Abstract
Acute hypoxia causes pulmonary vasoconstriction in part by inhibiting voltage-gated K+ (Kv) channel activity in pulmonary artery smooth muscle cells (PASMC). The hypoxia-mediated decrease in Kv currents (IK(V)) is selective to PASMC; hypoxia has little effect on IK(V) in mesenteric artery smooth muscle cells (MASMC). Functional Kv channels are homo- and/or hetero-tetramers of pore-forming α subunits and regulatory β subunits. KCNA5 is a Kv channel α subunit that forms functional Kv channels in PASMC and regulates resting membrane potential. Here, we show that acute hypoxia selectively inhibits IK(V) through KCNA5 channels in PASMC. Overexpression of the human KCNA5 gene increased IK(V) and caused membrane hyperpolarization in HEK-293, COS-7, and rat MASMC and PASMC. Acute hypoxia did not affect IK(V) in KCNA5-transfected HEK-293 and COS-7. However, overexpression of KCNA5 in PASMC conferred its sensitivity to hypoxia. Reduction of Po2 from 145 to 35 mmHg reduced IK(V) by ~40% in rat PASMC transfected with human KCNA5, but had no effect on IK(V) in KCNA5-transfected rat MASMC (or HEK and COS cells). These results indicate that KCNA5 is an important Kv channel that regulates resting membrane potential, and acute hypoxia selectively reduced KCNA5 channel activity in PASMC relative to MASMC and other cell types. Since Kv channels (including KCNA5) are ubiquitously expressed in PASMC and MASMC, the observation from this study indicates that a hypoxia-sensitive mechanism essential for inhibiting KCNA5 channel activity is exclusively present in PASMC. The divergent effect of hypoxia on IK(V) in PASMC and MASMC may also be due to different expression levels of KCNA5 channels.
Keywords: membrane potential, K+ channels, vascular smooth muscle, pulmonary
Introduction
Hypoxic pulmonary vasoconstriction is a critical physiological mechanism that directs blood flow away from poorly ventilated regions in the lung in order to maintain optimal ventilation-perfusion ratio for the maximal oxygenation of the venous blood in pulmonary artery. One of the potential mechanisms involved in hypoxic pulmonary vasoconstriction is acute hypoxia-mediated inhibition of voltage-gated K+ (Kv) channels in pulmonary artery smooth muscle cells (PASMC) (42, 43, 56). The subsequent membrane depolarization opens voltage-dependent Ca2+ channels (VDCC), increases cytoplasmic free Ca2+ concentration ([Ca2+]cyt), triggers PASMC contraction, and causes hypoxic pulmonary vasoconstriction. The vasoconstrictive response to hypoxia only occurs in the pulmonary vasculature; hypoxia, or hypoxemia, causes systemic vasodilation in vivo and has little contractile effect on isolated mesenteric arteries in vitro (28, 58). The hypoxia-induced functional inhibition of Kv channels is also selective to PASMC, because hypoxia has little effect on Kv channel activity in systemic artery smooth muscle cells (SMC), such as mesenteric artery smooth muscle cells (MASMC) (28, 56).
Functional Kv channels in native cells are either homo- or hetero-tetramers composed of the pore forming α subunits and cytoplasmic regulatory β subunits (12). In PASMC and systemic (e.g., cerebral, coronary, renal, and mesenteric) arterial SMC, multiple Kv channel α and β subunits are expressed (2, 6, 49, 50). Therefore, the K+ currents involved in determining the resting membrane potential (Em) are believed to result from activities of these various Kv channels as well as voltage-independent K+ channels (14, 19, 55). Since hypoxia-mediated inhibition of Kv channels was first reported in carotid body (glomus) cells (26), many investigators have attempted to identify the Kv channel subunits that are responsible for sensing changes in oxygen tension (Po2) in PASMC and other oxygen-sensitive tissues and cells (3–8, 19, 22, 25, 27, 28, 35–43, 50, 55–58).
Using heterologous transfection systems, we now know that various α subunit homotetramers (e.g., formed by Kv1.2, Kv2.1, or Kv3.1b) and heterotetramers (e.g., formed by Kv1.2/Kv1.5 or Kv2.1/Kv9.3) as well as α/β subunit homo- or heterotetramers (e.g., Kv4.2/Kvβ1.2, Kv1.5/Kvβ1.2) are sensitive to hypoxia (22, 38–40). KCNA5 (i.e., Kv1.5) is a pore-forming subunit that forms hetero- or homo-tetrameric Kv channels in many cells types including PASMC (4, 6, 8, 23, 44). Normal expression and function of KCNA5 channels in PASMC are necessary for the regulation of resting membrane potential and pulmonary vascular tone (6, 21). In vivo gene transfer of KCNA5 to lung tissues and pulmonary arteries improves pulmonary hemodynamics and induces regression of pulmonary vascular medial hypertrophy in rats with chronic hypoxia-mediated pulmonary hypertension (44), suggesting that enhancing KCNA5 protein expression may represent a potential therapeutic approach for pulmonary arterial hypertension. However, homotetrameric KCNA5 channels have been demonstrated to be insensitive to acute hypoxia in mouse L cells transiently transfected with the KCNA5 gene (22). Therefore, it remains unclear whether homotetrameric KCNA5 channels serve as hypoxia-sensitive Kv channels in PASMC.
In this study we investigated a) whether acute hypoxia reduces Kv currents (IK(V)) in different cell types (PASMC, MASMC, HEK-293 and COS-7 cells) transiently transfected with the human KCNA5 gene; and b) whether the sensitivity of the homomeric KCNA5 channels to acute hypoxia is a unique property of PASMC, or whether hypoxia-mediated effect on KCNA5 channel activity is dependent of the cells in which the KCNA5 gene is transfected. The present study demonstrates that expression of homomeric KCNA5 in various cell types (e.g., rat PASMC, rat MASMC, HEK-293, and COS-7) generates a typical Kv current that is sensitive to acute hypoxia only in rat PASMC, although the current (due to overexpressed KCNA5 channels) causes membrane hyperpolarization and is inhibited by 4-aminopyridine (a Kv channel blocker) in all cell types. These results suggest that, while expressed ubiquitously in both pulmonary and systemic arterial smooth muscle cells, the KCNA5 channel is a downstream effector used by an exclusive oxygen sensing mechanism present only in PASMC to reduce Kv currents and cause membrane depolarization during acute hypoxia.
MATERIALS AND METHODS
Cell preparation and culture.
Rat PASMC and MASMC were prepared from pulmonary arteries of male Sprague-Dawley rats (41). Briefly, the isolated pulmonary and mesenteric arteries were incubated for 20 min in Hanks’ balanced salt solution (HBSS) containing 1.5 mg/ml collagenase (Worthington Biochemical, Lakewood, NJ). Adventitia and endothelium were carefully removed after the incubation. The remaining smooth muscle was digested for 45–50 min with 1.5 mg/ml collagenase and 0.5 mg/ml elastase (Sigma, St. Louis, MO) at 37ºC. PASMC were sedimented by centrifugation, resuspended in fresh media, and plated. HEK-293 (human embryonic kidney epithelial cells) and COS-7 (monkey kidney fibroblast-like cells) cells (ATCC, Manassas, VA), rat PASMC, and rat MASMC were cultured in high glucose (4.5 g/l) DMEM supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (BioFluids, Camarillo, CA) and incubated in 5% CO2 at 37ºC in a humidified atmosphere.
Constructs.
In the KCNA5/pBK construct (kindly provided by Dr. M. Tamkun from Colorado State University), the coding sequence of the human KCNA5 gene was subcloned into XbaI and KpnI sites of multiple cloning site (MCS) of the phagemid expression vector pBK-CMV (Stratagene, La Jolla, CA). For electrophysiological experiments, a KCNA5/GFP construct was designed to visualize the transfected cells. In the KCNA5/GFP construct, the coding sequence of the human KCNA5 gene was subcloned into EcoRI and XbaI sites of MCS of the pCMS-EGFP mammalian expression vector (Clontech, Palo Alto, CA). The enhanced green fluorescent protein (EGFP) gene, a red-shifted variant of wild-type GFP from Aquorea victoria, is expressed separately from the gene of interest in the pCMS-EGFP vector and is used as a transfection marker.
Transfection of KCNA5.
Cells were transiently transfected with the expression constructs using Lipofectamine reagent according to the manufacturer’s instructions. Briefly, cells were first split and then cultured for 24 hrs. Transfection was performed on 50–80% confluent cells at 37ºC in serum-free Opti-MEM I medium (Invitrogen, Carlsbad, CA) with 1.6 μg/ml DNA and 4 μl/ml of Lipofectamine reagent. After 5–7 hrs of exposure to the transfection medium, cells were refed with construct-free serum-containing medium (10% FBS-DMEM) and incubated 12–24 hrs before experiments. The transfection efficiency was consistently greater than 30% (9).
Western blot analysis.
Cells were collected in tubes, centrifuged, and washed with cold PBS. Cell pellets were resuspended in 20–100 μl lysis buffer [1% Triton-100, 150 mM NaCl, 5 mM EDTA, and 50 mM Tris-HCl (pH 7.4)] supplemented with 1× Protease Inhibitor Cocktail (Sigma) and 100 μg/ml PMSF prior to use, then incubated in the lysis buffer for 30 min on ice. Resulting cell lysates were centrifuged at 14,000 rpm for 15 min and the insoluble fraction was discarded. The protein concentrations in the supernatant were determined by the Coomassie Plus protein assay (Pierce Biotechnology, Rockford, IL) using BSA as a standard. Proteins were mixed and boiled in SDS-PAGE sample buffer for 2 min. The protein samples separated on 8% SDS-PAGE were transferred to nitrocellulose membranes by electroblotting in a Mini Trans-Blot Cell transfer apparatus (Bio-Rad, Hercules, CA). After incubation for 1 hr at 22–24ºC in a blocking buffer (0.1% Tween 20 in PBS) containing 5% nonfat dry milk powder, the membranes were incubated with a polyclonal rabbit anti-KCNA5 antibody (Alomone Labs, Jerusalem, Israel) overnight at 4ºC. The membranes were then washed with the blocking buffer and incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature. After washing unbound antibodies with the blocking buffer the bound antibodies were detected using an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ).
Electrophysiological measurement.
Whole-cell and single channel K+ currents were recorded with an Axopatch-1D amplifier and a DigiData 1200 interface (Axon Instruments, Foster City, CA) using patch-clamp techniques. Patch pipettes (2–3 MΩ) were fabricated on an electrode puller (Sutter Instrument Company, Novato, CA) using borosilicate glass tubes and fire polished on a microforge (Narishige Scientific Instruments, Japan). Command voltage protocols and data acquisition were performed using pCLAMP-8 software (Axon Instruments). All experiments were performed at room temperature (22–24ºC). For recording optimal whole-cell Kv currents (IK(V)), cells were superfused with a standard extracellular solution containing (mM) 141 NaCl, 4.7 KCl, 3.0 MgCl2, 10 HEPES, 1 EGTA, and 10 glucose (pH 7.4). The pipette (intracellular) solution contained (mM) 135 KCl, 4 MgCl2, 10 HEPES, 10 EGTA, and 5 Na2ATP (pH 7.2). Under whole-cell configuration, the resting membrane potential (Em) was measured in all cell types in current-clamp (I=0) mode.
For cell-attached recording of single-channel Kv currents (iK(V)), the pipette (extracellular) solution contained (mM): 135 KCl, 10 HEPES, 1.2 MgCl2, 10 glucose, and 5 EGTA (pH 7.2). The bath solution was the same as described for the whole-cell IK(V) recording. The amplitude of single-channel iK(V) in cell-attached membrane patches was determined with Fetchan and pStat analysis programs (Axon Instruments). The pipette solution used to measure iK(V) contained high (135 mM) [K+] so that the K+ equilibrium potential (EK) would be close to 0 mV. The current (iK(V))-voltage relationship results are presented as a function of the current amplitude against the command potential (Ecomm) applied to the patched membrane. Because of a negative resting Em, the actual transmembrane potential across the patched membrane (Epatch) is equal to the difference between the Ecomm and resting Em [Epatch = (−Ecomm − Em)]. This is why the single channel current-voltage curves do not reverse at EK (≈ 0 mV), but reverse at a potential equals to −Em.
Green fluorescence emitted at 507 nm was used to visualize the cells transfected with KCNA5/GFP or pCMS-EGFP constructs by an inverted Nikon microscope (Eclipse/TE200) with the TE-FM epi-fluorescence attachment. The cell images were acquired with an Image Intensifier Tube/Philips 1381 system (Stanford Photonics Electronic Imaging Technologies, Palo Alto, CA).
Single-cell RT-PCR.
To determine the mRNA expression of exogenous human KCNA5 in rat PASMC transfected with human KCNA5 gene at the single-cell level, multiplex single-cell RT-PCR was performed according to a modified protocol previously described by Comer et al. (16). Briefly, after recording IK(V), the whole cell was carefully aspirated into a collection pipette, which contained 12 μl of the pipette solution supplemented with 10 μM dNTP and 0.5 U/μl RNase inhibitor. The content in the pipette was then expelled immediately into a 0.2-ml PCR tube which contained 8 μl of the solution composed of 10 mM Tris-HCl, 50 mM KCl, 2.5 mM MgCl2, 10 mM dithiothreitol, 1.25 mM oligo(dT), 0.5 mM dNTPs, and 5 U AMV reverse transcriptase XL. The reverse transcription (RT) was performed for 60 min at 42°C. Then, first-round PCR with 45 cycles was performed in the same tube by the addition of 80 μl of the pre-mix PCR buffer containing 10 mM Tris-HCl, 50 mM KCl, 2.5 mM MgCl2, 20 nM of each sense and antisense primers (First primers) for all the genes of interest, and 5 units of Taq polymerase (RNA PCR kit, Takara). Two-μl aliquots of the first-round PCR products were re-amplified by the second-round PCR with 25–30 cycles, which was separately carried out using fully nested gene-specific primers (Nested primers) for each target gene. Second-round PCR-amplified products were separated on 1.5% agarose gel and visualized with GelStar gel staining. β-actin mRNA was used an internal control. The sense and antisense primers were specifically designed from the coding region of human KCNA5 gene (NM_002234) (Table 1) to amplify human KCNA5; the primers do not completely match with rat KCNA5.
Table 1.
Oligonucleotide sequences of the primers used for RT-PCR
| Standard Names (Accession No.)* | Predicted Size (bp) | Sense/Antisense | Location (nt) | Gene (chromosome) |
|---|---|---|---|---|
| Rat Primer Sequences | ||||
| Kvβ1.1 (KCNAB1) | 192 | 5′-GCAACAAGCCCTACAGCAAA-3′/ | 1500–1519 | 2q31 |
| (NM_017303) | 5′-AGAGACGACAACGATGGTGA-3′ | 1672–1691 | ||
| Kvβ2.1 (KCNAB2) | 433 | 5′-TGGAGTACGTGGATGTGGTT-3′/ | 1038–1057 | 5q36 |
| (NM_017304) | 5′-CGGTTTGACTTCCTTGACGT-3′ | 1451–1470 | ||
| Kvβ3.1 (KCNAB3) | 308 | 5′-AGAAATAGTCCGTTGTGGGC-3′/ | 1595–1614 | 10q24 |
| (NM_031652) | 5′-AAGAGCGATTAGGTCCCGTA-3′ | 1883–1902 | ||
| β-Actin | 244 | 5′-AGTGTGACGTTGACATCCGT-5′/ | 932–951 | 12p11 |
| (NM_03114) | 5′-TTCGTCCTCATGCTACTCAG-3′ | 1156–1175 | ||
| Human Primer Sequences | ||||
| Kv1.5 (KCNA5) | 335§ | 5′-CATTCCCTACTCCACTGC-3′/ | 1564–1581 | 12p13 |
| (NM_002234)] | 5′-AGTCCAGCGGAAGGTCA-3′ | 1882–1898 | ||
| First Primers | 292‡ | 5′-AGCTTCGACGGTATCCTCTA-3′/ | 740–759 | |
| 5′-AGAAGGTGATGATGGAGATG-3′ | 1012–1031 | |||
| Nested primers | 274‡ | 5′-AGCTTCGACGGTATCCTCTA-3′/ | 740–759 | |
| 5′-TGAGGATAACCAAGACCGAG-3′ | 994–1013 | |||
| Kvβ1.1 (KCNAB1) | 197 | 5′-AGGCTGCAGCTCGAGTATGT-3′/ | 776–795 | 3q26.1 |
| (NM_003471) | 5′-ACCGGTGGGATCATATTGAA-3′ | 953–972 | ||
| Kvβ2.1 (KCNAB2) | 195 | 5′-TGGGCAATAAACCCTACAGC-3′/ | 1242–1261 | 1p36.3 |
| (NM_003636) | 5′-CAGCGACTTGGGAGATCATT-3′ | 1417–1436 | ||
| Kvβ3.1 (KCNAB3) | 193 | 5′-GTGGTGTTCGGGTATCCTGT-3′/ | 264–283 | 17p13.1 |
| (NM_004732) | 5′-TGATCTCCTCCAACCTTTGC-3′ | 437–456 | ||
| GAPDH | 198 | 5′-GATGACATCAAGAAGGTGGTG-3′/ | 841–861 | 12p13 |
| (NM_002046) | 5′-CTGTTGCTTAAACCGATGTCG-3′ | 1018–1038 | ||
RNA extraction and regular RT-PCR.
Total RNA was isolated from rat PASMC, rat MASMC, HEK-293, and COS-7 cells, and RT-PCR was performed according to protocols described previously (41). The sequences of sense and antisense primers (Table 1) were specifically designed from the coding regions of various rat and human Kv channel α and β subunits. Primer fidelity and specificity were examined using a BLAST program. As a control for integrity of total RNA, primers specific for β-actin or GAPDH were used. The net intensity values of the PCR product bands, measured by a Kodak Electrophoresis Documentation System (Eastman Kodak Company; Rochester, NY), were normalized to the net intensity values of the β-actin or GAPDH signals; the ratios are expressed as arbitrary units for quantitative comparisons.
Acute hypoxic treatment.
Acute hypoxia was established as described previously (56). Briefly, normoxic conditions were established by bubbling the superfusion solution with room air to achieve Po2 ranging from 140 to 149 mmHg at 24°C. Hypoxia was established by directly dissolving 0.8 mM sodium dithionite (Na2S2O4, Sigma), an oxygen scavenger that combines with oxygen and decreases Po2 in solution, in the extracellular solution to achieve a Po2 ranging from 22 to 40 mmHg. An oxygen electrode (Microelectrodes, Londonderry, NH) was positioned in the cell chamber on the microscope stage to continuously monitor the Po2. Sodium dithionite had no effect on Kv channel activity unless accompanied by a reduction in Po2. Rigorously bubbling the Na2S2O4 (0.8 mM)-containing solution with room air for 20–30 min increased the Po2 to approximately 145 mmHg. Application of the Na2S2O4-containing normoxic solution to KCNA5-transfected cells did not affect the KCNA5 currents.
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed using unpaired Student’s t test or ANOVA. Differences were considered to be significant when P<0.05.
RESULTS
Overexpression of KCNA5 increases IK(V) and induces membrane hyperpolarization.
In order to visibly recognize transfected cells, we made a GFP-containing construct carrying the human KCNA5 gene (Fig. 1A). We first transfected KCNA5 to HEK-293 cells. KCNA5 mRNA (Fig. 1Ba) and protein (Fig. 1Bb) levels were both markedly increased in HEK-293 cells transiently transfected with KCNA5. The time course of KCNA5 protein expression showed that the level of the channel protein dramatically increased 15 hrs after initial transfection and lasted for up to 80 hrs (Fig. 1Bb and c). Overexpression of KCNA5 proteins in HEK-293 increased the amplitude of IK(V) through KCNA5 channels (IKCNA5) (Fig. 1Ca and b). The voltage threshold for activating KCNA5 channels was between −40 and −50 mV, and IKCNA5 showed neither inward rectification nor inactivation (Fig. 1Ca and b). Furthermore, the resting membrane potential (Em) in KCNA5-transfected cells was more negative than in the empty vector-transfected cells (Fig. 1Cc); the membrane hyperpolarization resulted obviously from the increased IKCNA5 as a result of overexpressed KCNA5 channels.
Figure 1.
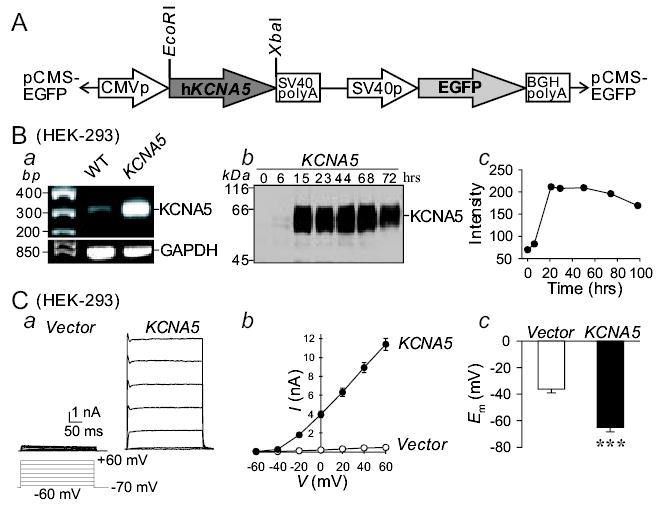
Overespression of the human KCNA5 gene in HEK-293 cells increases whole-cell IK(V) and causes membrane hyperpolarization. A: Map of the construct used to transfect KCNA5. B: The mRNA (a) and protein (b) expression levels of human KCNA5 channels in HEK-293 cells transiently transfected with human KCNA5. The time course (c) of KCNA5 protein levels in cells immediately before (0 hr) or 6, 15, 23, 44, 68, and 72 hrs after initial transfection. C: Representative currents (a), elicited by depolarizing the cells from a holding potential of −70 mV to a series of test potentials ranging from −60 to +60 mV in 20 mV increments, in HEK-293 cells transfected with an empty vector (Vector) or the KCNA5 vector. Summarized current-voltage (I-V) relationship curves (b) and membrane potential, Em (c), in the empty vector- (n=12–19) or KCNA5-transfected cells (n=12–23). *** P<0.001 vs. Vector.
In addition to HEK-293 cells, we were also able to efficiently transfect the human KCNA5 to COS-7 cells as well as rat PASMC and MASMC. Consistent with the results obtained from HEK-293 cells, overexpression of KCNA5 also dramatically increased IK(V) (Fig. 2A) and caused membrane hyperpolarization (Fig. 2B) in COS-7, rat PASMC and MASMC.
Figure 2.
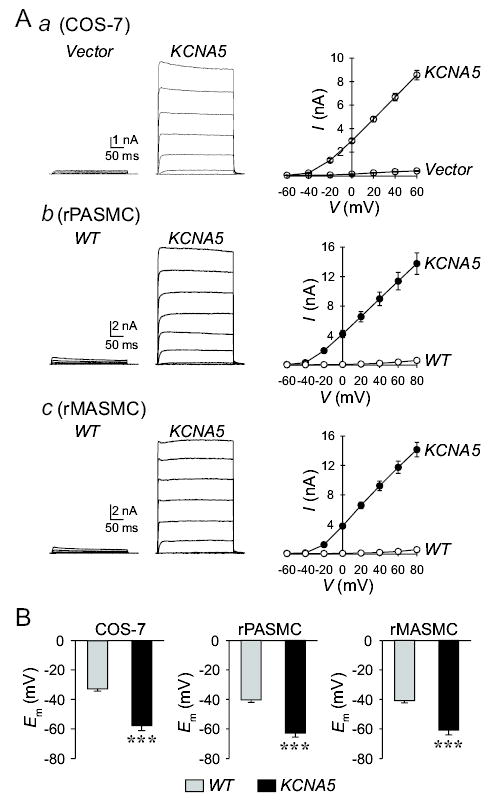
Overexpression of human KCNA5 in COS-7, rat PASMC and MASMC increases IK(V) and causes membrane hyperpolarization. A: Representative whole-cell currents (left panels), elicited by depolarizing the cells from a holding potential of −70 mV to a series of test potentials ranging from −60 to +60 mV in 20 mV increments, in COS-7 (a, n=10), and rat PASMC (b, n=9) and MASMC (c, n=9) transiently transfected with empty vector or with human KCNA5. Summarized current-voltage (I-V) relationship curves are shown in the right panels for empty vector- or KCNA5-transfected cells. B: Summarized data showing membrane potential (Em) in wild-type (WT, gray bars, n=8–9) cells and cells transiently transfected with KCNA5 (solid bars, n=9–10). *** P<0.001 vs. WT; the actual P values are 6.8874×10−6, 6.5943×10−6, and 4.26825×10−5 for COS-7, rat PASMC and rat MASMC, respectively.
Inability of acute hypoxia to inhibit homotetrameric KCNA5 currents in HEK-293 and COS-7 cells.
Functional Kv channels are either homogeneous or heterogeneous α4:β4 tetramers. In HEK-293 and COS-7 cells transiently transfected with KCNA5, IK(V) was mainly generated by K+ efflux through KCNA5 homotetrameric channels. Extracellular application of 4-aminopyridine (4-AP), a Kv channel blocker, significantly and reversibly reduced IKCNA5 in HEK-293 and COS-7 cells (Fig. 3A and B, upper panels). However, reducing Po2 from 154±5 to 32±4 mmHg had little effect on IKCNA5 in these cells (Fig. 3A and B, lower panels). These results are consistent with the report that acute hypoxia had negligible effect on homomeric IKCNA5 in other transfection systems (22). Inhibition of KCNA5 channels with 4-AP in KCNA5-transfected cells caused membrane depolarization in all cell types (data not shown), suggesting that overexpressed KCNA5 channels functionally participate in the regulation of resting membrane potential.
Figure 3.
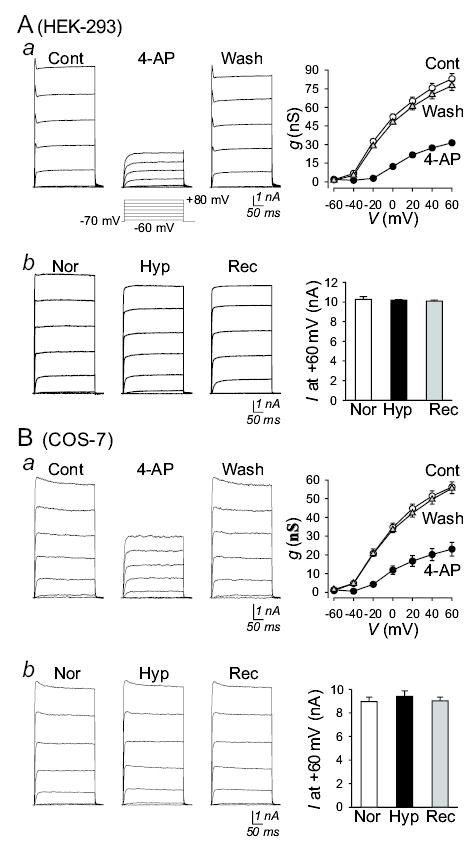
Inability of acute hypoxia to reduce IKCNA5 in HEK-293 and COS-7 cells. Representative whole-cell currents (left panels), elicited by depolarizing the cells from a holding potential of −70 mV to a series of potentials ranging from −60 mV to +60 mV in 20 mV-increments, in KCNA5-transfected HEK-293 (A) and COS-7 (B) cells before (Cont), during (4-AP) and after (Wash) extracellular application of 3 mM 4-AP under normoxic conditions (a), as well as before (Nor), during (Hyp) and after (Rec) reducing Po2 in the superfusate (b). Normalized conductance-voltage (g-V) relationship curves (Aa and Ba, right panels; averaged from multiple cells) were best fitted using the Boltzman equation. Summarized amplitudes of currents at +60 mV (Ab and Bb; averaged from n=6 cells) are shown in the right panels; no significant difference is observed between Nor and Hyp (the actually P values are 0.74488 and 0.48802 for Ab and Bb, respectively).
Acute hypoxia selectively inhibits homomeric KCNA5 channels in rat PASMC.
It is still unclear how acute hypoxia inhibits Kv channels in oxygen-sensitive cells. Hypoxia may reduce IK(V) directly by inhibiting Kv channel function (via the pore-forming α and/or the regulatory β subunits) (22, 42, 43, 56) and/or indirectly by an intermediate produced via a specific oxygen-sensing mechanism in PASMC (3, 5, 36, 57). In addition, the regulatory Kv channel β subunits may serve as an O2 sensor for hypoxia-induced inhibition of Kv channel activity (17, 32). The next set of experiments was designed to examine a) whether acute hypoxia inhibits homomeric KCNA5 channels and b) whether the effect of acute hypoxia on IKCNA5 is selective to PASMC.
As shown in Figure 4, transient transfection of the human KCNA5 gene into rat PASMC increased KCNA5 mRNA expression (Fig. 4A), and produced a single-channel current (iK(V)) with a conductance of 16±5 pS, (n=8) (Fig. 4Ba and Bb). In contrast to HEK-293 and COS-7 cells, acute hypoxia (Po2 = 35±2 mmHg) significantly reduced IKCNA5 in PASMC and the inhibitory effect was reversible upon restoration of extracellular Po2 to 145–150 mmHg (Fig. 4Bc). In these experiments, the whole-cell KCNA5 currents (IKCNA5) (Fig. 4Bc) and the single-channel KCNA5 currents (iKCNA5) (Fig. 4Ba) were recorded from the same rat PASMC (transfected with human KCNA5 gene) in which the single-cell RT-PCR (Fig. 4A) was conducted to elucidate the high expression level of exogenous (human) KCNA5. The primers used in this experiment were specifically designed for amplifying the human KCNA5 transcript; the primers do not completely match with rat KCNA5 (Fig. 4C). The sense (20 bp) and antisense (20 bp) primers contained 5 (25%) and 4 (20%) nucleotides, respectively, that did not match with the corresponding sequence in rat KCNA5 (Fig. 4C, underlined). Therefore, the single-cell RT-PCR products should contain little rat KCNA5 transcripts.
Figure 4.
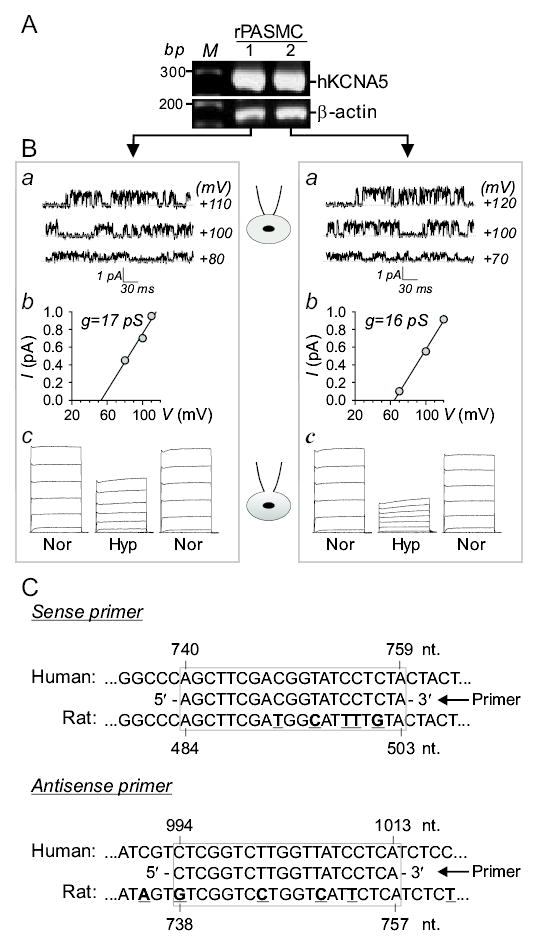
Acute hypoxia reversibly reduces KCNA5 currents in PASMC. Single-cell RT-PCR amplified products for KCNA5 and β-actin (A) and the corresponding single-channel IK(V) (Ba) in two rat PASMC (left and right panels) transiently transfected with the human KCNA5 gene. The single-channel current-voltage (i-V) curves (Bb) of IKCNA5 on cell-attached patches before breaking-in (Ba) are shown in Bb. IKCNA5 (c), elicited by depolarization from a holding potential of −70 mV to potentials ranging from −60 to +60 mV, in the same cells before (Nor, Po2=143–146 mmHg), during (Hyp) and after (Nor) hypoxic challenges (Po2=28–42 mmHg). Data are representative from 8 cells. C: Alignment of the nested primer sequence (specifically designed for human KCNA5 gene) with the sequences of human (NM_00234) and rat (NM_012972) KCNA5 genes. The antisense primer sequence shown here is the reverse complement of the actual antisense primer sequence (5′-TGAGGATAACCAAGACCGAG-3′; nt. 994-1013) shown in Table 1. Underlined and bold letters indicate the nucleotide variations in the rat KCNA5 gene sequence compared to the nested primer sequence designed for human KCNA5 gene.
Furthermore, the hypoxia-mediated decrease in IKCNA5 was selective to PASMC; acute hypoxia had no effect on IKCNA5 in human KCNA5-transfected rat MASMC (Fig. 5), although the amplitude and kinetics of the exogenously transfected KCNA5 channels were comparable in PASMC and MASMC. These results are consistent with our previous report that acute hypoxia selectively reduces native IK(V) in rat PASMC, but not in rat MASMC (56). The selective inhibition of native IK(V) by acute hypoxia has previously been demonstrated by other investigators in canine and rat PASMC and renal arterial smooth muscle cells (3, 42, 43, 56).
Figure 5.
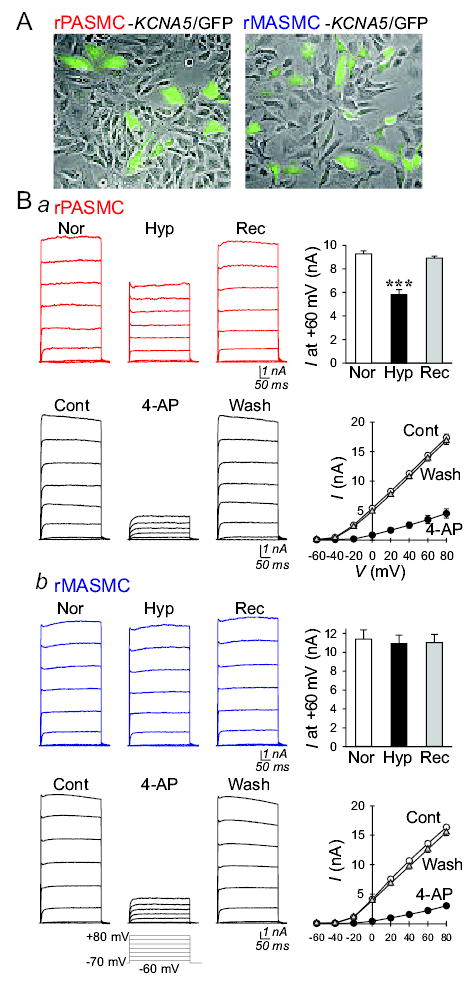
Comparison of acute hypoxia-induced effects on IKCNA5 in rat (r) PASMC and MASMC. A: Identification of cells transiently transfected with human KCNA5/GFP by green fluorescence. B: Representative IKCNA5 (left panels), elicited by depolarizing the cells from a holding potential of −70 mV to a series of potentials ranging from −60 mV to +60 mV in 20 mV-increment, in human KCNA5-transfected rPASMC (Ba) or rMASMC (Bb) before (Nor), during (Hyp) and after (Rec) exposure to hypoxia (Po2=28–43 mmHg) or before (Cont), during (4-AP) and after (Wash) extracellular application of 3 mM 4-aminopyridine (4-AP). Right panels: Summarized data showing current amplitudes at +60 mV (bar graphs) or the current-voltage relationship (I-V) curves in human KCNA5-transfected rPASMC (n=6) and rMASMC (n=6) before, during and after exposure to hypoxia or before (Cont, open circles), during (4-AP, closed circles) and after (Wash, open triangles) extracellular application of 4-AP. ***P<0.001 vs. Nor and Rec bars (the actual P values are 0.000411 and 0.7432978 for Ba and Bb, respectively).
In the aforementioned experiments, hypoxia was established by applying the superfusate containing 0.8 mM Na2S2O4, which reduced Po2 to 22–40 mmHg and stably maintained the low Po2 in the superfusate (in a sealed beaker) for several hours. Application of the hypoxic superfusate (Po2 at 22–40 mmHg) significantly reduced IKCNA5 in rat PASMC (but not in rat MASMC, HEK-293 and COS-7 cells) transfected with human KCNA5 gene (Figs. 4Bc and 5B). Since we used Na2S2O4 (0.8 mM) to reduce Po2 in the superfusate, we also examined whether Na2S2O4 per se affected KCNA5 channel activity. Constantly bubbling the 0.8 mM Na2S2O4-containing solution with room air for 20–30 min increased the solution’s Po2 to approximately 145 mmHg. Perfusion of the Na2S2O4-containing normoxic solution through the cell chamber, as shown in Figure 6A, did not change Po2 in the superfusate applied to cells (which was determined by an oxygen electrode positioned closely to the cells examined). Extracellular application of the Na2S2O4-containing normoxic solution did not alter the amplitude of IKCNA5 in rat PASMC transfected with human KCNA5 (Fig. 6B). These results indicate that the inhibitory effect of the Na2S2O4-containing hypoxic solution on IKCNA5 is due to hypoxia; Na2S2O4 has no effect on Kv channel activity unless accompanied by a reduction in Po2.
Figure 6.
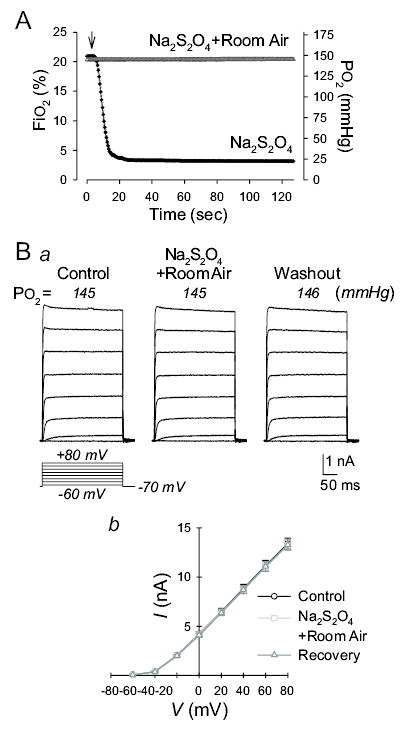
Effect of sodium dithionite (Na2S2O4) without reduction of Po2 on IKCNA5 in rat PASMC. A: Fractional O2 concentration (FiO2) and O2 tension (Po2) determined by an oxygen electrode positioned in the cell chamber, which was superfused with the Na2S2O4-contianing solution continuously bubbled with room air (Na2S2O4+Room air) or with the Na2S2O4-contianing solution without room air bubbling (Na2S2O4). Arrow indicates the time when the perfusion pump was turned on. B: Representative IKCNA5 (a), elicited by depolarizing the cells from a holding potential of −70 mV to a series of potentials ranging from −60 mV to +60 mV in 20 mV-increment, in human KCNA5-transfected rat PASMC before (Control), during (Na2S2O4) and after (Washout) exposure to Na2S2O4-containing normoxic solution (bubbled with room air). Po2 values showed on the oxygen meter while the currents were recorded are indicated in the parentheses. Summarized data (b) showing the current-voltage relationship (I-V) curves in human KCNA5-transfected rat PASMC (n=9) before, during and after exposure to the Na2S2O4-containing normoxic solution (Po2, 145–146 mmHg). The P value for the current amplitudes at +80 mV in cells superfused with Control and Na2S2O4-containing normoxic solution is 0.816699.
Comparable expression levels of various Kv channel β subunits in PASMC and MASMC.
Cytoplasmic auxiliary Kv channel β subunits, with more than 45% homology to NADPH oxidase (32), have been proposed as sensors for hypoxia-mediated inhibition of Kv channels in various oxygen-sensitive cells (17, 18, 22, 30, 45). Co-transfection of Kv channel β subunits with certain α subunits confers the hypoxia sensitivity onto some α subunit homotetramers (17, 40). The expression level of Kv channel β subunits is positively proportional to the vasoconstrictive response to hypoxia in small pulmonary arteries and arterioles compared with large pulmonary arteries (17). In order to examine whether the selective inhibitory effect of hypoxia on KCNA5 channels in PASMC was related to a potentially higher expression level of Kv channel β channels, we compared mRNA expression levels of Kv channel β subunits (e.g., Kvβ1.1, Kvβ2.1, and Kvβ3.1) between rat PASMC and MASMC using primers specifically designed for rat Kv channel β subunits, and between HEK-293 and COS-7 cells using primers specifically designed for human Kv channel β units.
As shown in Figure 7A, rat PASMC and MASMC expressed comparable levels of rat Kvβ1.1, Kvβ2.1, and Kvβ3.1 subunits, whereas acute hypoxia reduced IKCNA5 only in PASMC. These data indicate that the differential response of KCNA5 channels to acute hypoxia in rat PASMC and MASMC appears not to result from a high expression level of Kv channel β subunits in PASMC. In contrast, COS-7 expressed higher level of human Kvβ3.1, but lower levels of human Kvβ1.1 and Kvβ2.1 than HEK-293 cells (Fig. 6B), whereas acute hypoxia had no effect on IKCNA5 in both COS-7 and HEK-293 cells. These data suggest that expression level of Kv channel β subunits is probably not associated with the hypoxic sensitivity of KCNA5 homotetrameric channels in this study.
Figure 7.
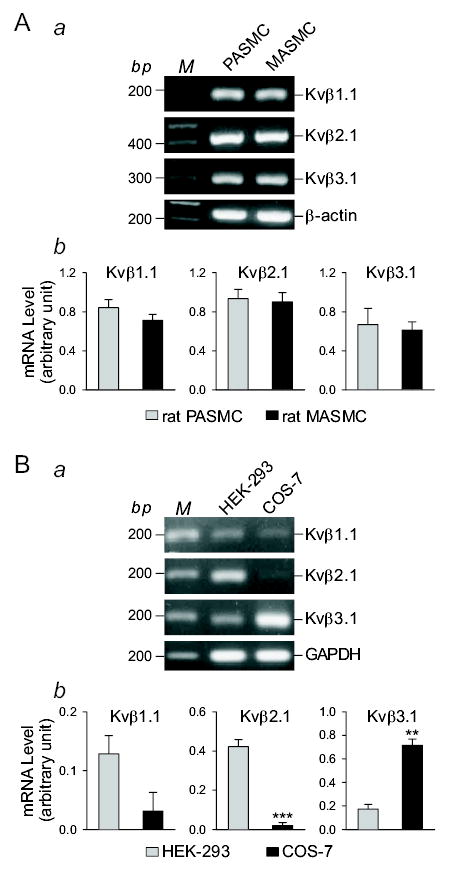
The mRNA expression levels of Kv channel β subunits are comparable in rat PASMC and MASMC. RT-PCR amplified products for rat (A) or human (B) Kvβ1.1, Kvβ2.1, and Kvβ3.1 in rat PASMC, rat MASMC, HEK-293, and COS-7 cells. “M,” 100 bp DNA ladder. RT-PCR amplified products of β-actin and GAPDH are shown as controls. Summarized data (n=15 in Ab, n=3 in Bb) showing the mRNA levels of different rat (A) or human (B) Kv channel β subunits in the cells tested. **P<0.01 vs. HEK-293, ***P<0.001 vs. PASMC/MASMC (A) or COS-7 (B). The actual P values are 0.43950, 0.71439, and 0.70196 for Kvβ1.1, Kvβ2.1, and Kvβ3.1, respectively in Ab and are 0.0927, 0.0004381, and 0.001354 for Kvβ1.1, Kvβ2.1, and Kvβ3.1, respectively in Bb.
It has to be emphasized that we used primers specifically designed for human KCNA5 for the RT-PCR experiments in HEK-293 (human embryonic kidney epithelial cells) and COS-7 (monkey kidney fibroblast-like cells). The different expression levels of Kv channel β subunits between HEK-293 and COS-7 cells might be related to the species difference.
DISCUSSION
Acute alveolar hypoxia causes pulmonary vasoconstriction, whereas hypoxia or hypoxemia causes vasodilation in coronary, renal, and cerebral arteries. Hypoxic pulmonary vasoconstriction is thus a unique property of the pulmonary vasculature, involving multiple mechanisms and cell types (e.g., fibroblasts, smooth muscle cells, and endothelial cells), to maintain an optimal ventilation-perfusion ratio for maximal oxygenation of the venous blood. In vitro experiments have demonstrated that acute hypoxia selectively constricts isolated pulmonary arteries in the presence (20) or absence (29, 58) of endothelial cells, and contracts single PASMC (34, 59), but has little effect on isolated systemic (e.g., mesenteric and renal arteries) and single mesenteric arterial smooth muscle cells. The unique oxygen- or hypoxia-sensitive contractile system present in the pulmonary vasculature or PASMC should include at least three components: a) an oxygen-sensing domain or complex that functions as a receptor; b) signal transduction cascades that may involve multiple pathways or molecules; and c) an efficient effector that leads to smooth muscle contraction.
One of the important cellular mechanisms mediating hypoxic pulmonary vasoconstriction is reduction of IK(V) in PASMC exposed to acute hypoxia, and subsequent membrane depolarization (3, 22, 31, 35, 37, 42, 43, 56). In this scenario, Kv channels function as an effector to induce the membrane depolarization that triggers Ca2+ influx through voltage-dependent Ca2+ channels, increasing [Ca2+]cyt and causing PASMC contraction and pulmonary vasoconstriction. In vitro experiments show that acute hypoxia selectively reduces native IK(V) in PASMC, but not in systemic arterial (e.g., mesenteric, renal, and coronary) smooth muscle cells (43, 48, 56). The selectivity of hypoxia-mediated Kv inhibition in PASMC is consistent with the selectivity of hypoxia-induced contraction in PASMC and vasoconstriction in pulmonary arteries (relative to systemic vascular smooth muscle cells and systemic arteries). The question is then whether the Kv channel itself (e.g., KCNA5 channel) is directly or indirectly affected by acute hypoxia in PASMC.
The results from the present study indicate that overexpression of KCNA5, a Kv channel α subunit ubiquitously expressed in various cell types including pulmonary and systemic arterial smooth muscle cells, increases IK(V) and causes membrane hyperpolarization in HEK-293, COS-7, rat PASMC, and rat MASMC. A similar role for native KCNA5 channels in regulating resting Em has been proposed in PASMC (7, 8, 21, 22), portal vein smooth muscle cells (15, 23), bronchial (1) and cerebral artery smooth muscle cells (13). Therefore, KCNA5 is an important Kv channel α subunit that participates in regulating resting Em. Secondly, the KCNA5 homotetrameric channels in MASMC, HEK-293, and COS-7 cells transiently transfected with the human KCNA5 gene are not sensitive to acute hypoxia. These observations suggest that a) KCNA5 is a critical effector for hypoxia to inhibit IK(V) in PASMC and to induce membrane depolarization, thereby increasing [Ca2+]cyt and causing PASMC contraction; and b) the oxygen-sensing mechanism(s) involved in triggering KCNA5 inhibition, which may be different from oxygen-sensing procedures involved in angiogenesis and erythropoiesis, is an intrinsic property of PASMC. Our findings concur with those of other groups that acute hypoxia preferentially inhibits oxygen-sensitive Kv channels, particularly KCNA5 and KCNB1, to promote hypoxic pulmonary vasoconstriction (6, 8, 21, 22, 44).
There are two schools of thoughts with regard to how acute hypoxia inhibits Kv channels in oxygen-sensitive cells. Hypoxia may reduce IK(V) i) directly by inhibiting Kv channel function (e.g., via conformational changes of the pore-forming α subunits, and interaction of the regulatory β subunits with the α subunits) (22, 42, 43, 56); and/or ii) indirectly by an intermediate released or synthesized via a specific oxygen-sensing complex in PASMC (e.g., oxygen radicals, oxidizing and reducing molecules, metabolic products and by-products) (3, 5, 36, 57). The regulatory Kv channel β subunits, which have been implicated as an oxygen sensor for hypoxia-induced inhibition of Kv channel activity (17, 32), could contribute to a) directly modulating Kv channel function (e.g., gating and inactivation kinetics) by direct interaction with the α subunits, and/or b) indirectly modulating native Kv channel function via their potential enzymatic activity, which could produce an “intermediate” Kv channel inhibitor during acute hypoxia (32, 46).
Given the fact that acute hypoxia selectively causes pulmonary vasoconstriction in vivo and selectively reduces IK(V) in PASMC in vitro, the potential mechanisms involved in hypoxia-mediated Kv channel inhibition have to be specific or intrinsic to PASMC or pulmonary arteries. That is, the “direct or indirect” effects of acute hypoxia must be from a) a specific intermediate that is synthesized or activated exclusively in PASMC during acute hypoxia; b) a motif or complex adjacent to the channel protein (or the pore-forming α subunit) that can be turned on or off in particular in PASMC by acute hypoxia; c) an oxygen- or hypoxia-sensitive Kv channel α and/or β subunit that is distinctively expressed in PASMC (relative to systemic arterial smooth muscle cells); and d) the redox-sensitive amino acid residuals (e.g., cysteine, methionine) that are explicitly present in PASMC Kv channels (α or β subunit) or modulated particularly by the redox status changes in PASMC but not in MASMC (25). It is unclear, however, whether the tertiary structure of Kv channels is different between PASMC and MASMC, and whether Kv channel genes expressed in PASMC have somatic mutations that make the Kv channels more sensitive to hypoxia or redox modulation. Furthermore, qualitative differences in mRNA and protein expression of Kv channel α and β subunits determined by RT-PCR and Western blot analyses have not been revealed so far between pulmonary and systemic arterial smooth muscle cells (e.g., MASMC). Therefore, current knowledge favors the contention that a PASMC-specific intermediate or modulator released or activated during acute hypoxia mediates the hypoxia-induced Kv channel inhibition.
It has been well documented that mitochondria serve as an oxygen-sensing intracellular organelle that is responsible for many hypoxia-induced effects (11, 33, 51–53). Although it remains debatable whether compromised mitochondrial metabolism (or metabolic inhibition) by hypoxia is a cause of sensitivity of PASMC to acute hypoxia, the changes in mitochondrial production or release of reactive oxygen species have been implicated in modulating Kv channel activity in PASMC (33). In cardiac and skeletal muscle (47) as well as the pulmonary vasculature (10, 24, 54), mitochondrial metabolism or ATP production through oxidative phosphorylation is not markedly affected by hypoxia until the Po2 drops to anoxic range (1–2 mmHg) (47), while hypoxic pulmonary vasoconstriction occurs at the Po2 range (e.g., 30–45 mmHg) that does not significantly change the mitochondrial ATP production. In contrast, inhibition of glycolysis using 2-deoxy-D-glucose selectively reduces native IK(V) in PASMC, but not in MASMC (57). These results suggest that metabolic inhibition exerts inhibitory effect on Kv channel activity, but whether hypoxia-mediated inhibition of Kv channels in PASMC results from compromised mitochondrial metabolism is unclear. Nonetheless, mitochondrial production of activated oxygen radicals (e.g., superoxide) has been demonstrated to be altered during acute hypoxia (33, 53), which then lead to direct or indirect (e.g., via altered cellular redox state) inhibition of Kv channel activity.
It has to be emphasized that acute hypoxia causes vasoconstriction only in pulmonary arteries but not in systemic arteries. In other words, the selective contractile effect of acute hypoxia on the pulmonary vasculature requires special attention when searching for potential mechanisms that are involved in hypoxic pulmonary vasoconstriction. Hypoxia may alter various cellular functions (e.g., those relating to angiogenesis, erythropoiesis, adaptation, and acclimatization), however, the mechanism involved in causing hypoxic pulmonary vasoconstriction should show selectivity to PASMC, in comparison to systemic arterial smooth muscle cells.
The data from this study provide evidence that a) a unique oxygen-sensing or hypoxia-sensitive mechanism exists exclusively in PASMC, but not in MASMC, b) KCNA5 (and other Kv channels) is an important effector Kv channel that responds to hypoxia via the PASMC-specific oxygen-sensing mechanism by causing membrane depolarization, and c) the expression level of KCNA5 (relative to other types of Kv channel subunits) may contribute to determining the sensitivity of a PASMC to hypoxia for mediating membrane depolarization and increase in [Ca2+]cyt (via enhanced activity of voltage-dependent Ca2+ channels). Whether depletion of Ca2+ from the sarcoplasmic reticulum or disrupted mitochondrial function abolish the acute hypoxia-induced decrease in IKCNA5 in PASMC remains to be elucidated. Nonetheless, we believe that, among the multiple mechanisms that underlie hypoxic pulmonary vasoconstriction, KCNA5 blockade by a PASMC-specific and oxygen-sensitive cellular process is an underlying cause of the membrane depolarization inherent to hypoxic pulmonary vasoconstriction.
Acknowledgments
We thank Dr. M. Tamkun for providing the Kv1.5 plasmid and Ms. A. Nicholson for technical assistance. This work was supported in part by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL54043, HL064945 and HL66012).
References
- 1.Adda S, Fleischmann BK, Freedman BD, Yu M-F, Hays DWP, Kotlikoff MI. Expression and function of voltage-dependent potassium channel genes in human airway smooth muscle. J Biol Chem. 1996;271:13239–13243. doi: 10.1074/jbc.271.22.13239. [DOI] [PubMed] [Google Scholar]
- 2.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, London B, Hampl V, Wu X, Nsair A, Puttagunta L, Hashimoto K, Waite RE, Michelakis E. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001;15:1801–1803. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci U S A. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv1.2, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer SL, Weir EK, Reeve HL, Michelakis E. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv Exp Med Biol. 2000;475:219–240. doi: 10.1007/0-306-46825-5_21. [DOI] [PubMed] [Google Scholar]
- 8.Archer SL, Wu X-C, Thébaud B, Nsair AI, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 9.Brevnova EE, Platoshyn O, Zhang S, Yuan JX-J. Overexpression of human KCNA5 increases IK(V) and enhances apoptosis. Am J Physiol Cell Physiol. 2004;287:C715–C722. doi: 10.1152/ajpcell.00050.2004. [DOI] [PubMed] [Google Scholar]
- 10.Buescher PC, Pearse DB, Pillai RP, Litt MC, Mitchell MC, Sylvester JT. Energy state and vasomotor tone in hypoxic pig lungs. J Appl Physiol. 1991;70:1874–1881. doi: 10.1152/jappl.1991.70.4.1874. [DOI] [PubMed] [Google Scholar]
- 11.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandy KG and Gutman GA. Voltage-gated K+ channels. In: Ligand- and Voltage-Gated Ion Channels, edited by North RA. Boca Raton, FL: CRC, 1995, p. 1–71.
- 13.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel (KVα1) subunits in terminal arterioles of rabbit. J Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992;262:H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- 15.Clément-Chomienne O, Ishii K, Walsh MP, Cole WC. Identification, cloning and expression of rabbit vascular smooth muscle Kv1.5 and comparison with native delayed rectifier K+ current. J Physiol. 1999;515:653–667. doi: 10.1111/j.1469-7793.1999.653ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comer AM, Gibbons HM, Qi J, Kawai Y, Win J, Lipski J. Detection of mRNA species in bulbospinal neurons isolated from the rostral ventrolateral medulla using single-cell RT-PCR. Brain Res Protoc. 1999;4:367–377. doi: 10.1016/s1385-299x(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 17.Coppock EA, Tamkun MM. Differential expression of KV channel α- and β-subunits in the bovine pulmonary arterial circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1350–L1360. doi: 10.1152/ajplung.2001.281.6.L1350. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Xu J, Bezanilla M, van Huizen R, Derin R, Li M. Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science. 1999;285:1565–1569. doi: 10.1126/science.285.5433.1565. [DOI] [PubMed] [Google Scholar]
- 19.Gurney AM, Osipenko ON, MacMillan D, Kempsill FEJ. Potassium channels underlying the resting potential of pulmonary artery smooth muscle cells. Clin Exp Pharmacol Physiol. 2002;29:330–333. doi: 10.1046/j.1440-1681.2002.03653.x. [DOI] [PubMed] [Google Scholar]
- 20.Harder DR, Madden JA, Dawson C. Hypoxic induction of Ca2+-dependent action potentials in small pulmonary arteries of the cat. J Appl Physiol. 1985;59:1389–1393. doi: 10.1152/jappl.1985.59.5.1389. [DOI] [PubMed] [Google Scholar]
- 21.Hong Z, Weir EK, Nelson DP, Olschewski A. Subacute hypoxia decreases voltage-activated potassium channel expression and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol. 2004;31:337–343. doi: 10.1165/rcmb.2003-0386OC. [DOI] [PubMed] [Google Scholar]
- 22.Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K+ channels expressed in the pulmonary vasculature. Circ Res. 1999;85:489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- 23.Kerr PM, Clément-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2-Kv1.5 channels underlie 4-aminopyridine–sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- 24.Leach RM, Sheehan DW, Chacko VP, Sylvester JT. Energy state, pH, and vasomotor tone during hypoxia in precontracted pulmonary and femoral arteries. Am J Physiol Lung Cell Mol Physiol. 2000;278:L294–L304. doi: 10.1152/ajplung.2000.278.2.L294. [DOI] [PubMed] [Google Scholar]
- 25.López-Barneo J. Oxygen-sensing by ion channel and the regulation of cellular functions. Trends Neurosci. 1996;19:435–440. doi: 10.1016/0166-2236(96)10050-3. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Lopez J, Gonzalez C, Urena J, Lopez-Barneo J. Low po2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-López J, González C, Ureña J, López-Barneo J. Low po2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden JA, Vadula KS, Kurup VP. Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol. 1992;263:L384–L393. doi: 10.1152/ajplung.1992.263.3.L384. [DOI] [PubMed] [Google Scholar]
- 29.Marshall C, Marshall BE. Hypoxic pulmonary vasoconstriction is not endothelium dependent. Proc Soc Exp Biol Med. 1992;201:267–270. doi: 10.3181/00379727-201-43506. [DOI] [PubMed] [Google Scholar]
- 30.Martens JR, Kwak Y-G, Tamkun MM. Modulation of KV channel α/β subunit interactions. Trends Cardiovasc Med. 1999;9:253–258. doi: 10.1016/s1050-1738(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 31.Mauban J, Remillard CV, Yuan JX-J. Hypoxic pulmonary vasoconstriction: Role of ion channels. J Appl Physiol. 2005;98:415–420. doi: 10.1152/japplphysiol.00732.2004. [DOI] [PubMed] [Google Scholar]
- 32.McCormack T, McCormack K. Shaker K+ channel β subunits belong to an NAD(P)H-dependent oxidoreductase superfamily. Cell. 1994;79:1133–1135. doi: 10.1016/0092-8674(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 33.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 34.Murray TR, Chen L, Marshall BE, Macarak EJ. Hypoxic contraction of cultured pulmonary vascular smooth muscle cells. Am J Respir Cell Mol Biol. 1990;3:457–465. doi: 10.1165/ajrcmb/3.5.457. [DOI] [PubMed] [Google Scholar]
- 35.Olschewski A, Hong Z, Nelson DP, Weir EK. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1143–L1150. doi: 10.1152/ajplung.00104.2002. [DOI] [PubMed] [Google Scholar]
- 36.Olschewski A, Hong Z, Peterson DA, Nelson DP, Porter VA, Weir EK. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am J Physiol Lung Cell Mol Physiol. 2004;286:L15–L22. doi: 10.1152/ajplung.00372.2002. [DOI] [PubMed] [Google Scholar]
- 37.Osipenko ON, Evans AM, Gurney AM. Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current. Br J Pharmacol. 1997;120:1461–1470. doi: 10.1038/sj.bjp.0701075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osipenko ON, Tate RJ, Gurney AM. Potential role for Kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–540. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- 39.Patel AJ, Lazdunski M, Honoré E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J. 1997;16:6615–6625. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez-García MT, López-López JR, González C. Kvβ1.2 subunit coexpression in HEK293 cells confers O2 sensitivity to Kv4.2 but not to Shaker channels. J Gen Physiol. 1999;113:897–907. doi: 10.1085/jgp.113.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX-J. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- 42.Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- 43.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 44.Pozeg ZI, Michelakis E, McMurtry MS, Thébaud B, Wu X-C, Dyck JRB, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 45.Rasmusson RL, Wang S, Castellino RC, Morales MJ, Strauss HC. The β subunit, Kvβ1.2, acts as a rapid open channel blocker of NH2 terminal deleted Kv1.4 α-subunits. Adv Exp Med Biol. 1997;430:29–37. doi: 10.1007/978-1-4615-5959-7_3. [DOI] [PubMed] [Google Scholar]
- 46.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 47.Richmond KN, Burnite S, Lynch RM. Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol. 1997;273:C1613–C1622. doi: 10.1152/ajpcell.1997.273.5.C1613. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu S, Bowman PS, Thorne G, III, Paul RJ. Effects of hypoxia on isometric force, intracellular Ca2+, pH, and energetics in porcine coronary artery. Circ Res. 2000;86:862–870. doi: 10.1161/01.res.86.8.862. [DOI] [PubMed] [Google Scholar]
- 49.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Juhaszova M, Rubin LJ, Yuan X-J. Hypoxia inhibits gene expression of voltage-gated K+ channel α subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 52.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 53.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: Redox events in oxygen sensing. J Appl Physiol. 2005;98:404–414. doi: 10.1152/japplphysiol.00722.2004. [DOI] [PubMed] [Google Scholar]
- 54.Wiener CM, Dunn A, Sylvester JT. ATP-dependent K+ channels modulate vasoconstrictor responses to severe hypoxia in isolated ferret rings. J Clin Invest. 1991;88:500–504. doi: 10.1172/JCI115331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan X-J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- 56.Yuan X-J, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- 57.Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. Deoxyglucose and reduced glutathione mimic effects of hypoxia on K+ and Ca2+ conductances in pulmonary artery cells. Am J Physiol. 1994;267:L52–L63. doi: 10.1152/ajplung.1994.267.1.L52. [DOI] [PubMed] [Google Scholar]
- 58.Yuan X-J, Tod ML, Rubin LJ, Blaustein MP. Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol. 1990;259:H281–H289. doi: 10.1152/ajpheart.1990.259.2.H281. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Carson RC, Zhang H, Gibson G, Thomas HM., 3rd Pulmonary artery smooth muscle cell [Ca2+]i and contraction: responses to diphenyleneiodonium and hypoxia. Am J Physiol. 1997;273:L603–L611. doi: 10.1152/ajplung.1997.273.3.L603. [DOI] [PubMed] [Google Scholar]


