Abstract
Signaling via G-protein coupled receptors is initiated by receptor-catalyzed nucleotide exchange on Gα subunits normally bound to GDP and Gβγ. Activated Gα·GTP then regulates effectors such as adenylyl cyclase. Except for Gβγ, no known regulators bind the adenylyl cyclase-stimulatory subunit Gαs in its GDP-bound state. We recently described a peptide, KB-752, that binds and enhances the nucleotide exchange rate of the adenylyl cyclase-inhibitory subunit Gαi. Herein, we report that KB-752 binds Gαs·GDP yet slows its rate of nucleotide exchange. KB-752 inhibits GTPγS-stimulated adenylyl cyclase activity in cell membranes, reflecting its opposing effects on nucleotide exchange by Gαi and Gαs.
Keywords: Adenylyl cyclase, Biosensors, G-proteins, Phage display, Signal transduction, Surface plasmon resonance
1. Introduction
G-proteins serve as crucial intermediaries of extracellularly-evoked signaling cascades critical to cellular physiology [1]. In the conventional model of heterotrimeric G-protein signaling, extracellular cues such as hormones and neurotransmitters activate seven transmembrane domain receptors (GPCRs) coupled to heterotrimers consisting of Gα·GDP bound to Gβγ in the inactive state [2]. Gβγ stabilizes the GDP-bound state and prevents spontaneous nucleotide exchange, thus serving as a guanine nucleotide dissociation inhibitor (GDI). In contrast, GPCR activation leads to exchange of GDP for GTP on Gα, thus serving as a guanine nucleotide exchange factor (GEF). Binding of GTP alters the conformation of three flexible “switch” regions within Gα, causing Gβγ dissociation [3]. Both Gα·GTP and freed Gβγ can subsequently regulate several downstream signaling components including adenylyl cylcases (ACs), phospholipases, and ion channels responsible for the elicited cellular responses [2]. Signal termination is achieved by the intrinsic GTP hydrolysis activity of Gα, regenerating Gα·GDP capable of reassociation with Gβγ and thereby preventing effector interactions. Given this bimodal nucleotide cycle, the lifetime of activated signaling is reliant on the duration of Gα in the GTP-bound state. In this fashion, G-proteins serve as critical temporal regulators of these pathways and thus understanding the molecular determinants underlying their nucleotide cycle is of particular interest.
Phage display is a powerful technique for identifying small peptides capable of binding protein targets in an unbiased manner. These peptides can then serve as tools for studying target protein binding surfaces, protein-protein interaction sites, and protein function and regulation [reviewed in [4]]. Phage display, along with similar approaches, has been used to investigate G-protein binding interfaces on GPCRs and effector binding regions on Gβγ subunits, as well as to identify peptides with regulatory properties including GEF and GDI activities [5-9]. We recently used phage display to identify a peptide that interacts with the AC inhibitory Gα subunit Gαi, specifically in its GDP-bound state. This peptide, KB-752, exerts GEF activity on Gαi, the molecular determinants for which were ascertained by the Gαi1·GDP/KB-752 crystal structure [10].
Here, we demonstrate that KB-752 is capable of binding the AC stimulatory Gα subunit, Gαs, in a GDP-selective manner similar to its interaction with Gαi. Analysis of mutations perturbing the Gαi1/KB-752 binding interface revealed a mutation retaining activity for Gαs, thus improving its Gα selectivity profile. Via N-terminal conjugation with the fluorescent dye FITC, KB-752 was found to act as a sensor for the GDP-bound conformation of Gαs. However, in contrast to its GEF activity toward Gαi, binding of KB-752 to Gαs·GDP was found to slow the rate of spontaneous nucleotide exchange in vitro, indicating that KB-752 is a GDI for Gαs. Furthermore, KB-752 was capable of inhibiting both GTPγS- and forskolin-stimulated generation of cyclic AMP by AC in cell membranes, consistent with the GDI activity of this peptide towards Gαs, a central participant in AC regulation. Our findings suggest that the KB-752 peptide, and variants thereof, will serve as valuable molecular tools to modulate cellular AC activity.
2. Materials and Methods
2.1. Materials.
Unless otherwise noted, all reagents were purchased from Sigma. Peptides were synthesized by Anaspec (San Jose, CA), except for the N-terminally FITC-labelled KB-752, the N-terminally palmitoylated KB-752 and its scrambled and W5A mutant counterparts which were synthesized by Dr. Michael Berne and the Tufts University Core Facility (www.tucf.org).
2.2. Protein purification.
Recombinant Gαi1 and Gαo proteins were expressed and purified as described [10]. His6-tagged bovine Gαs (short splice variant) was purified from BL21(DE3) E. coli essentially as previously described [11]. Briefly, protein expression from the prokaryotic plasmid pPRO-EXHTb-Gαs was induced at an OD600 = 0.6 with 0.1 mM IPTG for 16-18 hours at 20°C. Gαs was then purified by sequential Ni2+ nitrilotriacetate, anion exchange, and size exclusion chromatographies [11].
2.3. Surface plasmon resonance (SPR) biosensor measurements.
All SPR binding assays were performed at 25°C on a BIAcore 3000. To analyze nucleotide-dependent binding of KB-752 to Gαs, an N-terminally biotinylated KB-752 (diluted to 0.1 μg/ml in BIA running buffer [10 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM MgCl2, and 0.005 % NP40]) was coupled to separate flow cells of streptavidin biosensor chips to a surface density of approximately 250, 500, or 1000 response units (this variation of surface density was used as an internal control to ensure accuracy in binding affinity calculations). Prior to injection, Gαs was diluted in BIA running buffer containing 100 μM GDP, 100 μM GDP plus 30 μM AlCl3 and 10 mM NaF, or 100 μM GTPγS and allowed to incubate at room temperature for 2-3 hours. Thirty microliters of Gα subunits were then simultaneously injected over flow cells at 5 μL/min followed by a 300 sec dissociation phase. Binding to a non-Gα-interacting peptide (C-tail of mNOTCH1, PSQITHIPEAFK; [12]) was subtracted from all binding curves to correct for nonspecific binding and buffer shifts created during injection. Surfaces were regenerated between each injection with two injections of 10 μL regeneration buffer (500 mM NaCl and 25 mM NaOH) at 20 μL/min. Binding curves and kinetic analyses were conducted using BIAevaluation software version 3.0. Binding affinities were calculated with the simultaneous association and dissociation rate analysis parameter using generated curves.
2.4. Fluorimetric binding assays.
Assays were conducted in buffer containing 10 mM Tris/HCl, pH 8.0, 1 mM EDTA, 10 mM MgCl2, 150 mM NaCl, and 50 μM GDP. Experiments conducted in the presence of AlF4- were performed by supplementing the above buffer with 10 mM NaF and 30 μM AlCl3. Gαs·GDP and Gαs·GDP·AlF4- were prepared as described for SPR. Fluorescence measurements were made using a LS-55B spectrofluorimeter (Perkin Elmer). Timecourse measurements were made at 5 second intervals using excitation and emission wavelengths of 494 nm and 515 nm, respectively, and slit widths of 5 nm. Emission scans were performed at a rate of 20 nm·min-1 using an excitation wavelength of 440 nm, with slit widths of 5 nm.
2.5. GTPγS exchange and steady-state GTPase assays.
GTPγS binding assays were conducted at 20 °C using a nitrocellulose filter binding method detailed previously [13]. Steady-state GTPase assays were carried out at 20 °C using a charcoal precipitation as described previously [14].
2.6. Adenylyl cyclase assays.
HEK293 cell monolayers were lysed with ice-cold hypotonic buffer (1 mM Na+-HEPES, pH 7.4, 2 mM EDTA). Scraped cell lysates were centrifuged at 30,000 × g for 20 min. The resulting crude membrane fraction was resuspended (1 mg/ml) with a glass-teflon handheld homogenizer (8-10 strokes) in storage buffer (15 mM Na+-HEPES, pH 7.4, 1 mM EDTA) and frozen at -70° C until assayed. Total protein levels were determined with a BCA protein assay kit from Pierce (Rockfield, IL). AC assays were carried out as described previously [15] with modifications. Briefly, frozen membranes were thawed and added (15-40 μg of protein/tube) to duplicate assay tubes containing the reaction mixture (15 mM Na+-HEPES, pH 7.4, 4.5 mM phosphocreatine, 5 mM MgCl2, 0.25 mM ATP, 0.5 mM isobutylmethylxanthine, 3 units of creatine phosphokinase) in a final volume of 100 μL. Incubations were carried out at 30° C, then terminated by the addition of 200 μL 3% trichloroacetic acid. Tubes were vortexed and centrifuged for 10 min at 14,000 x g. cAMP in the supernatant was quantified using a competitive binding assay previously described [15] with minor modifications. Duplicate samples of supernatants (15 μl) were added to reaction tubes. [3H]cAMP (∼1 nM final concentration) and cAMP binding protein (ca. 150 mg) were diluted in cAMP assay buffer (100 mM Tris/HCl pH 7.4, 100 mM NaCl, 5 mM EDTA) and then added to each well for a total volume of 550 μL. The tubes were incubated on ice for 2 hr and harvested by filtration (Packard Unifilter GF/C) using a 96-well Packard Filtermate Cell harvester (Meriden, CT). The filters were allowed to dry, and Microscint O scintillation fluid was added. Filter radioactivity was determined using a Packard TopCount scintillation/luminescence detector. cAMP concentrations in each sample were estimated in duplicate from a standard curve ranging from 0.1 to 300 pmol of cAMP per assay.
3. Results and Discussion
3.1 Nucleotide selective interaction between KB-752 and Gαs:
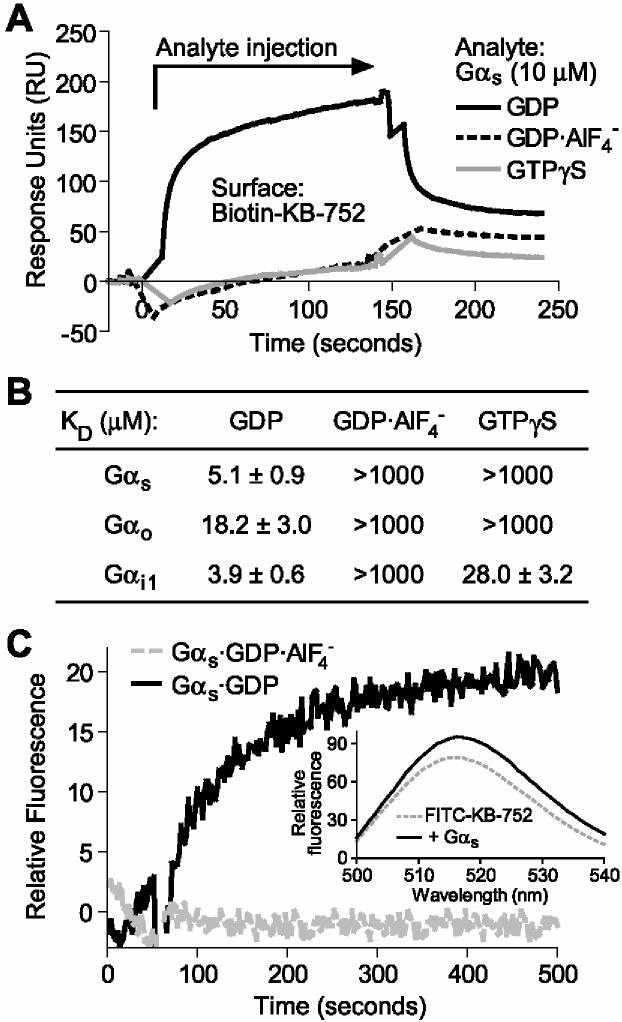
We previously demonstrated that the phage display-derived peptide KB-752 (SRVTWYDFLMEDTKSR) interacts preferentially with the GDP-bound conformation of Gαi1 (KD of ∼4 μM; [10]). We have since found that KB-752 also interacts with Gαs in a GDP-selective manner (Figure 1). In SPR binding assays, immobilized KB-752 peptide showed a robust interaction with recombinant Gαs in the presence of GDP; no detectable interaction was seen with Gαs in either the GDP·AlF4-- or GTPγS-bound states (Figure 1A). Using simultaneous kon and koff rate calculations, the apparent dissociation constant (KD) for the KB-752/Gαs·GDP interaction was found to be 5.1 ± 0.9 μM (Figure 1B). These results indicate that KB-752 interacts selectively with the inactive, GDP-bound state of Gαs with an affinity similar to that for its original target interactor, Gαi1·GDP.
Figure 1.
Nucleotide-dependent binding of KB-752 to Gαs. (A) 10 μM of Gαs (“Analyte”), in each of the indicated nucleotide bound states, was injected over immobilized KB-752 and binding measured by SPR. Non-specific binding to a control biotinylated peptide surface was subtracted from each curve. (B) Gαs, Gαo, and Gαi1 were separately injected at increasing concentrations (0.01 to 50 μM) over immobilized KB-752 to determine the dissociation constants (KD) for each interaction pair as obtained from simultaneous kon/koff analyses (n = 4-6 for each state). (C) Time course of FITC-KB-752 binding to Gαs. 100 nM FITC-KB-752 was equilibrated in buffer containing either GDP (black trace) or GDP·AlF4- (grey trace) and background fluorescence subtracted. At 50 seconds, 1 μM Gαs·GDP (black trace) or 1 μM Gα·GDP·AlF4- (grey trace) was added. (Inset) Emission scans of FITC-KB-752 in the absence and presence of Gαs·GDP obtained using an excitation wavelength of 440 nm.
The nucleotide state-selective nature of the Gα/KB-752 interaction suggests a possible application of peptides such as KB-752 as specific sensors for Gα conformational state. As proof-of-principle for such an application, we tested a KB-752 variant containing a covalently-linked N-terminal fluorescein isothiocyanate (FITC) group. Adding Gαs·GDP to a solution containing FITC-KB-752 resulted in an increase in fluorescence intensity above baseline (Figure 1C). Importantly, addition of Gαs·GDP·AlF4- showed no change in fluorescence response. These results suggest that fluorescent dye-labelled KB-752 can serve as a biosensor for the GDP-bound conformation of Gαs. Analysis of the emission spectrum of FITC-KB-752 prior to and following the addition of Gαs·GDP indicates an ∼20% increase in overall fluorescence intensity yield (Figure 1C inset). Thus, while this modified peptide represents a potentially novel tool for studying Gαs activation/deactivation dynamics in vitro [16], further development strategies to increase the change in fluorescence quantum yield will be required for its utility in in vivo applications.
3.2 GDI activity of KB-752 toward Gαs:
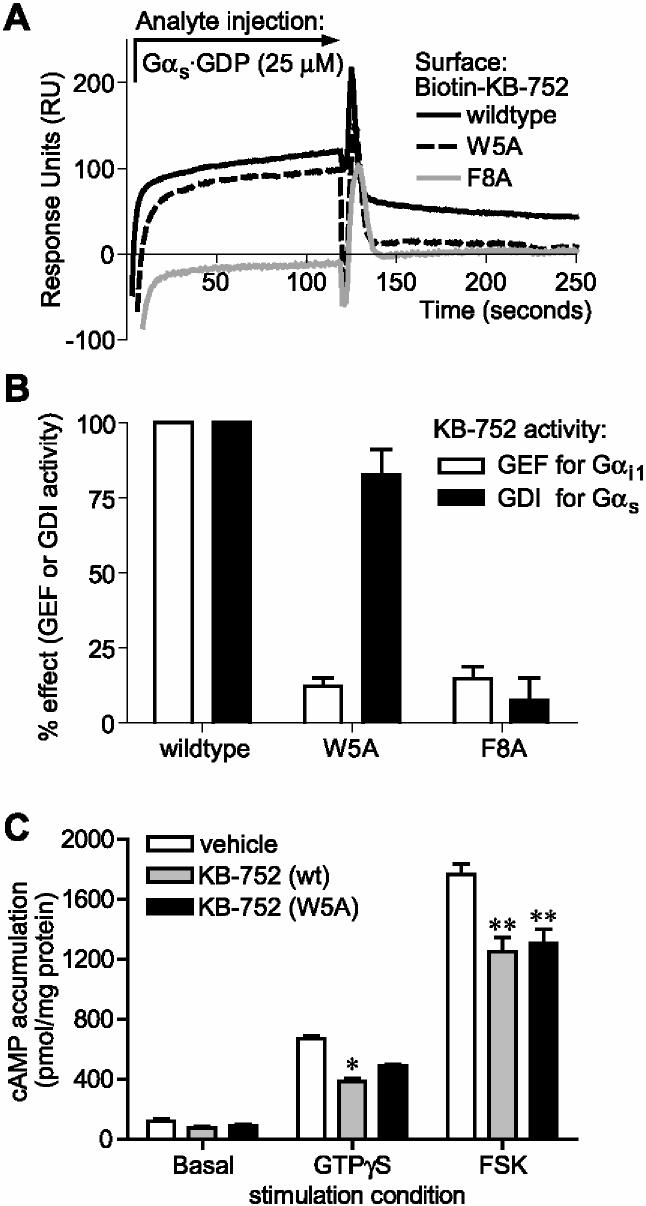
KB-752 serves as a guanine nucleotide exchange factor for Gαi1, Gαi2, and Gαi3 subunits [10]. In contrast, KB-752 slowed the rate of spontaneous nucleotide exchange by Gαs, as measured by the binding of [35S]GTPγS (Figure 2A). The effective concentration for 50% of maximal GDI activity (EC50) toward Gαs was 4.5 ± 1.9 μM (Figure 2B), agreeing closely with the observed binding affinity. To confirm this GDI activity, we also measured the effect of KB-752 on the steady-state GTPase rate of Gαs. Given that GDP release is the rate-limiting step in the guanine nucleotide cycle for isolated Gα subunits [17], any modulation of nucleotide exchange is reflected in the overall steady-state rate [14]. KB-752 association was found to reduce the steady-state GTPase rate of Gαs (Figure 2C), further supporting the discovery of GDI activity for this peptide/Gα pairing.
Figure 2.
. GDI activity of KB-752 on Gαs assessed by GTPγS binding and steady-state GTPase measurements. (A) 50 nM Gαs was incubated in the absence or presence of 5 μM KB-752 for 15 minutes at room temperature prior to addition of [35S]GTPγS. Duplicate reaction aliquots were removed at indicated times and protein-bound radioactivity counted. (B) 50 nM Gαs or Gαo was incubated with the indicated concentrations of KB-752 as in (A) and GTPγS binding reactions were incubated at 20 °C for 5 minutes. (C) 100 nM Gαo or Gαs was incubated with 10 μM KB-752 for 15 minutes at room temperature prior to the addition of [γ-32P]GTP and measurement of released [32P] inorganic phosphate.
Gβγ serves as the prototypical GDI in the conventional model of GPCR-mediated signal transduction. Gβγ binds Gα·GDP and couples the resulting heterotrimer to receptor, as well as preventing spontaneous GDP release [18,19]. More recently, we and others have described the GoLoco motif within several proteins that binds Gαi family members and slows spontaneous release of GDP [11,20-22]. However, the GoLoco motif does not interact with nor serve as a GDI for Gαs [20], and no similar protein domain has been identified for Gαs to date. Thus, KB-752 serves as a unique peptide capable of serving as a GDI for Gαs.
3.3 Inhibition of AC activity by KB-752:
Gαs·GTP stimulates the ability of AC to generate cAMP from ATP, whereas Gαi family members inhibit AC activity when in their GTP-bound state. Thus, we hypothesized that KB-752, by virtue of its GDI activity on Gαs and GEF activity on Gαi [10], could inhibit AC activity by modulating the balance of stimulatory and inhibitory inputs from Gα subunits. To test this idea, we incubated HEK293 cell membranes with an N-terminally palmitoylated version of KB-752 and examined cAMP accumulation. Palmitoylated KB-752 peptide was found to inhibit GTPγS-stimulated cAMP accumulation, whereas a sequence-scrambled, control palmitoylated peptide did not significantly affect cAMP accumulation (Figure 3). KB-752 had no effect on basal cAMP accumulation. These results suggest that KB-752 can modulate G-protein activation in a cellular context and in a manner consistent with its effects measured using purified Gα subunits.
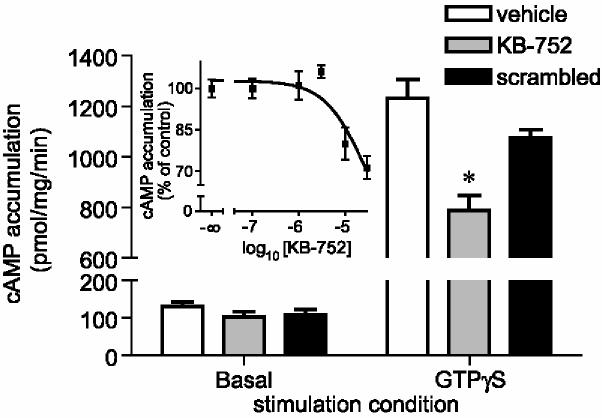
Figure 3.
. Effect of KB-752 on adenylyl cyclase activity. Basal or 10 μM GTPγS-stimulated cAMP accumulation over 10 minutes was measured from HEK293 cell membrane preparations in the absence (vehicle) or presence of 30 μM wildtype, palmitoylated KB-752 or a sequence-scrambled version. *, p < 0.05 compared to vehicle; one-way ANOVA followed by Dunnett's (n = 4). Inset: HEK293 membrane preparations were stimulated with 10 μM GTPγS in the presence of indicated molar concentrations of wildtype palmitoylated KB-752 and cAMP accumulation was measured following 10 minutes. Data are shown as the mean ± SEM of 4 independent experiments and expressed as percent of cAMP generated in the absence of peptide.
3.4 Generation of a KB-752 variant with improved Gα selectivity:
Our study of the structural determinants of KB-752 binding to Gαi1·GDP indicated that residues W5, F8, and E11 within KB-752 make critical contacts with Gαi1; for example, mutating either the W5 or F8 positions to alanine results in a loss of Gαi1 binding and GEF activity on Gαi1 [10]. We investigated the effects of these two mutations on the KB-752/Gαs interaction. Whereas the W5A mutant peptide retained Gαs association and GDI activity (albeit with an accelerated off-rate; Figure 4A), the F8A mutation abrogated both binding and GDI activity toward Gαs (Figure 4A,B). These results suggest that F8, but not W5, is critical to the interaction of KB-752 with Gαs. Our findings identify KB-752(W5A) as a Gαs-selective variant of KB-752 that retains the ability to inhibit GTPγS-stimulated AC activation (Figure 4C), although more weakly than that of wildtype, presumably reflecting its loss of Gαi-directed GEF activity and/or reduction in Gαs affinity. Both wildtype and W5A mutant palmitoylated KB-752 peptides were also capable of inhibiting forskolin-stimulated AC activity (Figure 4C); this inhibition is likely related to Gαs-directed GDI activity, as Gαs and forskolin are known to activate various AC isoforms in a synergistic fashion [23,24].
Figure 4.
. Effects of alanine substitutions to KB-752 binding, GDI activity, and attenuation of AC activation. (A) N-terminally biotinylated KB-752 mutant (W5A, F8A) or wildtype peptides were each immobilized to a density of ∼1000 response units (RUs) on separate streptavidin-coated flow cells and 25 μM of GDP-bound Gαs (“Analyte”) was injected simultaneously over all surfaces. (B) Purified Gαs or Gαi1 was incubated with the indicated KB-752 peptide and [35S]GTPγS binding was measured following incubation for 5 minutes at 20 °C. (C) cAMP accumulation over 10 minutes at 30 °C was measured from HEK293 cell membrane preparations stimulated with 10 μM GTPγS or 30 μM forskolin (FSK) in the absence (vehicle) or presence of 30 μM wildtype, palmitoylated KB-752 (wt) or the tryptophan-5 substituted version (W5A). *, p < 0.05; **, p < 0.01 compared to vehicle; one-way ANOVA followed by Dunnett's (n = 3).
Although attempts to crystallize a Gαs·GDP/KB-752 complex have not yet succeeded, several predictions can be made as to the structural determinants of this interaction. First, in light of its nucleotide-dependent nature, the Gαs·GDP/KB-752 interaction presumably involves one or more of the three switch regions of Gαs that undergo conformational changes throughout the nucleotide cycle [25]. Second, while the structural determinants for KB-752 binding to Gαs may partially overlap with those for Gαi1 binding (e.g., F8 in KB-752 is critical for binding both targets), the exact interactions and relative positions of the KB-752 binding interface likely differs. This is underscored by the W5A mutant which retains activity towards Gαs. This tryptophan residue participates in critical Gαi1 contacts and underlies the GDP-dependent binding [10]. The hydrophobic binding pocket in Gαi1 responsible for KB-752 binding (i.e., the cleft between the α2 and α3 helices) represents a highly conserved region within Gα subunits, including Gαs. Thus, while the F8 residue of KB-752 is still critical for interaction with Gαs (Figure 4), the dispensability of W5 suggests a different interaction site with Gαs versus Gαi1, perhaps a shift in peptide orientation within the α2/α3 hydrophobic groove. Third, KB-752 may make direct contact with the bound GDP within Gαs and/or bind in a manner that occludes its release. This mode of binding is seen in GoLoco-induced inhibition of GDP release from Gαi1 [21]. Alternatively, KB-752-mediated GDI activity could arise by the repositioning of the switch regions such as to inhibit GDP release.
In conclusion, we have found that KB-752 binds to the AC-stimulatory Gαs protein in a GDP-selective manner. We had previously shown that KB-752 interacts with Gαi1 in a similar nucleotide-dependent manner [10]. Whereas KB-752 has GEF activity for Gαi1, we demonstrate here that KB-752 possesses the diametrically-opposite biochemical activity for Gαs, namely GDI activity. These opposing activities on Gαi and Gαs result in an inhibition of cAMP production via G protein-modulated AC activity in cell membrane preparations. We serendipitously found a mutation (W5A) that prevents binding of KB-752 to Gαi1, thereby creating a more selective peptide that could be used to perturb Gαs signaling without affecting Gαi-mediated processes. Our observation that the Gαs-selective KB-752(W5A) variant retains the ability to decrease GTPγS- and forskolin-stimulated AC activity suggests that the predominant mechanism of action of KB-752 as an AC inhibitor resides in its GDI activity towards Gαs. Although the sequence of KB-752 does not correspond to any naturally-occurring sequence [10], its nucleotide-dependent binding and GDI activity make it a potentially powerful molecular tool for studying Gαs signaling.
4. Acknowledgements
We thank Chau H. Nguyen (Purdue Univ.) for technical assistance and Dr. Francis Willard (UNC) for design and first proof-of-concept data for the FITC-KB-752 peptide as well as critical appraisal of this manuscript. This work was supported by NIH grants R01 GM062338 and P01 GM065533 to D.P.S. and R01 MH060397 to V.J.W.
Footnotes
- AC
- adenylyl cyclase [E.C. 4.6.1.1]
- AMP
- adenosine monophosphate
- cAMP
- cyclic AMP
- FITC
- fluorescein isothiocyanate
- FSK
- forskolin
- GDI
- guanine nucleotide dissociation inhibitor
- GEF
- guanine nucleotide exchange factor
- GPCR
- G-protein coupled receptor
- GTPγS
- gamma-thiol guanosine triphosphate
- SPR
- surface plasmon resonance
REFERENCES
- 1.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–72. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 3.Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–78. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 4.Rodi DJ, Makowski L, Kay BK. One from column A and two from column B: the benefits of phage display in molecular-recognition studies. Curr Opin Chem Biol. 2002;6:92–6. doi: 10.1016/s1367-5931(01)00287-3. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist A, et al. Antagonists of the receptor-G protein interface block Gi-coupled signal transduction. J Biol Chem. 1998;273:14912–9. doi: 10.1074/jbc.273.24.14912. [DOI] [PubMed] [Google Scholar]
- 6.Hessling J, Lohse MJ, Klotz KN. Peptide G protein agonists from a phage display library. Biochem Pharmacol. 2003;65:961–7. doi: 10.1016/s0006-2952(02)01653-2. [DOI] [PubMed] [Google Scholar]
- 7.Ja WW, Roberts RW. In vitro selection of state-specific peptide modulators of G protein signaling using mRNA display. Biochemistry. 2004;43:9265–75. doi: 10.1021/bi0498398. [DOI] [PubMed] [Google Scholar]
- 8.Martin EL, Rens-Domiano S, Schatz PJ, Hamm HE. Potent peptide analogues of a G protein receptor-binding region obtained with a combinatorial library. J Biol Chem. 1996;271:361–6. doi: 10.1074/jbc.271.1.361. [DOI] [PubMed] [Google Scholar]
- 9.Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA, Taussig R, Smrcka AV. Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein betagamma subunits is used for recognition of a subclass of effectors. Embo J. 2001;20:767–76. doi: 10.1093/emboj/20.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston CA, et al. Structure of G-alpha-i1 bound to a GDP-selective peptide provides insight into guanine nucleotide exchange. Structure. 2005;13:1069–80. doi: 10.1016/j.str.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimple RJ, De Vries L, Tronchere H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP. RGS12 and RGS14 GoLoco motifs are G-alpha-i interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem. 2001;276:29275–81. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- 12.Snow BE, Brothers GM, Siderovski DP. Molecular cloning of regulators of G-protein signaling family members and characterization of binding specificity of RGS12 PDZ domain. Methods Enzymol. 2002;344:740–61. doi: 10.1016/s0076-6879(02)44752-0. [DOI] [PubMed] [Google Scholar]
- 13.Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, Siderovski DP, Gonczy P. RIC-8 is required for GPR-1/2-dependent G-alpha function during asymmetric division of C. elegans embryos. Cell. 2004;119:219–30. doi: 10.1016/j.cell.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Ross EM. Quantitative assays for GTPase-activating proteins. Methods Enzymol. 2002;344:601–17. doi: 10.1016/s0076-6879(02)44743-x. [DOI] [PubMed] [Google Scholar]
- 15.Cumbay MG, Watts VJ. Novel regulatory properties of human type 9 adenylate cyclase. J Pharmacol Exp Ther. 2004;310:108–15. doi: 10.1124/jpet.104.065748. [DOI] [PubMed] [Google Scholar]
- 16.Kimple RJ, Jones MB, Shutes A, Yerxa BR, Siderovski DP, Willard FS. Established and emerging fluorescence-based assays for G-protein function: heterotrimeric G-protein alpha subunits and regulator of G-protein signaling (RGS) proteins. Comb Chem High Throughput Screen. 2003;6:399–407. doi: 10.2174/138620703106298491. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson KM, Higashijima T, Smigel MD, Gilman AG. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J Biol Chem. 1986;261:7393–9. [PubMed] [Google Scholar]
- 18.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell. 1995;83:1047–58. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 19.Higashijima T, Ferguson KM, Sternweis PC, Smigel MD, Gilman AG. Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. J Biol Chem. 1987;262:762–6. [PubMed] [Google Scholar]
- 20.Willard FS, Kimple RJ, Siderovski DP. Return of the GDI: the GoLoco motif in cell division. Annu Rev Biochem. 2004;73:925–951. doi: 10.1146/annurev.biochem.73.011303.073756. [DOI] [PubMed] [Google Scholar]
- 21.Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP. Structural determinants for GoLoco-induced inhibition of nucleotide release by G-alpha subunits. Nature. 2002;416:878–81. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 22.Cismowski MJ, Takesono A, Bernard ML, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-proteins. Life Sci. 2001;68:2301–8. doi: 10.1016/s0024-3205(01)01019-0. [DOI] [PubMed] [Google Scholar]
- 23.Darfler FJ, Mahan LC, Koachman AM, Insel PA. Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J Biol Chem. 1982;257:11901–7. [PubMed] [Google Scholar]
- 24.Wong SK, Martin BR. The role of a guanine nucleotide-binding protein in the activation of rat liver plasma-membrane adenylate cyclase by forskolin. Biochem J. 1983;216:753–9. doi: 10.1042/bj2160753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wall MA, Posner BA, Sprang SR. Structural basis of activity and subunit recognition in G protein heterotrimers. Structure. 1998;6:1169–83. doi: 10.1016/s0969-2126(98)00117-8. [DOI] [PubMed] [Google Scholar]