Abstract
Methylphenidate (MPH) is the most commonly used drug to treat attention deficit/hyperactivity disorder (ADHD) in children effectively and safely. In spite of its widespread application throughout one of the most plastic and sensitive phases of brain development, very little is known to date about its long-term effects on brain structure and function. Hence, this short review updates the influence of MPH on brain development, since recent human and animal studies suggest that MPH alters the dopaminergic system with long-term effects beyond the termination of treatment.
Animal studies imply that the effects of MPH may depend on the neural responder system: Whereas structural and functional parameters are improved by MPH in animals with psychomotor impairments, they remain unaltered or get worse in healthy controls. While recent behavioural studies do not fully support such a differential effect of MPH in ADHD, the animal studies certainly prompt for further investigation of this issue. Furthermore, the abuse of MPH, when (rarely) intravenously applied, may even impair the maturation of dopaminergic fibres in subcortical brain areas. This argues for careful clinical assessment and diagnostics of ADHD symptomatology not only in conjunction with the prescription of MPH. Hence, one should be assured that MPH is only given to children with clear ADHD symptomatology leading to psychosocial impairment. The animal data suggest that under these conditions MPH is supportive for brain development and the related behaviour in children with ADHD.
Background and rationale
Attention deficit/hyperactivity disorder (ADHD) is one of the most common behavioural disorders in childhood and may persist into adulthood. According to conservative estimates, its prevalence is around 3–5% [1]. Including subclinical cases with less stringent criteria used the percentage may rise up to 17% [2]. Although the aetiology of the disorder is not yet fully understood, a high heritability with vulnerability genes as basis seems to explain most of the behavioural variance, although obstetric complications and psychosocial adversity may play a role, too [see recent reviews by [1,3,4]]. In the last years, models of the neurobiological background of ADHD have become both more substantial and more complicated by a wealth of studies which showed that several, rather than one or a few, neuronal systems are likely to be involved, and that circuits contributing to motor regulation, executive functions, attention and delay of reinforcement, i.a., may be impaired in ADHD patients [rev. in [1,4,5]]. Animal models have played an important role in gathering this knowledge [rev. in [6,7]].
Methylphenidate (MPH) is the most widely used drug and the golden standard to treat ADHD [rev. [1,8]]. Its efficacy and safety has been documented in many studies [9] However, there is still a gap of knowledge concerning the influence of MPH on brain development and its long-term effect on brain structure and function.
Childhood and adolescence are a highly plastic and sensitive period of brain maturation, during which environmental and pharmacological influences exert strong effects on neural structure and function [see [10,11] for rev.]. Especially, cognitive, motivational and emotional functions mature intensively during this period of life. Such functions are subserved by brain areas that are characterised by a selective innervation of dopamine (DA), i.e. the prefrontal cortex (PFC), nucleus accumbens (NAc) and amygdala. The DAergic innervation of these areas matures late and passes through a phase of drastic anatomical and physiological upheaval during periadolescence [11-13]. Thus, MPH, considered to act as a DA agonist by blocking the DA and, to a weaker extent, noradrenaline transporters [14-16] might influence this process. Although no neurotoxic action of MPH has been reported so far [17-19], it is quite likely that pharmacological interference with the maturing DA system may lastingly change the developmental outcome [8,20,21].
Unfortunately, to date, there exist only few studies investigating the long-term plastic neuronal effects of MPH. But it is known that neurotransmitters and their agonists exert a strong morphogenetic influence on single neurons and nervous tissues [22-26], and even small environmental events can lastingly shape the brain if applied over a longer period [27-31](Lehmann, Grund et al., unpublished observations). Indeed, some studies have already shown that early treatment with clinical doses of MPH persistently changes DAergic parameters in rodents [20,32-34]. We therefore dedicate this mini-review to the behavioural and neurobiological long-term effects of MPH in humans and experimental animals.
Dopamine function and dysfunction in ADHD
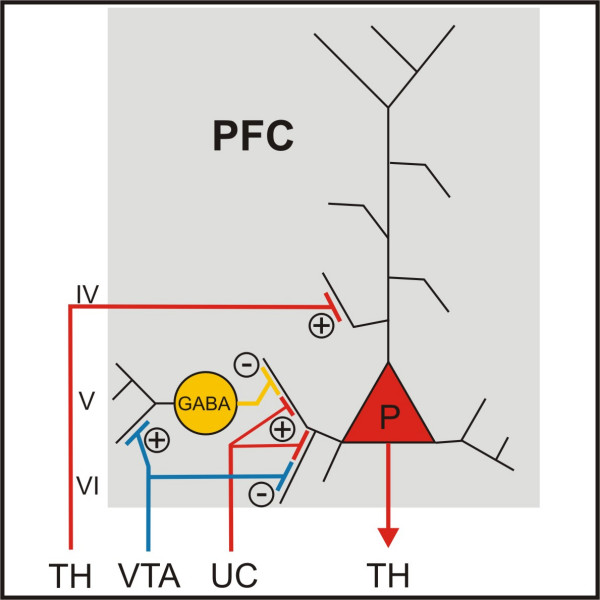
As an indirect DA agonist, MPH presumably enhances DAergic transmission in the very same brain areas that play such an important role for cognition and emotion, and two of them – the PFC and the NAc – are considered to be principally involved in the aetiology of ADHD. Genetic research and in vivo imaging observations have put the focus on DA dysfunction in ADHD by documenting increased dopa decarboxylase activity in the midbrain [35], decreased sensitivity of the DA receptor type 4 and increased density of the DA transporter (DAT) in the striatum/NAc [36-42]. In the PFC, there is a reduced DA storage in ADHD patients [43], and it has been shown that MPH increases the extracellular DA concentration in the PFC [44,45]. This cannot, however, be achieved in a straightforward way, since neither DAT nor D2 receptors are present in detectable or even sufficient amounts in the PFC [46-49]. Instead, it has been shown that MPH blocks not only the DAT, but also the noradrenaline transporter (NAT) [15], and that DA is cleared by the NAT in the PFC [50]. Since DA serves as a switch between cortical input into the PFC (with low DA transmission) and thalamic input (with high DA transmission, fig. 1), the functional consequence will be a behaviour that is more driven by information coming from non-cortical regions, rather than by intrinsic cortical information [109,110].
Figure 1.
Neuronal connections of Prefrontal Cortex. Dopamine (DA) fibres arising in the ventral tegmental area terminate on GABAergic interneurons and glutamatergic pyramidal cells in the PFC. DA serves as a switch between cortical (with low DA transmission) and thalamic input (with high DA transmission) [117,118]. +-signs signify an excitation and – signs signify an inhibition. IV, V and VI = layer IV, V and VI; P = pyramidal cell; PFC = prefrontal cortex; TH = thalamus; UC = u-shaped cortical connections; VTA = ventral tegmental area.
There is an ongoing debate on the DAergic pathology of the NAc in ADHD (fig. 2). Both a lack and an excess of DA transmission seem to be supported by the available experimental evidence. The higher DAT density in ADHD patients, which should improve DA clearance from the synaptic cleft [28-30], the action of MPH as an indirect DA agonist, and imaging data demonstrating increased extracellular DA concentrations in the striata of healthy controls after MPH treatment [51], all argue for a reduced striatal DAergic transmission in ADHD. The opposing view assuming an accumbal DA hyperfunction in ADHD, in contrast, maintains that DAT density may be regarded as a measure of DA fibre density [31-34]. It further proposes the fact that there are two different kinds of DA transmission in the striatum [52]: Firing of DA neurons leads to a phasic release of DA in relatively high concentrations. The transmitter is cleared by the very effective DAT, so only very low concentrations of DA remain in the extracellular space. This tonic transmission is, however, still strong enough to activate autosynaptic D2 receptors which inhibit phasic DA firing. By blocking the DAT, MPH may increase the tonic extracellular DA concentrations and thus decrease the phasic transmission [53]. The observation that DA antagonists increase the positive effect of MPH on motor behaviour [54,55], but prevent its enhancement of cognitive capacities [56], further supports this hypothesis. In this view, the two impairments in PFC and NAc are probably even causally related, since alterations of DA metabolism in the PFC reciprocally change the DA activity in the striatum [57-62].
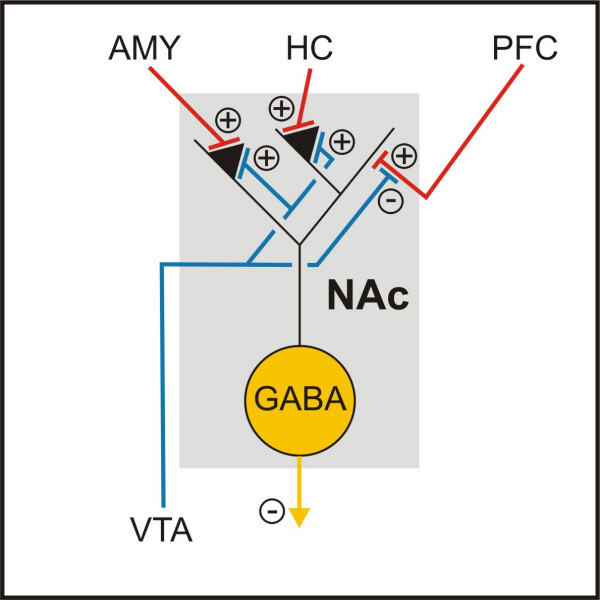
Figure 2.
Neuronal connections of Nucleus Accumbens. Glutamatergic afferences from the prefrontal cortex, hippocampus and amygdala terminate on GABAergic medium spiny neurons and are modulated pre- and postsynaptically by dopamine. The glutamatergic input from the hippocampus and the amygdala drives the medium spiny neurons into a depolarized state and the input from the prefrontal cortex is capable of triggering action potentials [119]. AMY = amygdala; HC = hippocampus; PFC = prefrontal cortex; NAc = nucleus accumbens; VTA = ventral tegmental area.
The DA projection to the amygdala matures in close coordination with that of the PFC, such that DA hypoinnervation of the PFC goes along with DA hyperinnervation of the amygdala (and entorhinal cortex) after early trauma [63]. Furthermore, the amygdala receives a strong projection from the PFC [64] which serves to put reflexive fear reactions under cognitive control (fig. 3) [65-67]. Although these neuronal effects after early trauma in gerbils should be considered only as a partial model of ADHD, it might be fruitful for further reasoning to remember that a high frequency of associated emotional problems has been reported in ADHD patients [9], but very little is known about DA function of the amygdala and its modifications by MPH in these cases.
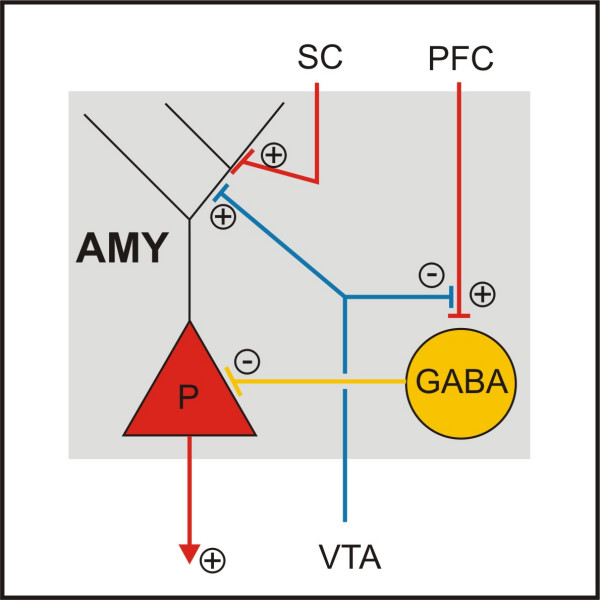
Figure 3.
Neuronal connections of Amygdala. Glutamatergic afferents from the prefrontal cortex (PFC) contact GABAergic interneurons in the basolateral amygdala, which then inhibit the firing of pyramidal cells, while afferents from sensory cortices terminate mainly on glutamatergic pyramidal cells. The PFC input is suppressed presynaptically, whereas the sensory cortical input is enhanced postsynaptically by DA [67,120]. AMY = amygdala; P = pyramidal cell; PFC = prefrontal cortex; VTA = ventral tegmental area, SC = sensory cortices.
Therapeutic effects of methylphenidate
The most commonly used genetic rodent model of ADHD is the spontaneously hypertensive rat (SHR) [rev. in [68-70]]. In this model, reduced DA transmission was found in the PFC and striatum [7,71]. In the NAc, D1 receptor densities were increased, while D2 receptor densities were lowered [32,72-74] – which is in line with the current conception of ADHD in humans as outlined above. Oral MPH treatment for two weeks significantly changes these receptor densities to normal values [32,74]. Accordingly, Russell and colleagues [7] reported that MPH treatment alleviates ADHD-like symptoms in this rodent model.
In our lab, we studied the long-term plastic effects of MPH in a model of hyperkinetic behaviour that bears some resemblance to ADHD, i.e. gerbils after an early traumatic experience [33,34]. Early trauma is not a typical, but a possible factor in the aetiology of ADHD [75]. A single high dose of methamphetamine (MA), administered on postnatal day 14, causes a syndrome in young-adult gerbils that is characterised by hyperactivity, increased fearfulness and impaired PFC function in both working memory and extinction [76,77]. Neuroanatomically, this is based on decreased prefrontal and accumbal, but increased entorhinal and amygdalar DA fibre densities [63,78,79]. Other neuromodulators like serotonin and acetylcholine adapt to this changed situation by altering their innervation densities, too [80,81].
In this animal model of early traumatic experience, we applied MPH both orally and (see below) intraperitoneally (i.p.) during adolescence (PD30–60) to isolation-reared, MA intoxicated gerbils. While the oral application was designed to match human medication, the i.p. application was meant to study the effects of MPH abuse. Two control groups were taken, one being left undisturbed, whereas the other received water. DA fibres were stained immunohistochemically, and their densities assessed by computerised image analysis in the PFC (anterior cingulate, prelimbic and infralimbic), ventral striatum (NAc core and shell, olfactory tubercle) and the central (lateral and medial) and basolateral amygdalar subnuclei. Additionally, cell proliferation rates in the hippocampal dentate gyrus were counted as a measure of long-term memory plasticity.
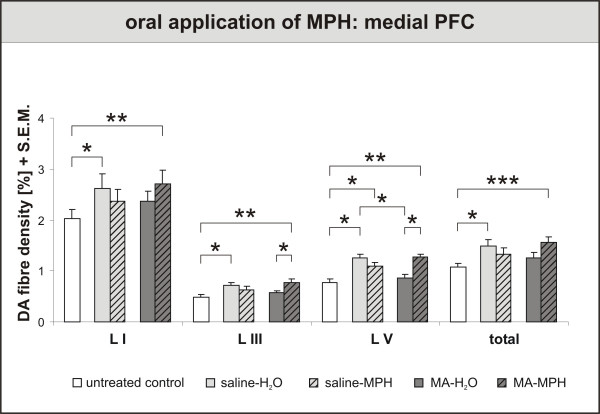
In the PFC, three effects are noteworthy (fig. 4). First, MA (= MA-H2O) impaired the maturation of DA fibres in the prelimbic cortex, as had been shown before [78]. Second, MPH treatment for 30 days returned DA fibre densities to control values in MA-traumatised (= MA-MPH) animals. In control animals, in contrast, MPH (= saline-MPH) did not change the DA fibre densities, or even rather reduced them. Third, application of water (= saline-H2O), i.e., pure handling, was highly effective in increasing the DA fibre densities in both the anterior cingulate and prelimbic cortices. As isolated rearing by itself allows only for a suppressed maturation of DA fibres, this latter finding suggests that handling is a beneficial, "therapeutic" intervention (Lehmann, Grund et al., unpublished observations).
Figure 4.
Oral application of Methylphenidate (MPH) – Effects on Prefrontal Cortex (PFC). Dopamine (DA) fibre density + S.E.M. is presented in lamina I, III and V of the PFC. Three effects are noteworthy. First, methamphetamine (MA) (= MA-H2O) impaired the maturation of DA fibres in layer V, as had been shown before [78]. Second, MPH treatment for 30 days returned DA fibre densities to control values in MA-traumatised (= MA-MPH) animals. In control animals, in contrast, MPH (= saline-MPH) did not change the DA fibre densities, or even rather reduced them. Third, application of water (= saline-H2O), i.e., pure handling, was highly effective in increasing the DA fibre densities in all layers. As isolated rearing by itself allows only for a suppressed maturation of DA fibres, this latter finding suggests that handling is a beneficial, "therapeutic" intervention (Lehmann, Grund et al., unpublished observations). For biostatistics two-way ANOVA with post-hoc contrast analysis among treated groups or pairwise comparisons with t-tests for untreated controls vs. treated groups were used for each lamina; significance values: *p < 0,05, **p < 0,01, ***p < 0,001.
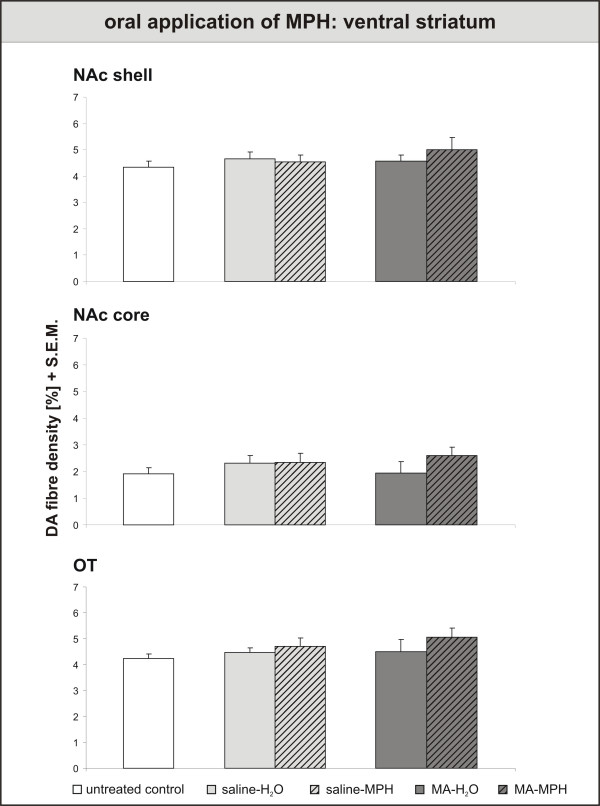
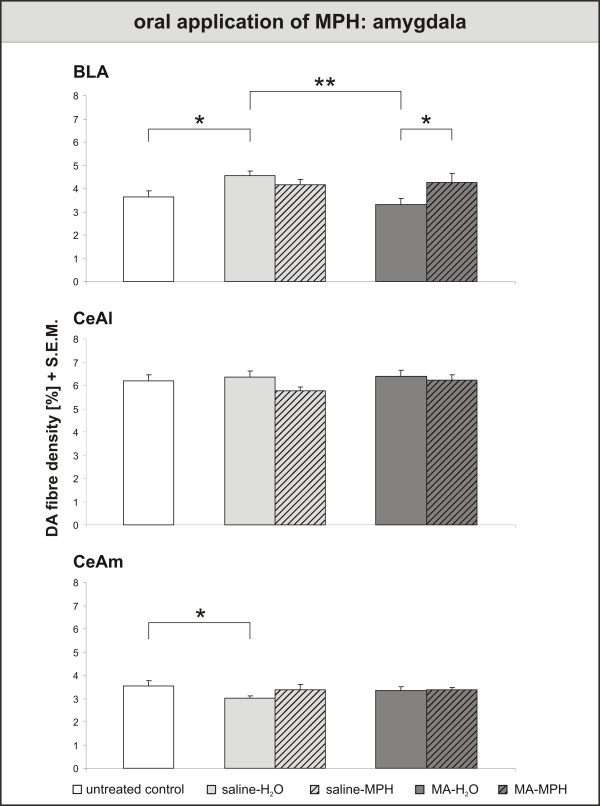
In contrast to the PFC results, neither handling nor MPH exerted any effects on the DA innervation of the ventral striatum (fig. 5). If anything, there seemed to be a slight, but non-significant rise in fibre densities after MPH treatment in MA-intoxicated animals. In the basolateral amygdala (fig. 6), results correspond to those in the PFC: Handling-induced rise in DA fibre density, MA-induced decrease and MPH-induced recovery. The DA innervation of the central amygdala did not react to MPH, but was lowered in the medial part by handling.
Figure 5.
Oral application of Methylphenidate – effects on Ventral Striatum. Dopamine (DA) fibre density + S.E.M. is presented in the nucleus accumbens shell, core and olfactory tubercle. In the nucleus accumbens, neither handling nor MPH exerted any effects on the DA innervation of the ventral striatum. For abbreviations, see fig. 4. Biostatistics: ANOVA with repeated measures and t-tests (see fig. 4).
Figure 6.
Oral application of Methylphenidate – effects on Amygdala. Dopamine (DA) fibre density + S.E.M. is presented in the basolateral, lateral and medial central amygdala. In the basolateral amygdala, results correspond to those in the PFC: Handling-induced rise in DA fibre density, MA-induced decrease and MPH-induced recovery. The DA innervation of the central amygdala did not react to MPH, but was lowered in the medial part by handling. For abbreviations, see fig. 4. Biostatistics: ANOVA with repeated measures and t-tests (see fig. 4); significance values: *p < 0,05, **p < 0,01.
That MPH restores the DA innervation of the gerbil PFC that was lesioned by MA indicates a beneficial effect of MPH treatment, which is confirmed by studies demonstrating improved attention and working memory in animals treated with MPH [82,83]. Since no impairments in long-term memory have been found in ADHD patients [84], and MPH, consequently, does not seem to influence this neuropsychological function [85-88], it is not surprising that we did not detect any effect of MPH on the hippocampal cell proliferation (data not shown). As numerous endo- and exogenous substances, including many psychopharmacological drugs, have been demonstrated to alter the dentate mitotic activity [rev. in [89], this result rather underlines the specificity of MPH as an enhancer of PFC/NAc-based function.
Concerning the clinical use of MPH, the reported findings in animals suggest that disturbed brain systems may react differently to the drug than those with a normal development. However, empirical evidence in humans with and without ADHD shows that the situation is rather complex. At the clinical behavioural level, Rapoport and Inoff-Germain [90] reported in an update that stimulants appear to have basically similar behavioural effects in normal and in hyperactive children, as had first been shown for dextroamphetamine [91,92]. But taking the neuropsychological level of behaviour into account, a different picture can be seen: MPH improved response inhibition in the healthy and ADHD group in one task and only in ADHD children in the other task [93]. Similarly, Elliott et al. [94] found that MPH influenced performance in two conflicting ways in healthy young adults; enhancing executive aspects of spatial function in novel tasks but impairing previously established performance. Further, beneficial effects of MPH on working memory seem to be greatest in the subjects with lower baseline working memory capacity [95]. Finally, looking at neural substrates of motor and cognitive control in fMRI, MPH seems to affect striatal activation differently in ADHD (positive) than in healthy (negative) children while increased frontal activation was seen in both groups [93]. A neurophysiological study with transcranial magnetic stimulation could demonstrate opposite effects of MPH on neuronal excitability in children with ADHD versus healthy controls [96].
In conclusion, a transfer from animal data on MPH to MPH treatment in human ADHD is always limited. Although the neuronal systems of ADHD patients work differently than those of healthy controls, some performing/behavioural output after MPH looks similar. However, distinct rather than unitary patterns of functional abnormality in ADHD have to be taken into consideration and a differential treatment approach seems to be adequate, which might work best for patients with an unequivocal ADHD symptomatology and psychosocial impairment, although only strong levels of response may be predicted by a few baseline characteristics (e.g. considerable inattentiveness [97]).
Against this backdrop, our results rather support the notion that psychomotor impaired individuals and healthy controls show indeed opposite responses to ADHD (fig. 4). This finding is further corroborated by behavioural animal studies which show that MPH treatment improves attention in bad performers, but has no effect on normal controls in the 5-choice serial reaction time task [82]. Furthermore, MPH did not induce locomotor sensitization in SHRs [98], but caused both locomotor sensitization and cross-sensitization to amphetamine in normal rat strains [98,99]. More studies will be needed to clarify this issue.
Although we did not find altered DA fibre densities in the NAc, MPH treatment indeed exerts significant and long-lasting functional effects in the NAc: In contrast to other stimulant drugs, MPH does not sensitise the rewarding effects of other drugs, but instead reduces the risk for substance abuse both in rats [100,101] and humans [[102], rev. in [103]], although there are conflicting results in rats [104]. Since clinical doses do not increase extracellular accumbal DA levels, but change noradrenaline concentrations [105-107], it seems likely that this beneficial effect is not mediated primarily via the DA system. Nevertheless, as mentioned above, accumbal DA receptor densities are altered by MPH treatment [32]. Furthermore, MPH treatment during adolescence lastingly decreases the DAT concentration in the NAc, while leaving the densities of the serotonin and noradrenaline receptors untouched [20]. Thus, the alterations occuring in the NAc are obviously rather of a physiological kind, possible due to the much earlier maturation of the accumbal than the prefrontal DA innervation [12,108,109].
The increased DA fibre density found in the basolateral amygdala of MPH-treated MA-intoxicated gerbils is a first hint that MPH affects neural systems beyond PFC and NAc. It corresponds to further results from the above mentioned behavioural study showing that MPH-treated rats respond stronger to aversive situations and show more anxiety-like behaviour [101].
Methylphenidate as a psychostimulant drug of abuse
Orally taken, MPH has no abuse potential because of its slow increase of the plasma level, while a "high" (with the associated risk of drug abuse) can be elicited by i.v. application [110]. Hence, when abused, MPH is usually applied intravenously [103,111]. This route of application dodges the pronounced hepatic first-pass metabolism that MPH is subjected to after oral consumption [112]. In consequence, higher plasma concentrations are reached about six times faster and with a much shorter half life [113], and extracellular DA concentrations in the NAc are higher [106]. Although the abuse of MPH is rather rare, it seems to be important to investigate if the potential long-term plastic effect of this kind of application differs from that of oral application.
To our knowledge, there is as yet no other animal study on the long-term effects of intraperitoneally or intravenously applied MPH except for one from our lab that we briefly summarise here [33,34]. We studied the plastic long-term effects of i.p. MPH on the DA innervation in the above described model. Two different concentrations of MPH were investigated, one (5 mg/kg) in the clinical range, the other (50 mg/kg) clearly beyond it. DA fibre densities were measured in the ventral striatum and amygdala. Data for the PFC could not be obtained out of technical problems, but previous experience with our model would suggest that DA fibres in the PFC react in a similar way to those in the NAc.
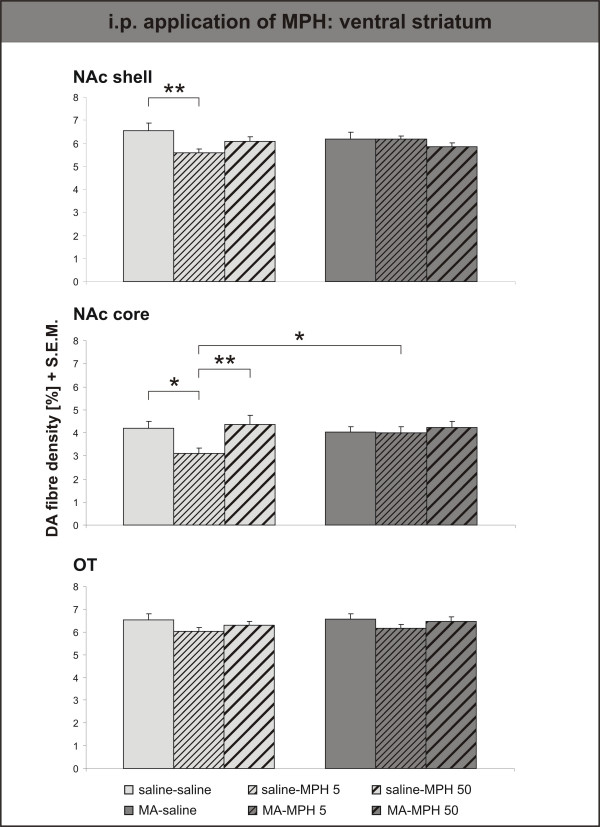
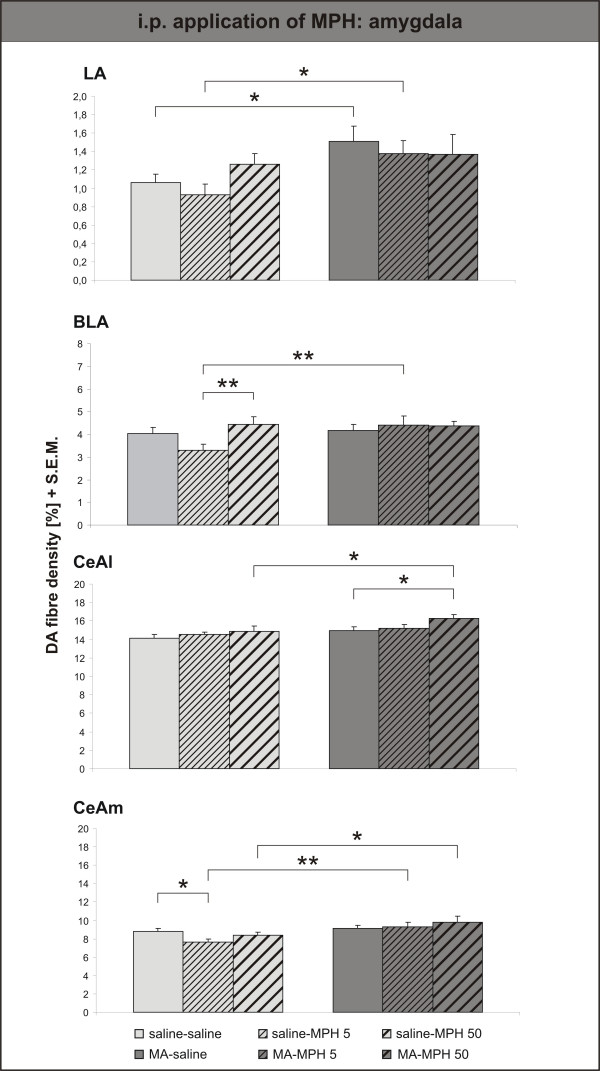
Surprisingly, i.p. MPH only had an effect in control animals, and only the lower, clinical dose (= saline-MPH 5) was effective in reducing the DA innervation density in both subterritories of the NAc (fig. 7). The DA innervation was unaltered by the higher dose of MPH (= saline-MPH 50), and even significantly denser than after treatment with the clinical dose in the NAc core. Similar, albeit not quite significant effects were observed in the lateral, basolateral and medial part of the central amygdala (fig. 8). Only in the lateral part of the central amygdala did the high dose of MPH (= MA-MPH 50) i.p. increase the DA fibre density in MA-intoxicated animals.
Figure 7.
Intraperitoneal application of Methylphenidate – Ventral Striatum. Dopamine (DA) fibre density + S.E.M. is presented in the nucleus accumbens (NAc) shell, core and olfactory tubercle. I.p. MPH only had an effect in control animals, and only the lower, clinical dose (= saline-MPH 5) was effective in reducing the DA innervation density in both subterritories of the NAc. The DA innervation was unaltered by the higher dose of MPH (= saline-MPH 50), and even significantly denser than after treatment with the clinical dose in the NAc core. For abbreviations, see fig. 4. Biostatistics: Two-way ANOVA with repeated measures; significance values: *p < 0,05, **p < 0,01.
Figure 8.
Intraperitoneal application of Methylphenidate – Amygdala. Dopamine (DA) fibre density + S.E.M. is presented in the lateral, basolateral, lateral and medial central amygdala. Similar, albeit not quite significant effects were observed in the lateral, basolateral and medial part of the central amygdala. Only in the lateral part of the central amygdala did the high dose of MPH (= MA-MPH 50) i.p. increase the DA fibre density in MA-intoxicated animals. For abbreviations, see fig. 4. Biostatistics: Two-way ANOVA with repeated measures; significance values: *p < 0,05, **p < 0,01.
Our results demonstrate that by i.p. application, MPH can indeed impair the postnatal maturation of DA fibres in the ventral striatum in a similar way as a single high dose of MA [79,109]. We can offer no explanation why the higher dose of MPH has no detectable effects in the anatomical dimension. It has been shown, however, that already a single dose of MPH in that range (30 mg/kg) sensitises rats for the locomotor-effect of amphetamine [105], thus making them more prone for a drug addiction. An up-regulation of the cAMP pathway in the NAc mediated via D2 receptors has been implicated in this kind of sensitization [114], so here again changes may rather be intracellular and physiological than anatomical.
Conclusion
MPH is an indispensable drug that beautifully fits the pharmacological demands to regulate DA dysfunctions in ADHD. In patients with this disorder, MPH simultaneously compensates a prefrontal DA hypofunction and probably restrains an accumbal DA hyperfunction in the long run. Animal studies suggest that this effect is supported in the PFC by enhancing the maturation of DA fibres [33,34], whereas adaptations of pre- and postsynaptic receptor densities are elicited in the NAc. Both clinical and preclinical studies converge to confirm that in subjects suffering from cognitive-motivational and neural impairments, MPH has long-term beneficial effects in several respects, e.g. by reducing the core symptoms of ADHD as well as the risk for substance abuse [e.g. [102,103,115]. A certain reservation must be deduced from the observation that both the DA innervation and the behavioural function of the amygdala are altered by MPH, making animals more fearful and sensitive to stressful stimuli [33,101]. However, since the behavioural study used normal rats, further investigations are needed to check whether adverse emotional effects are also evoked by MPH in animal models of ADHD.
This latter consideration directly leads us to one of two important caveats concerning the use of MPH: Behavioural studies show that MPH is ineffective in rodents without attentional impairments [82], as far as attention is concerned. In contrast, MPH elicits locomotor sensitization in non-hyperactive rat strains, whereas it has no such effect in SHRs [98]. The assessment of DA fibre densities confirms that these are only improved in previously traumatised animals, but unchanged or possibly even reduced in healthy controls [33,34]. Transformed to a clinical perspective, this might suggest that physicians are possibly dealing with (at least partly) quantitatively and/or qualitatively different responder systems when treating the brains of children with or without ADHD [see also [7,116]]. This perspective is supported by different effects of MPH on neuronal excitability (measured with transcranial magnetic stimulation) in healthy persons compared with ADHD patients [96]. However, as discussed above, there are also partly conflicting data [90-95], making it impossible to arrive at a firm conclusion so far.
Finally, being a psychostimulant, MPH has unfortunately also been discovered by some as a drug of abuse that is intravenously applied. First results on the long-term effect of such abuse in animals has shown equivocal results, with negative effects similar to methamphetamine in low but no effect with high doses of MPH [33,79]. It remains to be checked whether it may even be neurotoxic under such conditions. Nevertheless, the wealth of human and animal information on MPH shows the great value of the drug which has to be handled with care to use it in the right way.
Competing interests
The author(s) declare that there are no competing interests.
Authors' contributions
All authors are involved in the idea and planning of the reported own studies as well as in the writing of the manuscript. The Bielefeld authors conducted the reported own animal experiments in their laboratory.
Acknowledgments
Acknowledgements
The present work was supported by grants of the deutsche Parkinson Vereinigung (dPV). The authors are indebted to Mrs. U. Schroeder and Mr. F. Bagorda for technical assistance.
Contributor Information
Thorsten Grund, Email: Thor.Grund@gmx.de.
Konrad Lehmann, Email: Konrad.Lehmann@Uni-Bielefeld.de.
Nathalie Bock, Email: nbock@gwdg.de.
Aribert Rothenberger, Email: arothen@gwdg.de.
Gertraud Teuchert-Noodt, Email: g.teuchert@uni-bielefeld.de.
References
- Rothenberger A, Dopfner M, Sergeant J, Steinhausen HC. ADHD – beyond core symptoms. Not only a European perspective. Eur Child Adolesc Psychiatry. 2004;13 [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, Weaver AL, Weber KJ, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156:217–224. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Ruocco LA, Sadile AG. Behavioural, pharmacological, morpho-functional molecular studies reveal a hyperfunctioning mesocortical dopamine system in an animal model of attention deficit and hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:683–689. doi: 10.1016/j.neubiorev.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Nutt DJ. Stimulants: use and abuse in the treatment of attention deficit hyperactivity disorder. Curr Opin Pharmacol. 2005;5:87–93. doi: 10.1016/j.coph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Rothenberger A, Danckaerts M, Dopfner M, Sergeant J, Steinhausen HC. EINAQ – a European educational initiative on Attention-Deficit Hyperactivity Disorder and associated problems. Eur Child Adolesc Psychiatry. 2004;13:I31–I35. doi: 10.1007/s00787-004-1003-9. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G, Czaniera R. Maturation of the dopamine innervation during postnatal development of the prefrontal cortex in gerbils (Meriones unguiculatus). A quantitative immunocytochemical study. J Hirnforsch. 1993;34:281–290. [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Froimowitz M, Patrick KS, Cody V. Conformational-analysis of methylphenidate and its structural relationship to other dopamine reuptake blockers such as CFT. Pharm Res. 1995;12:1430–1434. doi: 10.1023/a:1016262815984. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Pan DF, Chen RY, Chaturvedi G, Ding YS. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996;58:L231–L239. doi: 10.1016/0024-3205(96)00052-5. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel S, Krause J. Wirkmechanismus von Methylphenidat. In: Voss Hv, editor. Kinderärztliche Praxis; Sonderheft "Unaufmerksam und hyperaktiv". Mainz: Kirchheim-Verlag; 2001. pp. 23–27. [Google Scholar]
- Wagner GC, Ricaurte GA, Johanson CE, Schuster CR, Seiden LS. Amphetamine induces depletion of dopamine and loss of dopamine uptake sites in caudate. Neurology. 1980;30:547–550. doi: 10.1212/wnl.30.5.547. [DOI] [PubMed] [Google Scholar]
- Zaczek R, Battaglia G, Contrera JF, Culp S, Desouza EB. Methylphenidate and pemoline do not cause depletion of rat-brain monoamine markers similar to that observed with methamphetamine. Toxicol Appl Pharmacol. 1989;100:227–233. doi: 10.1016/0041-008x(89)90309-8. [DOI] [PubMed] [Google Scholar]
- Yuan J, McCann U, Ricaurte G. Methylphenidate and brain dopamine neurotoxicity. Brain Res. 1997;767:172–175. doi: 10.1016/s0006-8993(97)00771-3. [DOI] [PubMed] [Google Scholar]
- Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adolesc Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Tennyson VM, Budininkas-Schoenebeck M, Gershon P. Effects of chronic reserpine treatment on development of maturity of the putamen in fetal rabbits. Brain Res Bull. 1982;9:651–662. doi: 10.1016/0361-9230(82)90169-1. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as morphogens. Prog Brain Res. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988;472:179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Teuchert-Noodt G. Neuronal degeneration and reorganization: a mutual principle in pathological and in healthy interactions of limbic and prefrontal circuits. J Neural Transm [Suppl] 2000;60:315–333. doi: 10.1007/978-3-7091-6301-6_22. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Murphy R, Azmitia EC. Stimulation of astroglial 5-HT1A receptors releases the serotonergic growth factor, protein S-100, and alters astroglial morphology. Brain Res. 1990;528:155–158. doi: 10.1016/0006-8993(90)90210-3. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Eterovic VA. Forty minutes of experience increase the weight and RNA content of cerebral cortex in periadolescent rats. Dev Psychobiol. 1986;19:511–519. doi: 10.1002/dev.420190604. [DOI] [PubMed] [Google Scholar]
- Winterfeld KT, Teuchert-Noodt G, Dawirs RR. Social environment alters both ontogeny of dopamine innervation of the medial prefrontal cortex and maturation of working memory in gerbils (Meriones unguiculatus) J Neurosci Res. 1998;52:201–209. doi: 10.1002/(SICI)1097-4547(19980415)52:2<201::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Keller A, Bagorda F, Hildebrandt K, Teuchert-Noodt G. Effects of Enriched and of Restricted Rearing on Both Neurogenesis and Synaptogenesis in the Hippocampal Dentate Gyrus of Adult Gerbils (Meriones unguiculatus) Neurology, Psychiatry and Brain Research. 2000;8:101–108. [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Papa M, Diewald L, Carey MP, Esposito FJ, Gironi Carnevale UA, Sadile AG. A rostro-caudal dissociation in the dorsal and ventral striatum of the juvenile SHR suggests an anterior hypo- and a posterior hyperfunctioning mesocorticolimbic system. Behav Brain Res. 2002;130:171–179. doi: 10.1016/s0166-4328(01)00421-1. [DOI] [PubMed] [Google Scholar]
- Grund T. Zum Einfluss von Methylphenidat (MPH; Ritalin®) auf die Reifung von Dopamin in limbo-präfrontalen Arealen von Meriones unguiculatus Bielefeld; Dissertation. 2005.
- Grund T, Teuchert-Noodt G, Busche A, Neddens J, Moll GH, Dawirs RR. Oral Methylphenidate During Prepuberty Prevents Pharmacologically-Induced (Preweaning) Suppressive Development of Dopamine Projections into Prefrontal Cortex and Amygdala. 2005. [DOI] [PubMed]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, et al. A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. Am J Hum Genet. 2002;70:1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354:2132–2133. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285:107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30:306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Marsteller DA, Gerasimov MR, Schiffer WK, Geiger JM, Barnett CR, Borg JS, et al. Acute handling stress modulates methylphenidate-induced catecholamine overflow in the medial prefrontal cortex. Neuropsychopharmacology. 2002;27:163–170. doi: 10.1016/S0893-133X(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann IS, Talmaciu RK, Ferro CP, Cubeddu LX. Sustained high release at rapid stimulation rates and reduced functional autoreceptors characterize prefrontal cortex dopamine terminals. J Pharmacol Exp Ther. 1988;245:761–772. [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Watson SJ., Jr Differential expression of autoreceptors in the ascending dopamine systems of the human brain. Proc Natl Acad Sci U S A. 1994;91:8297–8301. doi: 10.1073/pnas.91.17.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Bergman DA, Murrin LC. Localization and quantification of the dopamine transporter: comparison of [3H]WIN 35,428 and [125I]RTI-55. Brain Res. 1995;690:217–224. doi: 10.1016/0006-8993(95)00614-v. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Silvagni A. Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol. 2004;16:121–128. doi: 10.1615/critrevneurobiol.v16.i12.130. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:U1–U5. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Transm Gen Sect. 1993;91:111–134. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras B. Methylphenidate elevates resting dopamine which lowers the impulse-triggered release of dopamine: a hypothesis. Behav Brain Res. 2002;130:79–83. doi: 10.1016/s0166-4328(01)00435-1. [DOI] [PubMed] [Google Scholar]
- Gittelman-Klein R, Klein DF, Katz S, Saraf K, Pollack E. Comparative effects of methylphenidate and thioridazine in hyperkinetic children. I. Clinical results. Arch Gen Psychiatry. 1976;33:1217–1231. doi: 10.1001/archpsyc.1976.01770100079008. [DOI] [PubMed] [Google Scholar]
- Weizman A, Weitz R, Szekely GA, Tyano S, Belmaker RH. Combination of neuroleptic and stimulant treatment in attention deficit disorder with hyperactivity. J Am Acad Child Psychiatry. 1984;23:295–298. doi: 10.1016/s0002-7138(09)60506-9. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J Neurochem. 1980;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Weinberger DR, Crawley JN. Microinjection of apomorphine into the prefrontal cortex of the rat reduces dopamine metabolite concentrations in microdialysate from the caudate nucleus. Biol Psychiatry. 1991;29:703–706. doi: 10.1016/0006-3223(91)90144-b. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A. Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci. 1992;12:3609–3618. doi: 10.1523/JNEUROSCI.12-09-03609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Clark WA, Goldstein M, Roth RH, Deutch AY. Effects of 6-hydroxydopamine lesions of the prefrontal cortex on tyrosine hydroxylase activity in mesolimbic and nigrostriatal dopamine systems. Neuroscience. 1992;48:831–839. doi: 10.1016/0306-4522(92)90271-3. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Busche A, Polascheck D, Lesting J, Neddens J, Teuchert-Noodt G. Developmentally induced imbalance of dopaminergic fibre densities in limbic brain regions of gerbils (Meriones unguiculatus) J Neural Transm. 2004;111:451–463. doi: 10.1007/s00702-004-0106-2. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The amygdala – A functional analysis. Oxford: Oxford University Press; 2000. pp. 31–115. [Google Scholar]
- Bertolucci-D'Angio M, Serrano A, Driscoll P, Scatton B. Involvement of mesocorticolimbic dopaminergic systems in emotional states. Prog Brain Res. 1990;85:405–416. doi: 10.1016/s0079-6123(08)62692-8. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Russell VA. Dopamine hypofunction possibly results from a defect in glutamate-stimulated release of dopamine in the nucleus accumbens shell of a rat model for attention deficit hyperactivity disorder – the spontaneously hypertensive rat. Neurosci Biobehav Rev. 2003;27:671–682. doi: 10.1016/j.neubiorev.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus-accumbens and caudate-putamen of an animal-model of attention-deficit hyperactivity disorder – the spontaneously hypertensive rat. Brain Res. 1995;676:343–351. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Up-regulation of dopamine receptors in the brain of the spontaneously hypertensive rat: an autoradiographic analysis. Neuroscience. 1993;52:135–141. doi: 10.1016/0306-4522(93)90188-l. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Fujita M, Ito Y, Okada T, Kusuoka H, Nishimura T. Brain dopamine transporter in spontaneously hypertensive rats. J Nucl Med. 1997;38:470–474. [PubMed] [Google Scholar]
- Carey MP, Diewald LM, Esposito FJ, Pellicano MP, Gironi Carnevale UA, Sergeant JA, et al. Differential distribution, affinity and plasticity of dopamine D-1 and D-2 receptors in the target sites of the mesolimbic system in an animal model of ADHD. Behav Brain Res. 1998;94:173–185. doi: 10.1016/s0166-4328(97)00178-2. [DOI] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Psychiatric diagnoses of maltreated children: preliminary findings. J Am Acad Child Adolesc Psychiatry. 1992;31:863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G, Czaniera R. Ontogeny of PFC-related behaviours is sensitive to a single non-invasive dose of methamphetamine in neonatal gerbils (Meriones unguiculatus) J Neural Transm. 1996;103:1235–1245. doi: 10.1007/BF01271184. [DOI] [PubMed] [Google Scholar]
- Polascheck D. Zum Einfluss epigenetischer Faktoren auf die Reifung aminerger Neurotransmitter im Corpus amygdaloideum und das Verhalten Eine quantitative Studie an Meriones unguiculatus Bielefeld; Dissertation. 2004.
- Dawirs RR, Teuchert-Noodt G, Czaniera R. The postnatal maturation of dopamine innervation in the prefrontal cortex of gerbils (Meriones unguiculatus) is sensitive to an early single dose of methamphetamine. A quantitative immunocytochemical study. J Brain Res. 1994;35:195–204. [PubMed] [Google Scholar]
- Neddens J, Lesting J, Dawirs RR, Teuchert-Noodt G. An early methamphetamine challenge suppresses the maturation of dopamine fibres in the nucleus accumbens of gerbils: on the significance of rearing conditions. J Neural Transm. 2002;109:141–155. doi: 10.1007/s007020200010. [DOI] [PubMed] [Google Scholar]
- Neddens J, Bagorda F, Busche A, Horstmann S, Moll GH, Dawirs RR, et al. Epigenetic factors differentially influence postnatal maturation of serotonin (5-HT) innervation in cerebral cortex of gerbils: interaction of rearing conditions and early methamphetamine challenge. Brain Res Dev Brain Res. 2003;146:119–130. doi: 10.1016/j.devbrainres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Hundsdorfer B, Hartmann T, Teuchert-Noodt G. The acetylcholine fiber density of the neocortex is altered by isolated rearing and early methamphetamine intoxication in rodents. Exp Neurol. 2004;189:131–140. doi: 10.1016/j.expneurol.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Jr, Sirvio J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa S, Hirabayashi S, Kobayashi M. [Memory functions in children with attention deficit/hyperactivity disorder – the effects of methylphenidate on them] No To Hattatsu. 2004;36:31–36. [PubMed] [Google Scholar]
- Barkley RA. Attention-Deficit Hyperactivity Disorder. New York: Guilforld Press; 1990. [Google Scholar]
- Pliszka SR. The neuropsychopharmacology of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1385–1390. doi: 10.1016/j.biopsych.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Walter J. Kann Ritalin (Methylphenidat) die Schulleistungen von Schülern mit Aufmerksamkeits- und Hyperaktivitätsproblemen verbessern? – Ein Literaturüberblick auf der Basis US-amerikanischer Forschung. Heilpädagogische Forschung. 2001;27:106–123. [Google Scholar]
- Walter J. Ritalin und Schulleistungen bei HKS: Befunde bei Langfrist- und Kombinationsbehandlungen. Sonderpädagogik. 2001;31:191–210. [Google Scholar]
- Lehmann K, Butz M, Teuchert-Noodt G. Offer and demand: proliferation and survival of neurons in the dentate gyrus. Eur J Neurosci. 2005;21:3205–3216. doi: 10.1111/j.1460-9568.2005.04156.x. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Inoff-Germain G. Responses to methylphenidate in Attention-Deficit/Hyperactivity Disorder and normal children: update 2002. J Atten Disord. 2002;6:S57–S60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199:560–563. doi: 10.1126/science.341313. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Rothenberger A. Methylphenidate and intracortical excitability: opposite effects in healthy subjects and attention-deficit hyperactivity disorder. Acta Psychiatr Scand. 2003;107:69–72. doi: 10.1034/j.1600-0447.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Van der Gaag RJ, Swaab-Barneveld H, Kuiper M. Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:1025–1032. doi: 10.1097/00004583-199508000-00012. [DOI] [PubMed] [Google Scholar]
- Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic pretreatment with methylphenidate induces cross-sensitization with amphetamine. Life Sci. 2003;73:2899–2911. doi: 10.1016/s0024-3205(03)00673-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:E201–E205. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Huss M, Lehmkuhl U. Methylphenidate and substance abuse: a review of pharmacology, animal, and clinical studies. J Atten Disord. 2002;6:S65–S71. doi: 10.1177/070674370200601s09. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Dynamic changes in sensitivity occur during the acute response to cocaine and methylphenidate. Psychopharmacology (Berl) 1999;147:96–103. doi: 10.1007/s002130051147. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, et al. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Lesting J, Neddens J, Busche A, Teuchert-Noodt G. Hemisphere-specific effects on serotonin but not dopamine innervation in the nucleus accumbens of gerbils caused by isolated rearing and a single early methamphetamine challenge. Brain Res. 2005;1035:168–176. doi: 10.1016/j.brainres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Faraj BA, Israili ZH, Perel JM, Jenkins ML, Holtzman SG, Cucinell SA, et al. Metabolism and disposition of methylphenidate-14C: Studies in man and animals. J Pharmacol Exp Ther. 1974;191:535–547. [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43:559–567. doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirot S, Glowinski J, Thierry AM. Excitatory responses evoked in prefrontal cortex by mediodorsal thalamic nucleus stimulation: influence of anaesthesia. Eur J Pharmacol. 1995;285:45–54. doi: 10.1016/0014-2999(95)00377-w. [DOI] [PubMed] [Google Scholar]
- Pirot S, Glowinski J, Thierry AM. Mediodorsal thalamic evoked responses in the rat prefrontal cortex: influence of the mesocortical DA system. Neuroreport. 1996;7:1437–1441. doi: 10.1097/00001756-199605310-00023. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]