WR) | NR | — | 28.3 ± 4.8 | 46 |
| Defective Copenhagen (dVV-Cop) | NR | — | 1.5 ± 0.4 | 10 |
| Defective Lederle (dVV-Led) | NR | — | 2.7 ± 0.9 | 19 |
| Defective Lister (dVV-L) | NR | — | 2.2 ± 0.7 | 7 |
| Defective Lister, p53 insert (dVV-L-p53) | NR | — | 1.5 ± 0.4 | NAd |
| MVA control (MVA-c) | NR | lacZ/gpt | 9.67 ± 1.99 | NA |
| MVA, p53 insert (MVA-p53) | NR | lacZ/gpt | 6.87 ± 0.53 | NA |
R, replicating; NR, nonreplicating.
Percentage of yield of corresponding replicating virus.
—, no insert or marker.
NA, not applicable.
Design and construction of the recombinant viruses.
It was important to determine the efficiency of defective Lister-based recombinant viruses in inducing immune responses and to compare the data with an established nonreplicating reference virus, MVA. We therefore constructed recombinant viruses based on the two strains. The p53 tumor suppressor protein, a possible target antigen for the treatment of cancer, was used as a model antigen (13, 28, 33) in the recombinant viruses. The constructs are outlined in Fig. 1. The unmodified insertion site, the vaccinia virus D4R gene region, is depicted in Fig. 1A. In the empty Lister-based defective virus, termed dVV-L, the D4R ORF is deleted and replaced by a small insert of noncoding DNA (R) (Fig. 1B). In the Lister-based virus carrying the foreign gene, termed dVV-L-p53, the D4R ORF is replaced by the mH5-p53 gene cassette, in which expression is driven by the strong early/late promoter mH5 (45).
In the MVA constructs, the foreign genes were integrated into the intergenic region located between the D4R and the D5R ORFs. Since MVA grows efficiently only in CEFs, the D4R ORF was not deleted and the marker genes lacZ and gpt were retained to allow accurate titration of the viruses. The virus carrying the mH5-p53 gene cassette was termed MVA-p53, and the control construct carrying only the marker gene was termed MVA-c (Fig. 1D and E). The construction of the viruses and the plasmids is described in Materials and Methods. All viruses had the expected genomic structure as characterized by Southern blotting (data not shown).
Expression of the p53 antigen and vaccinia virus vector components by the viral constructs under permissive conditions.
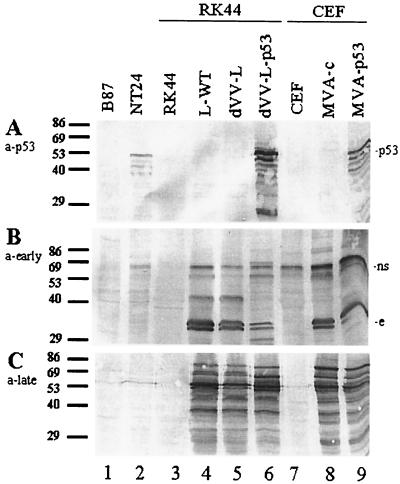
We first analyzed expression of the p53 antigen by the recombinant viruses in the permissive cells by Western blotting using a monoclonal anti-p53 antibody. The dVV-L-p53 virus induced high levels of expression in complementing RK-D4R-44.20 cells (Fig. 2A, lane 6); infection of chicken cells with the MVA-p53 construct also resulted in high expression levels (lane 9), while the lysates of cells infected with the control viruses dVV-L and MVA-c (lanes 5 and 8) and the lysates of the noninfected cells (lanes 3 and 7) did not show this band. The cell line NT24 overexpressing p53 served as a positive control for p53 expression (lane 2), and the p53-negative parental cell line B87 served as the negative control (lane 1).
FIG. 2.
Functional characterization of recombinant viruses under permissive conditions by SDS-PAGE and Western blotting. Cell lysates of RK44 cells or CEFs (equivalent to 105 cells infected at an MOI of 1 for 36 h) were analyzed using a human p53-specific monoclonal antibody (a-p53) (A), an anti-vaccinia virus early protein antiserum (a-early) (B), and an anti-vaccinia virus late protein-specific antiserum (a-late) (C). Lane 1, noninfected B87 cells (p53-negative parental cell line). Lane 2, noninfected p53-positve NT24 cells. Lane 3, noninfected RK44 cells. Lane 4, wild-type Lister (L-WT)-infected RK44 cells. Lane 5, dVV Lister (dVV-L)-infected RK44 cells. Lane 6, dVV-L-p53-infected RK44 cells. Lane 7, noninfected CEFs. Lane 8, MVA control virus (MVA-c)-infected CEFs. Lane 9, MVA-p53-infected CEFs. Numbers at the left are sizes of marker proteins in kilodaltons. ns, non-vaccinia virus-specific band; e, early band. The results are representative of those from three independent experiments.
Next, the expression of the vector components in the cell lysates was examined using vaccinia virus early- and late-specific antisera. The vaccinia virus early antiserum was produced by immunization of chickens with dVV-infected CEFs. Under these conditions, dVV induces only expression of early genes, allowing specific induction of anti-vaccinia virus antibodies directed against early proteins. The late sera were produced by immunization of guinea pigs with purified psoralen-inactivated vaccinia virus (see Materials and Methods). Using the early antiserum, few early-specific bands were observed in all vaccinia virus-infected cell lysates (Fig. 2B, lanes 4 to 6, 8, and 9). A strong non-vaccinia virus-specific cellular band in the 70-kDa range (Fig. 2B, upper part) was also detected, which is strongly upregulated in vaccinia virus-infected CEFs (lanes 8 and 9) and induces antibodies that are cross-reactive with a protein of similar size in CV-1 cells. These bands are likely to be heat shock proteins migrating in this molecular mass range that are induced by vaccinia virus infection (37).
Using the late antiserum, strong late vaccinia virus-specific bands were observed only in the infected cells (Fig. 2C, lanes 4 to 6, 8, and 9), confirming a uniform infection of the different cells. The noninfected cells did not react with the antiserum (lanes 1 to 3 and 7). Under permissive conditions, both viruses express a similar spectrum of antigens.
Expression of the p53 antigen and the vector components under restricted conditions.
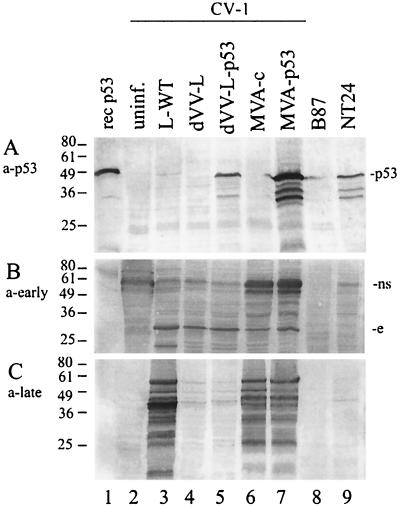
In order to also check expression under restricted conditions, the monkey kidney cell line CV-1, which does not support multiplication of defective viruses and supports MVA only to a limited extent (semipermissive), was infected with the p53 viruses and the controls (Fig. 3A). Purified recombinant p53, derived from E. coli, and a lysate from NT24 cells were included as positive controls (lanes 1 and 9, respectively). Expression of p53 was readily detected in lysates of dVV-L-p53-infected cells (Fig. 3A, lane 5) but not in noninfected, wild-type virus-infected, or dVV-L-infected cells (lanes 2 to 4). Since the Lister construct dVV-L-p53 does not replicate and therefore does not express late genes in CV-1 cells, only the early part of the mH5 early/late promoter contributes to the p53 expression level. Expression is lower than that seen in the MVA-p53 construct (lane 7), which replicates in CV-1 cells to a limited extent (3, 7). Therefore, both the early and late parts of the promoter contribute to the p53 antigen level.
FIG. 3.
Functional characterization of recombinant viruses under conditions nonpermissive for viral replication by SDS-PAGE and Western blotting. Cell lysates of CV-1 cells (equivalent to 105 cells infected at an MOI of 1 for 72 h) were analyzed using a human p53-specific monoclonal antibody (a-p53) (A), an anti-vaccinia virus early protein antiserum (a-early) (B), and an anti-vaccinia virus late protein-specific antiserum (a-late) (C). Lane 1, 100 ng of recombinant (rec) human p53. Lane 2, lysates of uninfected (uninf.) cells. Lane 3, wild-type Lister (L-WT)-infected cells. Lane 4, dVV Lister (dVV-L)-infected cells. Lane 5, dVV-L-p53-infected cells. Lane 6, MVA control virus (MVA-c)-infected cells. Lane 7, MVA-p53-infected cells. Lane 8, p53-negative B87 cells. Lane 9, p53-positive NT24 cells. Numbers at the left are sizes of marker proteins in kilodaltons. ns, nonspecific band; e, early band. The results are representative of those from three independent experiments.
To extend the Western blot analysis, expression of early and late vector antigens under nonpermissive conditions (that mimic the situation in a vaccinee) by the nonreplicating viruses was analyzed with the specific vaccinia virus early and late antisera (see above). To detect the viral antigens, Western blots of lysates of infected cells were incubated with the anti-vaccinia virus sera (Fig. 3B and C). Using the early antiserum, a strong vaccinia virus-specific band in the 30-kDa range was seen in the lower part of the blot for all vaccinia virus-infected cells (Fig. 3B, lanes 3 to 7). This immunodominant early protein may correspond to a strong early band of similar size seen after radiolabeling of nonpermissive cells with defective virus (14). Again, the strong non-vaccinia virus-specific cellular band in the 70-kDa range (Fig. 3B, upper part) was also detected in this cell line.
As expected for uracil DNA glycosylase-defective viruses, late proteins could not be detected in cells infected with the dVV-L-p53 and dVV-L constructs (Fig. 3C, lanes 4 and 5), reflecting the genetic block after early protein synthesis (14). In contrast, late antigens were detected with the two MVA constructs (Fig. 3C, lanes 6 and 7), consistent with the block of MVA in morphogenesis in mammalian cells (39). The wild-type-Lister-infected cells also showed a strong reaction with the late antiserum (lane 3), while the lysates of the noninfected cells (lanes 2, 8, and 9) did not react. This characterization confirmed the expected expression patterns induced by the different viruses.
Induction of humoral immune responses.
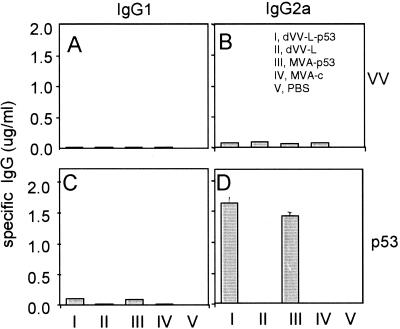
To compare the humoral immune responses induced by dVVs and MVA-based viruses, BALB/c mice were immunized once intramuscularly with 106 PFU of dVV-L-p53, MVA-p53, and the corresponding control viruses. The absolute amounts of p53- and virion-specific antibodies in the sera were subsequently determined by IgG1, IgG2a, IgG2b, and IgG3 isotype-specific ELISAs. Antibodies specific for the virion were detected in small amounts in the sera of mice immunized with all four vectors but not in the PBS control. The isotype that was mainly induced was IgG2a (Fig. 4B). No significant amounts of virion-specific antibodies of the IgG1, IgG2b, and IgG3 isotypes (all <30 ng/ml) were found.
FIG. 4.
The nonreplicating viruses induce an efficient humoral immune response of the Th1 phenotype against the recombinant p53 antigen. Mice were immunized once intramuscularly with 106 PFU of the indicated viruses. The vaccinia virion (VV) and the p53-specific antibodies were determined by analysis of a series of serum dilutions in ELISAs using IgG1- and IgG2a-specific antisera for detection. The absolute amounts were determined by plotting against an isotype-specific standard curve. Error bars represent standard deviations. The results are representative of those from three independent experiments.
When tested for p53 specificity, the sera of mice immunized with the control constructs or PBS did not react (<30 ng/ml). In contrast, MVA-p53 induced antibodies of all isotypes (Fig. 4, bars III). Even larger amounts of p53-specific antibodies of all isotypes were detected in the sera of dVV-L-p53-immunized mice (Fig. 4C and D). The sera of both dVV- and MVA-immunized mice showed the classical picture of a cell-mediated antibody profile. The most prominent isotype was IgG2a, which was approximately 15-, 3- to 7-, and 24-fold more abundant than IgG1, IgG2b, and IgG3, respectively. In addition, the p53-specific antibodies were in both cases approximately 10-fold more abundant than the virion-specific antibodies, indicating that the main humoral immune response is directed against the expressed foreign gene driven by the strong mH5 promoter. This result therefore demonstrates that both viral systems induce an efficient antibody response predominantly against the recombinant p53 antigen. Antibodies against virion components were substantially lower with all constructs, and higher levels of virion-specific antibodies require boosting. Since isotype switching took place and the antibody profile of a cell-mediated immune response was observed, this indicates that both vector systems also prime cellular components of the immune system.
Induction of cellular immune responses.
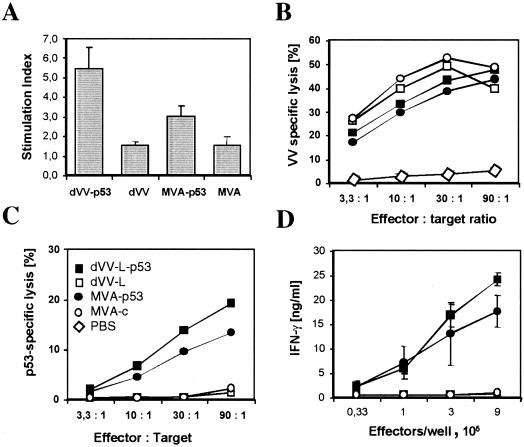
To test for the induction of cellular immunity, we immunized BALB/c mice with 106 PFU of vaccinia virus intravenously. Using T-cell-enriched splenocytes from immunized mice, p53-specific proliferation was assessed with purified recombinant p53 (see also Fig. 3A, lane 1) for restimulation (see Materials and Methods). As shown in Fig. 5A, no significant proliferation was found when the control constructs dVV-L and MVA-c were used for immunization. The construct MVA-p53 induced only a weak proliferation, whereas dVV-L-p53 showed a statistically significant proliferation with a stimulation index of 5.5. Therefore, both p53-expressing viruses prime T cells, with dVV-L-p53 being more efficient.
FIG. 5.
dVVs induce an efficient cellular immune response against p53 and vaccinia virus components. Mice were immunized once intramuscularly (A) or intravenously (B to D) with 106 PFU of dVV-L-p53, dVV-L, MVA-p53, and MVA-c or PBS as indicated in panel C and used for the tests. All results shown are representative of those from at least three independent experiments. (A) The p53-specific proliferation of B-cell-depleted splenocyte suspensions was determined upon restimulation with recombinant human p53 (1 μg/ml), and the stimulation index was determined. Error bars represent the standard errors of the means. (B) Induction of vaccinia virus-specific CTLs. Upon restimulation of a splenocyte suspension with vaccinia virus (MOI, 0.05), lysis of infected target cells was determined. The observed specific lysis with respect to the effector-to-target cell ratio is shown. Specific lysis of <3% was observed for noninfected B87 target cells. (C) Induction of p53-specific CTLs. The observed specific lysis, with respect to the effector-to-target cell ratio, is shown. Specific lysis of <3% was observed for p53-negative B87 target cells. (D) Induction of p53-specific IFN-γ secretion upon restimulation of splenocytes with recombinant p53-expressing vaccinia virus vp53. NT24 cells were added at the concentrations indicated, and IFN secretion was determined by ELISA. Absolute amounts were subsequently determined by comparison to an IFN standard curve. The amount of IFN-γ secreted upon restimulation with p53-negative B87 cells was <2 ng/ml for all cell concentrations.
A special feature of viral vectors is the priming of CD8+ T cells and the induction of CTLs. To assess the ability of the dVVs to induce CTLs, BALB/c mice were immunized intravenously with 106 PFU of the viruses. As shown in Fig. 5B, high levels of vaccinia virus-specific target cell lysis were observed for all four constructs. No lysis was observed for PBS-immunized mice or on noninfected targets. Therefore, not only MVA-based viruses but also the dVV Lister constructs efficiently induce vaccinia virus-specific CTLs upon immunization.
Next, we tested for p53-specific lysis by using the p53-expressing murine cell line NT24 as a target; this cell line expresses high levels of human p53 (Fig. 1B, lane 9). As shown in Fig. 5C, specific lysis was observed only after immunization with the p53-expressing viruses. No lysis was detected with the control vectors and NT24 cells or the p53-negative murine cell line B87 as targets. Therefore, as observed for antigen-specific CTL induction, the dVV Lister system proved to be as efficient as the MVA system.
To further substantiate that both systems are able to prime p53-specific CD8+ T cells, we also tested for p53-specific IFN-γ secretion in cultures stimulated with NT24 cells. IFN-γ is the main cytokine secreted by activated CD8+ T cells. As shown in Fig. 5D, both p53-expressing viruses induced T cells with the ability to secrete IFN-γ as measured by restimulation with NT24 cells. No IFN-γ secretion was observed using the control vectors dVV-L and MVA-c upon restimulation with NT24 cells or for all constructs using B87 cells (9 × 105 effector cells/well, <3 ng/ml).
In conclusion, these results demonstrate that defective viruses based on the Lister strain can induce cellular immune responses, including the priming of CTLs, to a similar extent as MVA-based viruses.
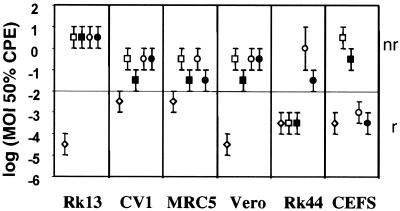
Induction of CPE in permissive and nonpermissive cell lines.
One of the most important aspects in the development of viral vectors for clinical use is safety. Due to their inability to replicate, dVVs should be better tolerated than the respective wild-type viruses. Since MVA is replication deficient in most cell lines, it was expected that dVVs and MVA would show similar CPE induction and have similar safety profiles. To test this assumption, a variety of vaccinia virus-susceptible cell lines (RK13, CV-1, MRC5, Vero, RK-D4R-44.20, and CEFs) were infected with the p53-expressing viruses and the controls at MOIs ranging from 101 to 10−6, grown for 6 days, and monitored daily for CPE such as cytoplasmic contraction and cell rounding. As expected, replicating wild-type Lister was the most aggressive strain (Fig. 6). All cell lines were lysed at MOIs of <10−2. In contrast, the dVVs showed lysis at an MOI of <10−2 only on the complementing cell line (Fig. 6, Rk44) and not on any of the other cell lines tested. The MVA viruses lysed only the chicken production cell line and none of the others (Fig. 6). In nonpermissive cell lines, both dVVs and MVA viruses induced CPE at similar MOIs, mainly in a range between 1 and 0.1, reflecting the fact that although they are replication deficient, these live viruses still infect the target cell and turn on viral protein synthesis, which finally leads to the death of the infected cell. The recombinant p53-expressing viruses induced CPE in almost every cell line at concentrations 1 order of magnitude lower. A likely explanation is that overexpression of the p53 gene additionally induces apoptosis, as was seen for replicating vaccinia viruses (42). In summary, the nonreplicating viruses were less virulent in cell culture, inducing a CPE at doses 5 orders of magnitude higher than those of the wild-type strain, and should therefore be safer in animals.
FIG. 6.
Induction of CPE by the different viruses in cell lines under permissive (replicating [r]) and nonpermissive (nonreplicating [nr]) conditions. The cell lines RK13, CV-1, MRC5, Vero, and RK-D4R-44.20 (Rk44) and CEFs were infected with 10-fold dilutions of the viruses (wild-type Lister [⋄], dVV-L-p53 [▪], dVV-L [□], MVA-p53 [•], and MVA-c [○]) at MOIs ranging from 10 to 10−6. The dilution with the lowest MOI where at least 50% CPE in a well occurred was determined. The indicated intervals represent the range of MOIs where at least 50% CPE was observed. The results represent those from three independent experiments.
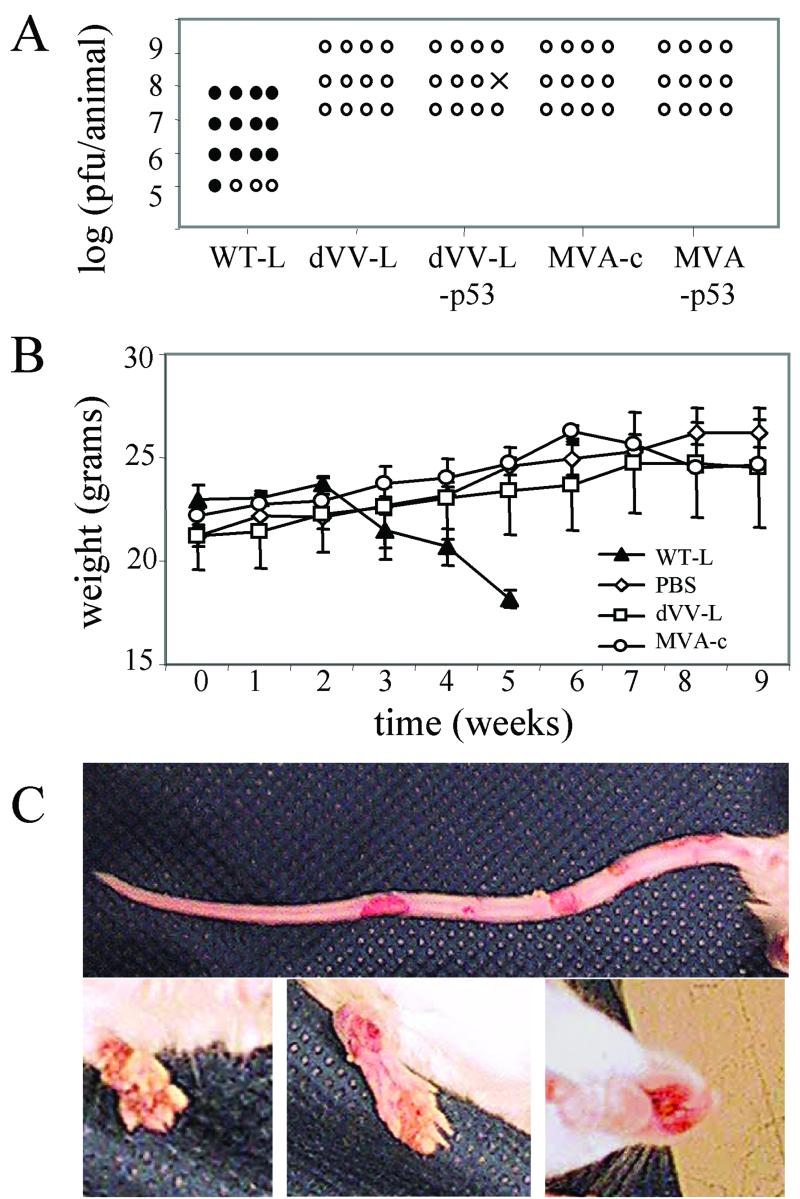
Safety in immunodeficient mice.
The main concern about the use of replication-competent viruses in immunotherapy is that severe adverse effects may occur in immunocompromised patients. To more thoroughly address the safety question in an in vivo model, groups of four immunodeficient BALB/c/SCID mice were challenged subcutaneously with high doses of the wild-type Lister strain or the nonreplicating Lister- or MVA-based vectors (Fig. 7). Using the wild-type Lister virus, doses of ≥106 PFU led to a progressive vaccinia virus infection within a 2-month observation period. At 4 to 8 weeks after challenge, the mice showed typical blister formation on the tail and footpad (Fig. 7C). Later, weight loss, sometimes accompanied by swelling of the mouth region, led to the death of the mice. While mice receiving the nonreplicating viruses gained weight over time similarly to those receiving the PBS control, mice challenged with the replicating virus started to lose weight after 2 weeks (Fig. 7B). Since no mouse recovered after the challenge with replicating Lister virus, the animals were sacrificed when they reached a critical weight of 15 g. When the observation period was extended to 6 months, doses of replicating Lister virus of 105 PFU also led to progressive vaccinia disease and death.
FIG. 7.
dVV vectors are safe in immunodeficient animals. (A) BALB/c/SCID mice were challenged with the indicated doses of wild-type Lister (WT-L), defective Lister (dVV-L), recombinant defective Lister expressing p53 (dVV-L-p53), MVA control (MVA-c), and recombinant MVA expressing p53 (MVA-p53) and monitored for signs of a progressive vaccinia virus infection. Results for healthy mice (open circles) and mice which showed signs of disease (closed circles) during a time period of 2 months are shown; one mouse died due to an unrelated event (×). The results represent those from two independent experiments. (B) Weight monitoring of BALB/c/SCID mice infected with the indicated viruses. After 2 weeks, wild-type virus (WT-L)-infected mice (106 PFU/animal) started to lose weight and die, while mice infected with the nonreplicating viruses (dVV-L or MVA-c at a dose of 109 PFU/animal) grew normally and gained weight over time, similar to the PBS control mice. (C) Typical symptoms of generalized vaccinia disease in SCID mice infected with the wild-type Lister vaccinia virus. Cutaneous lesions are seen on the tail (upper panel) and on the paws and the mouth region (lower panels).
In contrast to the replicating Lister strain, the MVA viruses were tolerated well. No signs of disease were observed over the observation period of 6 months with doses of as high as 109 PFU. Notably, no wild-type MVA was used in these experiments; the MVA control is a recombinant virus with a lacZ/gpt marker gene inserted into the D4R-D5R intergenic region. Insertion into this region has a slight attenuating effect, resulting in diminished growth compared to the wild-type virus.
Importantly, the defective Lister strain-based viruses also did not induce any signs of progressive disease. Both types of nonreplicating viruses were tolerated without any visible signs of discomfort of the mice at doses of 107 and 108 PFU. The highest dose of 109 PFU was accompanied by mild signs of sickness in the first few days, which disappeared later. At 4 weeks after challenge a lesion was observed at the injection site, which subsequently healed. In summary, not only in the in vitro system but also in vivo in immunodeficient animals, dVVs based on the Lister strain are as well tolerated as the MVA-based viruses.
DISCUSSION
The advantage of using defective viruses is that viral replication occurs only in a permanent complementing cell line. If used as vaccines, these vectors will retain their infectivity and the ability to synthesize viral early proteins but will fail to complete the replication cycle, thereby eliminating the risk of a disseminated or progressive vaccinia virus infection in an immunocompromised host. In addition, the risk of environmental spread of the virus is eliminated. This may occur, for example, when avipoxviruses are used to vaccinate nonpermissive hosts (41). There are also several practical advantages of the defective virus technology platform. During generation of the vectors, recombinations and titrations can be performed on a fast-growing stable cell line and thereby the cumbersome preparation of CEFs necessary for the other nonreplicating poxviral systems can be avoided. Furthermore, the formation of lytic plaques is far more pronounced on RK-D4R-44.20 cells than for MVA-based viruses on CEFs, thereby facilitating the screening especially of marker-free recombinants. Third, we showed that a variety of vaccinia virus strains can be made defective by deletion of the uracil DNA glycosylase gene. Since vaccinia virus strains can show extensive genomic differences, this feature allows rapid construction of additional defective strains, broadening the basis for future gene delivery vectors with improved properties.
MVA can still grow in BHK cells (3, 7) and regains full growth potential in Vero cells when passaged in this cell line (19). This is surprising, because the large deletions characteristic for MVA (2, 24) seemed to suggest that the restriction in host range and virulence was mainly due to these deletions, including the loss of a host range gene and many immune modulatory genes. The numerous single-nucleotide differences found between the MVA sequence and the Copenhagen sequence (2) suggest that regain of function (replication in a specific host) is also due to reversions on the nucleotide level. It will be interesting to see whether an MVA strain first adapted to growth in mammalian cells and then passaged in mouse brain also regains virulence. In contrast to MVA, dVVs with an essential gene deleted cannot regain replication and virulence functions upon passaging in a chosen host. Therefore, the defective genotype principally excludes safety risks in severely immunocompromised vaccinated subjects.
Using in vitro and in vivo safety assays, we showed that defective viruses are safe live viral vectors. The wild-type Lister vaccine strain induced a progressive vaccinia virus infection in immunodeficient mice. This disease could completely be avoided by the introduction of the defective phenotype. The safety of the vector was increased by at least 4 orders of magnitude, suggesting that, similar to the case for MVA, it should also be well tolerated in immunocompromised hosts. The only noticeable adverse effect was toxicity at the site of injection at the highest dose, which was also seen with MVA. Therefore, these results suggest that the defective viruses can be used safely in immunocompromised patients, a precondition important for potential application in the immunotherapy of cancer and chronic diseases.
The immune response induced by MVA is well characterized; this virus induces a Th1 type of response with low antibody titers against the viral vector (31). Here we show that the immunity induced by dVVs is similar to that induced by MVA vectors. The defective viruses induced a humoral immunity with the phenotype of a cell-mediated immune response. The vectors also efficiently primed CTL responses. Since MVA was formerly used for primary smallpox vaccinations even of immunocompromised patients, these results, together with the excellent safety profile, suggest that the defective Lister viruses may be used for the same applications.
Currently, recombinant MVA-based constructs are developed as vaccines for a variety of diseases. Progress has been made towards the clinical assessment of MVA-based vectors in the treatment of HIV infections, malaria, measles, or cancer, either alone or in combination with DNA vaccines (1, 6, 36, 43). Here we show that a recombinant p53-expressing defective virus, dVV-p53, induced significant levels of humoral and cellular immune responses against p53. The levels of immunity induced were comparable to or even slightly better than those induced by the MVA-based constructs, indicating that the dVV Lister system is an excellent alternative to MVA or to other nonreplicating poxviral vectors. One important advantage of the defective Lister system is the possibility of producing sufficient amounts of the virus in a permanent stable cell line. Virus production in permanent cells allows cell banking and rigorous quality control, increasing product safety. The product safety aspect is especially important for the production of live vaccines, where no final virus inactivation steps are possible. In contrast, biologicals produced in primary cell cultures, such as chicken fibroblasts, may be contaminated by adventitious viruses such as retroviruses.
Since permanent cell lines can be grown in large fermentors, upscaling is easier and more cost-effective than production in primary cells. The complementing cell line, for instance, grows in high cell densities in microcarrier cultures, and upscaled batches of defective viruses of up to 50 liters in volume have been produced (M. Reiter, unpublished data). A defective Lister-based immunotherapy vector, having vector properties similar to those of a corresponding MVA construct, has the advantage of easy upscaling due to state-of-the-art production possibilities and superior product safety. Therefore, the dVV Lister technology platform fulfills the most important aspects for clinical use as a live vaccine vector.
An exciting new perspective is the development of safer smallpox vaccines based on nonreplicating vaccinia virus vectors. As shown decades ago, prevaccination of patients with a highly attenuated vaccinia virus strain followed by vaccination with a standard vaccine strain reduces side effects dramatically (17). The approach resulted, however, in reduced titers of neutralizing antibodies (21), probably trading safety for efficacy. Prevaccination was used in the last years of smallpox eradication in Germany with MVA strain as prevaccine and the Lister/Elstree strain as the standard vaccination strain (20, 38). The dVV system might be a more suitable smallpox prevaccine for immunocompromised subjects. It was highly protective in a preclinical challenge model (16), induced antibodies and CTLs similarly to MVA, and was as safe as MVA-based recombinants in immunodeficient mice. In addition, reversion to virulence can principally be excluded because the vector lacks an essential gene, which restricts its host range to a complementing cell line. Nevertheless, a prevaccination regimen involving the currently used replicating strains in combination with a nonreplicating prevaccine may still have severe consequences in HIV-infected or otherwise severely immunocompromised persons (32). Therefore, strategies to enhance the levels of neutralizing antibodies, using, for instance, nonreplicating vaccinia virus strains twice or in combination with cross-protective viral antigens, should be further explored to develop a safe smallpox vaccine.
Acknowledgments
We thank R. Drillien and J. Esposito for the Copenhagen strains, A. Levine for the plasmid pC53-SN3 and the cell lines 10(3)Tx4BT87 and 10(3)273.1NT24, G. Antoine for sequencing of plasmids, and I. Livey and G. Holzer for critically reading the manuscript. We thank also N. Naetar, C. Egger, K. Schmid, and J. Mayrhofer for expert technical assistance and P. Kogoj for photographs.
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 3.Carroll, M. W., and B. Moss. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238:198-211. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, W. I., J. Tartaglia, and E. Paoletti. 1992. Poxvirus recombinants as live vaccines, p. 123-162. In M. M. Binns and G. L. Smith (ed.), Recombinant poxviruses. CRC Press, Boca Raton, Fla.
- 6.Drexler, I., E. Antunes, M. Schmitz, T. Wolfel, C. Huber, V. Erfle, P. Rieber, M. Theobald, and G. Sutter. 1999. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 59:4955-4963. [PubMed] [Google Scholar]
- 7.Drexler, I., K. Heller, B. Wahren, V. Erfle, and G. Sutter. 1998. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 79:347-352. [DOI] [PubMed] [Google Scholar]
- 8.Falkner, F. G., and B. Moss. 1988. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J. Virol. 62:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkner, F. G., and B. Moss. 1990. Transient dominant selection of recombinant vaccinia viruses. J. Virol. 64:3108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson, D. A., T. V. Inglesby, J. G. Bartlett, M. S. Ascher, E. Eitzen, P. B. Jahrling, J. Hauer, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. Perl, P. K. Russell, K. Tonat, et al. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127-2137. [DOI] [PubMed] [Google Scholar]
- 11.Henderson, D. A., and B. Moss. 1999. Smallpox and vaccinia, p. 74-97. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines. W. B. Saunders, Philadelphia, Pa.
- 12.Hill, A. V., J. Elvin, A. C. Willis, M. Aidoo, C. E. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, A. R. Townsen, et al. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360:434-439. [DOI] [PubMed] [Google Scholar]
- 13.Hollstein, M., D. Sidransky, B. Vogelstein, and C. C. Harris. 1991. p53 mutations in human cancers. Science 253:49-53. [DOI] [PubMed] [Google Scholar]
- 14.Holzer, G. W., and F. G. Falkner. 1997. Construction of a vaccinia virus deficient in the essential DNA repair enzyme uracil DNA glycosylase by a complementing cell line. J. Virol. 71:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzer, G. W., W. Gritschenberger, J. A. Mayrhofer, V. Wieser, F. Dorner, and F. G. Falkner. 1998. Dominant host range selection of vaccinia recombinants by rescue of an essential gene. Virology 249:160-166. [DOI] [PubMed] [Google Scholar]
- 16.Holzer, G. W., G. Remp, G. Antoine, M. Pfleiderer, O. M. Enzersberger, W. Emsenhuber, T. Hammerle, F. Gruber, C. Urban, F. G. Falkner, and F. Dorner. 1999. Highly efficient induction of protective immunity by a vaccinia virus vector defective in late gene expression. J. Virol. 73:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempe, C. H., V. Fulginiti, M. Minamitani, and H. Shinefield. 1968. Smallpox vaccination of eczema patients with a strain of attenuated live vaccinia (CVI-78). Pediatrics 42:980-985. [PubMed] [Google Scholar]
- 18.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayr, A. 2001. Altered strain of the modified vaccinia virus Ankara (MVA). WO 01/68820 A1. World Intellectual Property Organization, Geneva, Switzerland.
- 20.Mayr, A., H. Stickl, H. K. Müller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentbl. Bakteriol. Hyg. Abt. 1 Orig. B 167:375-390. [PubMed] [Google Scholar]
- 21.McIntosh, K., J. D. Cherry, A. S. Benenson, J. D. Connor, D. W. Alling, U. T. Rolfe, W. A. Todd, J. E. Schanberger, and M. J. Mattheis. 1977. Clinical and serologic study of four smallpox vaccines comparing variations of dose and route of administration. Standard percutaneous revaccination of children who receive primary percutaneous vaccination. J. Infect. Dis. 135:155-166. [DOI] [PubMed] [Google Scholar]
- 22.McKinney, D. M., R. Skvoretz, M. Qin, G. Ishioka, and A. Sette. 2000. Characterization of an in situ IFN-gamma ELISA assay which is able to detect specific peptide responses from freshly isolated splenocytes induced by DNA minigene immunization. J. Immunol. Methods 237:105-117. [DOI] [PubMed] [Google Scholar]
- 23.Melief, C. J., R. E. Toes, J. P. Medema, S. H. van der Burg, F. Ossendorp, and R. Offringa. 2000. Strategies for immunotherapy of cancer. Adv. Immunol. 75:235-282. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 25.Midgley, C. A., C. J. Fisher, J. Bartek, B. Vojtesek, D. Lane, and D. M. Barnes. 1992. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli. J. Cell Sci. 101:183-189. [DOI] [PubMed] [Google Scholar]
- 26.Mondelli, M. U., F. Bortolotti, P. Pontisso, E. G. Rondanelli, R. Williams, G. Realdi, A. Alberti, and A. L. Eddleston. 1987. Definition of hepatitis B virus (HBV)-specific target antigens recognized by cytotoxic T cells in acute HBV infection. Clin. Exp. Immunol. 68:242-250. [PMC free article] [PubMed] [Google Scholar]
- 27.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Offringa, R., M. P. Vierboom, S. H. van der Burg, L. Erdile, and C. J. Melief. 2000. p53: a potential target antigen for immunotherapy of cancer. Ann. N.Y. Acad. Sci. 910:223-233. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti, E., J. Tartaglia, and J. Taylor. 1994. Safe and effective poxvirus vectors—NYVAC and ALVAC. Dev. Biol. Stand. 82:65-69. [PubMed] [Google Scholar]
- 30.Penna, A., P. Fowler, A. Bertoletti, S. Guilhot, B. Moss, R. F. Margolskee, A. Cavalli, A. Valli, F. Fiaccadori, F. V. Chisari, et al. 1992. Hepatitis B virus (HBV)-specific cytotoxic T-cell (CTL) response in humans: characterization of HLA class II-restricted CTLs that recognize endogenously synthesized HBV envelope antigens. J. Virol. 66:1193-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez, J. C., M. M. Gherardi, and M. Esteban. 2000. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J. Virol. 74:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673-676. [DOI] [PubMed] [Google Scholar]
- 33.Roth, J., D. Dittmer, D. Rea, J. Tartaglia, E. Paoletti, and A. J. Levine. 1996. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc. Natl. Acad. Sci. USA 93:4781-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Scheiflinger, F., F. Dorner, and F. G. Falkner. 1998. Transient marker stabilisation: a general procedure to construct marker-free recombinant vaccinia virus. Arch. Virol. 143:467-474. [DOI] [PubMed] [Google Scholar]
- 36.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 37.Sedger, L., and J. Ruby. 1994. Heat shock response to vaccinia virus infection. J. Virol. 68:4685-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stickl, H., V. Hochstein-Mintzel, A. Mayr, H. C. Huber, H. Schäfer, and A. Holzner. 1974. MVA-Stufenimpfung gegen Pocken. Dtsch. Med. Wschr. 99:2386-2392. [DOI] [PubMed] [Google Scholar]
- 39.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davis, J. van der Hoeven, B. Meignier, M. Riviere, et al. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217-232. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, J., and E. Paoletti. 1988. Fowlpox virus as a vector in non-avian species. Vaccine 6:466-468. [DOI] [PubMed] [Google Scholar]
- 42.Timiryasova, T. M., B. Chen, P. Haghighat, and I. Fodor. 1999. Vaccinia virus-mediated expression of wild-type p53 suppresses glioma cell growth and induces apoptosis. Int. J. Oncol. 14:845-854. [DOI] [PubMed] [Google Scholar]
- 43.Weidinger, G., M. Ohlmann, B. Schlereth, G. Sutter, and S. Niewiesk. 2001. Vaccination with recombinant modified vaccinia virus Ankara protects against measles virus infection in the mouse and cotton rat model. Vaccine 19:2764-2768. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1998. Requirements for the use of animal cells as in vitro substrates for the production of biologicals. Technical Report Series, no. 878. World Health Organization, Geneva, Switzerland.
- 45.Wyatt, L. S., S. T. Shors, B. R. Murphy, and B. Moss. 1996. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine 14:1451-1458. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel, R. M., and H. Hengartner. 1994. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol. Today 15:262-268. [DOI] [PubMed] [Google Scholar]