Summary
Advances in medical technology have increased the number of individuals who survive cardiac arrest/cardiopulmonary resuscitation (CPR). This increased incidence of survival has created a population of patients with behavioral and physiologic impairments. We used temperature manipulations to characterize the contribution of central nervous system damage to behavioral deficits elicited by 8 minutes of cardiac arrest/CPR in a mouse model. Once sensorimotor deficits were resolved, we examined anxiety-like behavior with the elevated plus maze and social interaction with an ovariectomized female. We hypothesized that anxiety-like behavior would increase and social interaction would decrease in mice subjected to cardiac arrest/CPR and that these changes would be attributable to central nervous system damage rather than damage to peripheral organs or changes orchestrated by the administration of epinephrine. Mice that were subjected to cardiac arrest/CPR while the peripheral organs, but not the brain, were protected by hypothermia exhibited increased anxiety-like behavior and decreased social interaction, whereas mice with hypothermic brains and peripheral organs during cardiac arrest/CPR did not exhibit behavioral impairments. The present study demonstrates that central nervous system damage from cardiac arrest/CPR results in increased anxiety and decreased social interaction and that these behavioral changes are not attributed to underlying sensorimotor deficits, dynamics of arrest and CPR, or peripheral organ damage.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Glucocorticoids, Sudden death, Anxiety, Social interaction
Cardiac arrest/cardiopulmonary resuscitation (CPR) results in one death every minute in the United States and approximately 250,000 deaths per year (American Heart Association, 2002). Increased public awareness of symptoms and advances in medical technology have increased the number of individuals who survive cardiac arrest/CPR through early defibrillation, temperature control, and drug therapies (Bunch et al., 2003; Hypothermia After Cardiac Arrest Study Group, 2002; Paradis et al., 2002). The increased incidence of survival presents a class of physiologic and behavioral deficits that are related to the anoxic/hypoxic state experienced during cardiac arrest/CPR (de Vos et al., 1999; Drysdale et al., 2000; Pusswald et al., 2000). Several laboratories have examined the molecular and cellular effects of cardiac arrest/CPR (Bottiger et al., 1999; Katz et al., 2001; Sadowski et al., 2002), but until recently, little was known about behavioral deficits in cardiac arrest/CPR survivors.
The effect of surviving cardiac arrest/CPR on quality of life is poorly understood. Several clinical reports suggest that cardiac arrest/CPR survivors experience a quality of life similar to the general population (Bunch et al., 2003; Graves et al., 1997). In contrast, others report that cardiac arrest/CPR survivors experience apathy (Reich et al., 1983), decreased quality of life (de Vos et al., 1999), dysfunctional psychosocial behavior (Miranda, 1994), increased social isolation (Sunnerhagen et al., 1996), and increased risk for posttraumatic stress disorder (Ladwig et al., 1999). One hypothesis is that behavioral dysfunctions in cardiac arrest/CPR survivors are the result of fear of an additional cardiac arrest (discussed in Ladwig et al., 1999). However, a clinical study that compared mean anxiety scores of cardiac arrest/CPR survivors with a reference population revealed that few patients altered their daily lives as the result of fear over a second arrest and had lower anxiety than the reference group (Ladwig et al., 1999). Furthermore, increased anxiety-like behavior after cardiac arrest/CPR has been documented in rats (Dhooper et al., 1997), suggesting that there may be a somatic cause underlying increased anxiety after cardiac arrest/CPR.
Cerebral damage after cardiac arrest/CPR is predominantly located in “watershed” regions of the brain because of intensification of neuronal injury during the hyporeperfusion experience of cardiovascular postresuscitation syndrome (Cerchiari et al., 1993). The hippocampus is one of the brain regions predominantly damaged during cardiac arrest/CPR (Kofler et al., in press) (Bottiger et al., 1999; Sadowski et al., 1999), and damage to the hippocampus has been documented to alter anxiety-like behavior (for review see, File et al., 2000) and interfere with social behavior (Bachevalier et al., 1999; Kogan et al., 2000).
Because cardiac arrest/CPR results in ischemia in peripheral organs, in addition to the central nervous system damage (CNS), and because damage to the heart, lungs, liver, or kidneys can alter behavior, it is difficult to ascribe behavioral dysfunctions specifically to CNS damage. One approach that produces global ischemia in the brain without peripheral organ damage is the four-vessel occlusion (4VO) model of global cerebral ischemia. However, one drawback of using 4VO as a model of ischemia is that it does not reproduce the effects of cardiovascular postresuscitation syndrome (Cerchiari et al., 1993). In the current study, we used body hypothermia to protect the peripheral organs of cardiac arrest/CPR animals against ischemia-induced damage (Kofler et al., in press) (Hachimi-Idrissi et al., 2001; Kato et al., 2002; Smith and Bleck, 2002), while maintaining the brain temperature of the animals at either normal temperature or at a slightly hyperthermic temperature during cardiac arrest/CPR. Hyperthermia after traumatic brain injury, including cardiac arrest, exacerbates neuronal damage and is of clinical concern because of treatment protocols in response to reactive hypothermia (Hickey et al., 2003; Thompson et al., 2003). By independently manipulating head and body temperatures, we were able to assess the involvement of CNS damage in cardiac arrest/CPR-induced behavioral deficits without the confounding issue of peripheral organ damage. Furthermore, in a subset of cardiac arrest/CPR animals, we used both brain and body hypothermia to assess the effects of potassium chloride (KCl) administration, epinephrine (EPI) administration, and the hemodynamics of reperfusion on behavior in the absence of CNS damage.
The goal of the present study was to characterize the range of behavioral deficits exhibited by mice that have been exposed to cardiac arrest/CPR and resuscitation and to determine if higher order deficits were the product of underlying sensorimotor disturbances or peripheral organ damage.
METHODS
Animals
Adult male C57BL/6 mice were housed individually in poly-carbonate cages (28 × 17 × 12 cm) from the onset of the study in rooms maintained on a 14:10 light:dark cycle (lights illuminated at 12:00 midnight Eastern Standard Time) at 20°C ± 4°C and relative humidity of 50 ± 5%. Tap water and food (LabDiet 5001; PMI Nutrition; Brentwood, MO, U.S.A.) were available ad libitum throughout the study. At the start of the experiment, mice were randomly assigned to one of six experimental groups:
SHAM surgery with head temperature lowered to 27°C (SHAM-hypo)
SHAM surgery with head temperature maintained at 37°C (SHAM-normo)
SHAM surgery with head temperature elevated to 39°C (SHAM-hyper)
Cardiac arrest/CPR with head temperature lowered to 27°C (CA-hypo)
Cardiac arrest/CPR with head temperature maintained at 37°C (CA-normo)
Cardiac arrest/CPR with head temperature elevated to 39°C (CA-hyper)
Cardiac arrest/cardiopulmonary resuscitation procedure
Mice were anesthetized with 3% halothane in air, and they were intubated and maintained on 1.5% halothane. A temperature probe was placed in the temporalis muscle on the left side of the head. A previous study validated this measure in rats (Jiang et al., 1991), and in a separate cohort of mice, we demonstrated that cortical temperature and temporalis temperature were highly correlated (r2 = 0.94215) over the range of temperatures experienced during our cardiac arrest/CPR and SHAM procedures (24°C to 39.5°C). Thus we used temporalis muscle temperature as an index of brain temperature. Another temperature probe was inserted into the rectum and provided a feedback to a heating blanket (Harvard Apparatus, Holliston, MA, U.S.A.) to maintain body temperature. A PE10 catheter was inserted into the right jugular vein for drug administration. A cannula (Fine Science, Foster City, CA, U.S.A.) was inserted into the right femoral artery and connected to a blood pressure transducer (Columbus Instruments, Columbus, OH, U.S.A.) to allow continuous monitoring of arterial blood pressure. The intubation tube was connected to a ventilator (Columbus Instruments, Columbus, OH, U.S.A.), and the mice were ventilated with a tidal volume of 120 μL and a respiratory rate of 160 breaths per minute. Mice were allowed to stabilize for 10 minutes, during which time blood pressure and temperatures were recorded at 1-minute intervals (Fig. 1). At the end of the acclimation period, body temperature was decreased to 27°C by circulating cold water through a coil system beneath the animal and placement of an alcohol patch on the ventrum. Head temperature was manipulated independently of body temperature through the use of a double lumen coil that was placed around the head and filled with circulating water to achieve a brain temperature of 27°C, 37°C, or 39°C. To induce cardiac arrest, cold KCl (50.0 μL, 0.5 M, 4°C) was injected via the jugular catheter, and the animal was detached from the ventilator. Slow rewarming via heating lamp and thermal blanket began when body temperature reached 27°C after approximately 4 minutes of arrest. At 7 minutes 45 seconds into the arrest period, the mouse was reattached to the ventilator and ventilated with 100% oxygen with a tidal volume of 150 μL and a respiratory rate of 190 breaths per minute. At 8 minutes after injection of KCl, 8 μg of epinephrine (EPI) in 0.5 cc saline, warmed to 37°C, was injected via the jugular vein catheter, and chest compressions (approximately 300 per minute) were initiated. Additional epinephrine was administered in increments of 0.5 μg every 30 seconds in conjunction with continued chest compressions until mice were resuscitated or until 16 μg epinephrine was administered. The total amount of epinephrine administered and the total duration of cardiac arrest/CPR time from KCl injection to the first minute of spontaneous mean blood pressure above 60 mmHg were recorded. Mice were maintained on 100% oxygen for 25 minutes after initiation of spontaneous circulation and then extubated, followed by the removal of catheters and suturing of wounds. An injection of 0.75 cc of prewarmed lactated ringers was administered subcutaneously immediately following the conclusion of the procedure. Mice were placed in a clean cage on a thermal barrier for an additional hour before being returned to the colony.
FIG. 1.

(A) Mean blood pressure was recorded for each animal beginning 10 minutes before cardiac arrest and continuing for 25 minutes after resuscitation (BS LN, baseline). First dotted line shows the point at which KCl or saline was injected into cardiac arrest/CPR or SHAM animals, respectively. Second dotted line shows the onset of reperfusion. SHAM animals maintained a stable blood pressure regardless of temporalis temperature. Cardiac arrest/CPR animals display a marked drop in blood pressure during the arrest and then rebound during reperfusion after epinephrine injection and chest compressions. (B) Temporalis temperature is highly correlated with brain temperature and the three temperature conditions are demonstrated: 27°C (hypo), 37°C (normo), and 39°C (hyper). (C) All groups were exposed to peripheral hypothermia as demonstrated by the rapid decline in rectal temperature and were then warmed to basal temperature. Data are presented as mean ± SD. CA/CPR, cardiac arrest/cardiopulmonary resuscitation.
The surgical preparations, anesthetic exposure, and temperature modulation described in the previous section were similar for cardiac arrest/CPR and SHAM animals, except that SHAM animals received a 50.0 μL injection of isotonic saline instead of KCl and a 0.5 mL injection of isotonic saline instead of epinephrine. The SHAM animals did not experience cardiac arrest/CPR and were not exposed to ischemia, epinephrine, or chest compressions.
Behavioral testing
All behavioral testing occurred during the light phase of the circadian cycle. The individual conducting and scoring behavioral tests was uninformed of experimental assignments. The behavioral apparatuses were cleaned with 70% ethyl alcohol between each use. Only animals that completed the entire behavioral testing protocol were included in the statistical analysis.
Latency to move one body length
Latency to move one body length was used as a measure of initiation of locomotor activity. Animals were placed on a flat surface in the center of a circle with a radius of approximately one body length (5 cm). Latency to move outside the circle (all four feet) was recorded. Animals not completing the task within 1 minute were assigned a latency of 60 seconds. Each animal was tested twice per session, and the mean latency was calculated. Latency to move was recorded at baseline and on postsurgical days 3 and 6.
Negative geotaxis
An inclined plane was used to assess negative geotaxis, a measure of coordination. The mouse was placed on the plane (45° incline) facing downward. Latency to turn 180° with paws parallel was recorded. Each animal was tested twice per session, and mean latency was calculated. Latency to turn was recorded at baseline and on postsurgical day 6.
Visual placement
To assess gross visual ability, mice were suspended by their tails approximately 10 cm above the surface of a table and lowered slowly toward the edge. If the animal responded by extending both front paws toward the table, it was assigned a score of 2. If only one paw was extended, it received a score of 1, and if neither paw was extended, it received a score of 0. Each animal was tested twice per session, and the mean score was calculated. Visual placement was recorded at baseline and on postsurgical days 3 and 6.
Locomotor activity
Locomotor activity was assessed in Flex Field photobeam activity systems (San Diego Instruments, San Diego, California, U.S.A.). The apparatus was enclosed in individual sound attenuating chambers equipped with a 15-W fluorescent white light and ventilating fan that also provided masking noise. A clear Plexiglas insert (40 × 40 × 37.5 cm) was fitted inside a metal frame consisting of 16 equally spaced infrared photocell detectors. The photocells were located 2 cm from the floor along two adjacent walls of the chamber. Interruptions in the infrared light sources by the experimental animal were recorded. Beam breaks were converted to distance traveled (cm). Data were also analyzed to determine how much activity occurred in the periphery versus the center (a 90 cm2 zone in the middle of the apparatus). Locomotor activity was assessed during 60-minute sessions at baseline and on postsurgical days 3 and 6.
Elevated plus maze
The elevated plus maze was used as a measure of anxiety-like behavior. The apparatus consisted of two open arms and two closed arms arranged in a “+” orientation. The arms were 65 cm long and 5 cm wide. The walls enclosing the closed arms were 15 cm high. The mouse was placed in the center of the apparatus facing an open arm, and the following measures were recorded: latency to enter arms, duration of time spent in closed and open arms, and frequency of arm entries. The 5-minute test was administered at baseline and on postsurgical day 7. The total number of fecal boli excreted during the 5-minute test was also recorded.
Social interaction test
Social motivation was assessed by placing an unfamiliar ovariectomized female mouse and the experimental male in a clean cage for 30 minutes. The session was videotaped and scored for latency to approach the female and duration of physical contact. The social interaction test was administered at baseline and on postsurgical day 7.
Organ collection and histology
Seven days after surgery, at the conclusion of the social interaction testing, animals were euthanized, a blood sample was collected, and organs were removed and prepared for histology. Brain, heart, lungs, kidneys, and liver were immersion fixed in 10% neutral buffered formalin. Organs were embedded in paraffin and sectioned (5 μm) on a microtome. Sections were stained with hematoxylin and eosin, as was previously described (Carson, 1997). Analysis of the heart, lungs, kidneys, and liver for necrosis was conducted by a veterinary pathologist (K. M. D. La Perle). Each organ was assessed for lesions by microscopic analysis. Lesions were characterized as follows: no significant microscopic lesions (NSML), minimal, mild, moderate, or marked. The prevalence of dystrophic neurons in the CA1 region of the hippocampus (Bregma −1.85 mm) was graded using the following scale, without knowledge of the experimental condition: normal = 0; <25% damage = 1; 26% to 50% damage = 2; 51% to 75% damage = 3; and 76% to 100% damage = 4. The pyramidal cell layer was visually assessed using 400× magnification, and the percentage of each visual field occupied by dystrophic neurons and alternate cell types (i.e., glia) was estimated. This process was repeated until the entire CA1 region was assessed; then the grade for each hemisphere was determined, and the two numbers were tallied to represent the level of damage for each animal. This procedure was adapted from previous studies of global ischemia (Calle et al., 1989; Hickey et al., 2003; Ooboshi et al., 2000).
Data analyses and statistics
Blood pressure, temporalis muscle temperature, and body temperature were compared using two way repeated measures ANOVA assessing effects of time and group. Total surgery time, total time in cardiac arrest, amount of epinephrine administered, and change in body mass were assessed using one-way ANOVA. The results of all the behavioral tests were analyzed in a four-step process. Differences in baseline performance by group were assessed using one-way ANOVA. Performance statistics examined the effect of repeated behavioral testing by comparing pre- and postsurgical performance using either a t-test or one-way ANOVA, depending upon the number of time points. Postsurgical performance was assessed for effects of temperature manipulations by comparing all SHAM groups with a one-way ANOVA. When no differences were found, all SHAM groups were collapsed into one control group for further comparison. We assessed the effects of cardiac arrest/CPR on behavioral outcomes by comparing the collapsed control group, CA-hypo, CA-normo, and CA-hyper groups using one-way ANOVA. In the case of significant differences, post hoc analyses were conducted using the Tukey test for all pairwise multiple comparisons. If conditions of normality or equal variance were not met, then nonparametric statistics were used. The Mann-Whitney rank sum test was used in place of the t-test, and a Kruskal-Wallis one-way ANOVA followed by Dunn’s test was substituted in place of the one-way ANOVA. To conduct a semiquantitative analysis of the qualitative analysis of peripheral organ pathology, we assigned the following ranks: NSML = 0, minimal = 1, mild = 2, moderate = 3, and marked = 4. If a lesion was graded as between two ranks, then the rank assigned was half-way between as well. Ranks assigned to kidney damage and hippocampal lesions were then tested with Kruskal-Wallis one-way ANOVA followed by Dunn’s test. Differences were considered significant at P < 0.05.
RESULTS
Surgical parameters
Mean arterial blood pressure differed based upon group (F5,899 = 52.01, P < 0.05) (Fig. 1A) and time (F19,899 = 536.23, P < 0.05) and as a consequence of an interaction between group and time (F95,899 = 132.04, P < 0.05). Although mean arterial blood pressure differed between the SHAM and cardiac arrest/CPR groups during the arrest and reperfusion periods (P < 0.05), there were no differences among arrest groups or SHAM groups at any time point. As expected, differences were observed for temporalis muscle temperature (group: F5,798 = 192.01, P < 0.05; time: F19,798 = 480.24, P < 0.05; group × time: F95,798 = 84.14, P < 0.05) (Fig. 1B). It is important to note that there were differences in temporalis muscle temperatures during the arrest period and the beginning of the reperfusion period between the SHAM-hypo/CA-hypo, SHAM-normo/CA-normo, and SHAM-hyper/CA-hyper groups. However, within each temperature category, there were no differences between SHAM and cardiac arrest/CPR groups. Body temperatures differed between groups and over time (group: F5,798 = 8.91, P < 0.05; time: F19,798 = 852.88, P < 0.05; group × time: F95,798 = 2.09, P < 0.05) (Fig. 1C). The effect of time is explained by the imposed temperature manipulation; however, the effect of group is a consequence of the manipulation of the temporalis muscle temperature. Because blood flow is intact in the SHAM animals, heating the head of the animal increases the peripheral body temperature slightly before rewarming begins, such that the SHAM-hyper group has a warmer body temperature at time points 6 through 20 as compared with the CA-hyper group. However, there were no body temperature differences between any of the SHAM groups or any of the cardiac arrest/CPR groups. Mean surgery time did not differ between groups (91.6 ± 14.0 minutes) nor did mean time of cardiac arrest/CPR (9.68 ± 1.09 minutes). In addition, the total amount of epinephrine administered did not differ based upon the cardiac arrest group (9.44 ± 1.72 μg). Furthermore, mean terminal body mass (25.84 ± 1.25 g) was not altered by SHAM surgery, cardiac arrest, or temperature manipulations.
Behavioral tests
To rule out preexisting group differences, baseline behavioral performance was assessed for all groups in all behavioral tests. No baseline differences in behavior as an effect of group were observed for any of the parameters tested (P > 0.05).
Latency to move one body length differed among SHAM animals as a function of time (H2 = 7.32, P < 0.05) with a decrease in median latency from pre- (2.25 seconds) to postsurgical testing on day 3 (1.00 second). There was no effect of repeated testing on the latency to turn. Visual placement scores decreased over the course of repeated testing (H2 = 8.9, P < 0.05), however there were no differences between individual time points, and all median values were identical (1.00). Postsurgical analysis of sensorimotor behavior established that there was no effect of alterations in head temperature during SHAM surgery on the latency to move one body length, latency to turn, or visual placement on either postsurgical days 3 or 6 (Figs. 2A–2E). Likewise, CA-hypo, CA-normo, and CA-hyper did not result in a deficit in latency to move one body length or latency to turn on days 3 or 6. A decrease in visual placement was identified in a comparison of visual placement on postsurgical day 3 for the collapsed control group, CA-hypo, CA-normo, and CA-hyper (H2 = 11.75, P < 0.05). Post hoc analysis did not attribute this difference to any specific group. The difference was transient and had resolved by postsurgical day 6.
FIG. 2.

Mice were tested before surgery and again on days 3 and 6 after surgery for sensorimotor abilities. SHAM mice did not differ by temperature condition, so they were combined into the control group. Cardiac arrest did not alter latency to move on either day 3 (A) or day 6 (B), nor did it alter latency to turn (C). A main effect of group is present on the day 3 retest of visual placement (D); however, this difference is not attributable to any specific group differences and had resolved by day 6 after surgery (E). Data are presented as mean ± SD. CA/CPR, cardiac arrest/cardiopulmonary resuscitation.
Analysis of ambulatory activity established that there was an effect of time (F2,71 = 9.13, P < 0.05) with a general decrease in mean distance traveled (cm; pre-test: 7,326.33 ± 1,333.81; day 3: 6,037.08 ± 977.92; day 6: 6,336.29 ± 925.5). Central tendency also differed as an effect of time (F2,71 = 4.32, P < 0.05) with a general decrease over repeated testing (pre-test: 13.88 ± 4.32; day 3: 11.10 ± 4.37; day 6: 10.72 ± 3.45). Postsurgical analysis established that there was no effect of temperature condition during SHAM surgery on postsurgical days 3 or 6 for total ambulatory activity or central tendency (Figs. 3A–3D). Increased ambulatory activity was demonstrated by the CA-hyper group (7,879.56 ± 1,786.00) on postsurgical day 3 as compared with either SHAM animals (6,037.08 ± 977.92) or CA-hypo (5,497.13 ± 685.84; F3,48 = 5.67, P < 0.05), but this effect was transient and resolved by postsurgical day 6. There was a general decrease in central tendency on postsurgical day 3 (F3,48 = 3.11, P < 0.05) but this effect was not attributable to any specific group difference and was absent by postsurgical day 6.
FIG. 3.

Locomotor activity was assessed during a 1-hour session before surgery and again on days 3 and 6 after surgery. Behavior of SHAM mice did not differ by temperature condition, so they were combined into the control group. Cardiac arrest during the hyperthermic head condition resulted in increased ambulatory activity on day 3 (A), but this difference was no longer detectable on day 6 (C). Central tendency was decreased as indicated by an overall effect of group on day 3 (B); however, this difference was not attributable to any specific group differences and was absent when tested at day 6 (D). *significant difference from control, P < 0.05. Data are presented as mean ± SD. CA/CPR, cardiac arrest/cardiopulmonary resuscitation.
Performance in the elevated plus maze was not affected by repeated testing or temperature conditions during SHAM surgery. Multiple facets of performance in the elevated plus maze were altered by the CA-normo and CA-hyper conditions relative to the SHAM group (Figs. 4A–4D). The number of total arm entries decreased, but this difference was not attributable to any specific group differences (F3,47 = 2.95, P < 0.05). The percentage of open arm entries was lower for the CA-normo (5.21 ± 7.30) and CA-hyper groups (8.40 ± 6.52) as compared with SHAMs (20.46 ± 12.02; F3,47 = 5.32, P < 0.05). The total time in the open arms was also reduced for the CA-normo group (17.38 ± 25.20 sec) as compared with SHAMs (60.06 ± 36.17 sec; F2,47 = 7.21, P < 0.05). The median number of fecal boli excreted during the time in the elevated plus maze was increased in the CA-hyper group (3.00 boli) as compared with SHAMs (1.00 bolus; H3 = 15.99, P < 0.05). There were no differences between SHAMs and CA-hypo in any of the behaviors assessed in the elevated plus maze (P > 0.05).
FIG. 4.

Anxiety-like behavior was assessed before surgery and again on day 7 after surgery. Behavior of SHAM mice did not differ by temperature condition, so they were combined into the control group. There was an overall effect of group for total arm entries, but this difference was not attributable to any specific group differences (A). Percentage of open arm entries were reduced for animals exposed to cardiac arrest/CPR with either a normothermic (normo) or hyperthermic (hyper) head temperature (B). Animals for which a normothermic head temperature was maintained during cardiac arrest/CPR spent significantly less time in the open arms compared with SHAM animals (C); however, animals with a hyperthermic head temperature during cardiac arrest/CPR produced significantly more fecal boli during the 5 minutes in the elevated plus maze (D). *significant difference from control, P < 0.05. Data are presented as mean ± SD. CA/CPR, cardiac arrest/cardiopulmonary resuscitation.
An examination of social interaction did not reveal any effect of either repeated testing or temperature manipulations during SHAM surgery. However, latency to initiate contact was increased in the CA-normo (11.63 ± 3.03 sec) and CA-hyper groups (11.78 ± 1.72 sec) as compared with either SHAMs (6.68 ± 1.64 sec) or CA-hypo (6.5 ± 1.72 sec; F2,38 = 30.23, P < 0.05) (Figs. 5A and 5B). In addition, total time in contact was reduced in the CA-normo (230.75 ± 33.60 sec) and CA-hyper groups (301.44 ± 64.92 sec) as compared with the SHAMs (400.54 ± 53.89 sec) or CA-hypo group (361.13 ± 35.92 sec; F2,40 = 34.27, P < 0.05). Furthermore, mice in the CA-normo group spent less time in contact than those in the CA-hyper group (P < 0.05).
FIG. 5.
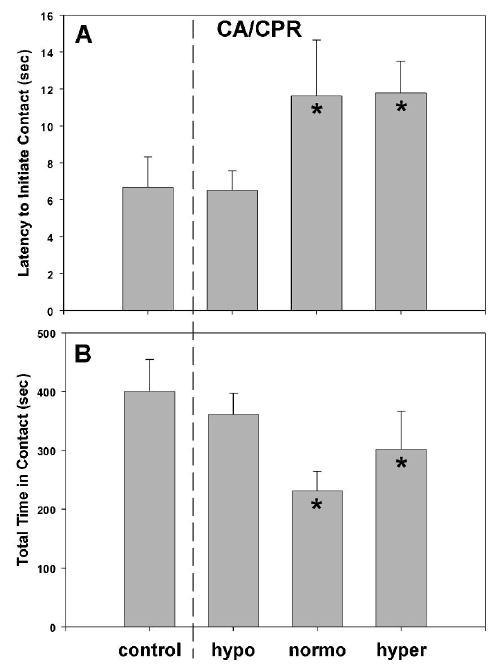
Social interaction was tested before surgery and on day 7 after surgery. SHAM mice did not differ by temperature condition, so they were combined into the control group. Animals exposed to cardiac arrest/CPR with a normothermic (normo) head temperature or a hyperthermic (hyper) head temperature exhibited an extended latency to the first contact with an ovariectomized female mouse (A) and also spent significantly less time in contact with the female then the control group (B). Furthermore, the CA-normo group spent significantly less time interacting than the hyper group. *significant difference from control, P < 0.05. Data are presented as mean ± SD. CA/CPR, cardiac arrest/cardiopulmonary resuscitation.
Analysis of peripheral organs
Histologic analysis of the heart, lungs, and liver established that there were no microscopic lesions regardless of temperature condition or surgical procedures. Analysis of the kidneys did not suggest that the SHAM surgery or temporalis temperature manipulations significantly damaged the kidneys. Despite hypothermic body temperature, cardiac arrest/CPR did produce tubular epithelial degeneration, necrosis, and regeneration within the kidneys (H3 = 11.30, P < 0.05). This difference was greater for the CA-hyper group (median injury rating of 2.0) as compared with SHAMs (median injury rating of 0.0, P < 0.05).
Neuronal analysis
Analysis of the CA1 region of the hippocampus did not suggest that the SHAM surgery or associated temporalis muscle temperature manipulations resulted in dystrophic neurons. Figure 6 is comprised of representative sections from the SHAM (A), CA-normo (B), and CA-hyper (C) groups. Cardiac arrest/CPR produced significant damage within CA1 (H3 = 38.6, P < 0.05) (Fig. 7). This difference was greater for both the CA-normo and CA-hyper groups (median injury ratings of 2 and 2.5, respectively) as compared with SHAMs (median injury rating of 0, P < 0.05) or the CA-hypo group (median injury rating of 0, P < 0.05).
FIG. 6.
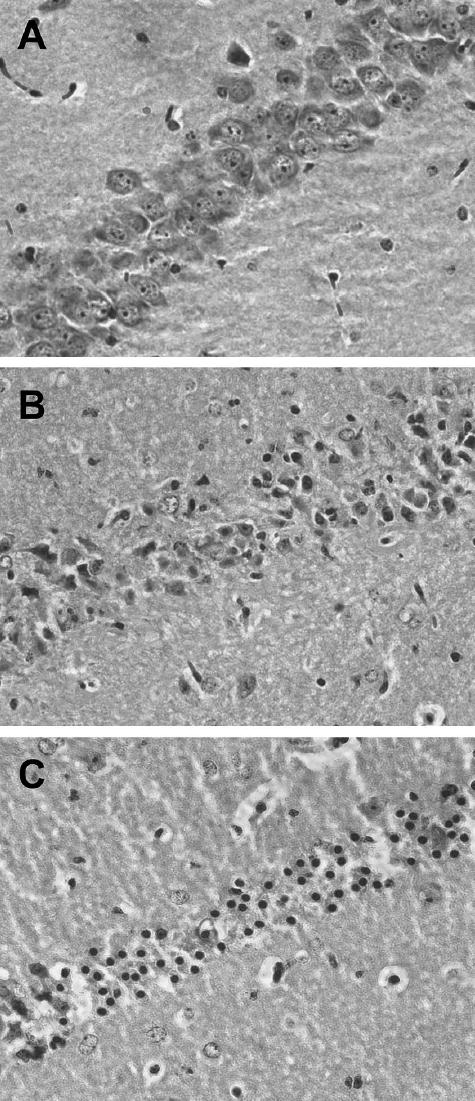
Photomicrographs (400×) of the CA1 region of the hippocampus from a SHAM animal (A) and cardiac arrest/CPR animals with normothermic (B) or hyperthermic (C) head temperatures. The following rank system was established to assess damage qualitatively: normal hippocampi = 0, illustrated in a SHAM animal (A); <25% damage in each hemisphere = 2 (B); between 25% and 50% damage = 4(C). Animals exposed to cardiac arrest with either a normothermic head or a hyperthermic head temperature had significantly more dystrophic neurons than either SHAMs or animals exposed to cardiac arrest with a hypothermic head. CPR, cardiopulmonary resuscitation.
FIG. 7.

The degree of neuronal damage in the CA1 region of the hippocampus was assessed using the following scale: normal = 0; <25% damage = 1; 25% to 50% damage = 2; 50% to 75% damage = 3; and 75% to 100% damage = 4. Cardiac arrest/CPR produced significant damage within CA1 for both the CA-normo and CA-hyper groups as compared with either the controls or the CA-hypo group. *significant difference from control, P < 0.05. Data are presented as mean ± SD. CA/CPR, cardiac arrest/cardiopulmonary resuscitation.
DISCUSSION
Our results demonstrate that cardiac arrest/CPR increases anxiety-like behavior and decreases social interaction. These alterations are not the result of sensorimotor disturbances because any detected sensorimotor deficits were resolved before assessment of anxiety and social behavior. Furthermore, our data suggest that KCl and EPI administered during the arrest procedure were not responsible for behavioral deficits observed in the CA-normo and CA-hyper animals. Specifically, the CA-hypo animals, which received the same amount of KCl and EPI as the other two cardiac arrest/CPR groups but did not sustain neuronal damage because of the maintenance of low brain temperature during arrest, did not exhibit behavioral deficits. Heart, liver, and lung tissue of all animals, regardless of cardiac arrest/CPR, was normal. Moderate kidney damage was present in animals that were exposed to the CA-hyper condition but not those exposed to the CA-normo condition, suggesting that behavioral changes cannot be attributed to kidney damage.
The sensorimotor deficits documented in this study were transient, as would be expected in less severe cardiac arrest/CPR (Bunch et al., 2003; Graves et al., 1997). We observed a general change in some behavioral measures during repeated testing of SHAM animals. Habituation is not uncommon during repeated behavioral testing (Hattori et al., 2000; McIlwain et al., 2001); however, the performance level demonstrated by all animals tested in the sensorimotor tasks suggests that they were performing at a level that would be considered normal for a mouse of similar age and sex (Hattori et al., 2000; McIlwain et al., 2001).
Mice in both the CA-normo and CA-hyper groups demonstrated anxiety-like behavior as measured in the elevated plus maze. This task is commonly used to assess anxiety-like behavior in mice (Pellow et al., 1985). Increased anxiety behaviors have been reported in the clinical population of cardiac arrest/CPR survivors (de Vos et al., 1999; Ladwig et al., 1999; Miranda, 1994) and in laboratory animal studies of cardiac arrest (Dhooper et al., 1997). Elevated anxiety levels may account for difficulties reported by the clinical population in maintaining employment, concentrating, and interacting with others (de Vos et al., 1999; Miranda, 1994; Sunnerhagen et al., 1996). Furthermore, underlying anxiety may account for some symptoms classified as depressive symptoms observed in cardiac arrest/CPR surviviors (de Vos et al., 1999; Sheps and Sheffield, 2001). Recognizing and treating these disorders is of particular importance for survivors of cardiac arrest/CPR because concomitant depressive symptoms in addition to cardiac dysfunction increase the risk of cardiac-related death (Connerney et al., 2001; Irvine et al., 1999; Sheps and Sheffield, 2001). It is important to note that psychosocial therapy after out-of-hospital cardiac arrest/CPR reduces the risk of subsequent cardiovascular death (Cowan et al., 2001), suggesting that increased recognition of mood disturbances may improve prognosis for cardiac arrest/CPR survivors.
An examination of social motivation in our mice revealed both an increase in latency to approach a novel female and a general decrease in overall contact (Fig. 5). This behavioral observation in mice reflects observations in the clinical literature (Miranda, 1994; Sunnerhagen et al., 1996). It was not possible to determine the influence of increased anxiety on the observed decrease in social interaction, but it is conceivable that this accounts for at least a portion of the change in social motivation. Further studies are necessary to determine if the neural substrate underlying these two behavioral deficits is a common or distinct pathway.
The cardiac arrest/CPR model described here uses hypothermia to protect peripheral organs while maintaining a normal to slightly elevated head temperature to focus on the brain damage resulting from cardiac arrest/CPR. We did observe some kidney damage in our mice, with the most predominant amount of kidney damage occurring in the CA-hyper group. Although these animals did not appear to increase their overall body temperature at a faster rate than the other groups, perhaps the elevated temperature of the blood in the brain (39°C) led to more damage during reperfusion. Regardless, behavioral deficits observed did not solely reflect the level of kidney damage observed, as supported by the observation that the median kidney damage in the CA-normo group was lower than that of the CA-hypo group, yet the CA-normo group displayed behavioral deficits and the CA-hypo group did not. Kidney damage after cardiac arrest/CPR may also reflect a predisposition of this strain of mice to kidney disorders (Robson et al., 2003; Rodriguez et al., 1996).
The use of temperature manipulations also facilitated the separation of effects of administration of KCl and EPI and cardiac arrest/CPR-induced changes in hemodynamics from resulting CNS damage. Mice in the CA-hypo group were exposed to conditions similar to the CA-normo and CA-hyper groups, with the exception of head temperature changes. Mice in the CA-hypo group did not exhibit behavioral deficits in any of the tests conducted compared with SHAM groups. These data suggest that deficits observed in the CA-normo and CA-hyper group were caused by alterations in the CNS. This supposition is supported by the observed damage in the hippocampal CA1 region for animals in either the CA-normo or CA-hyper group but not those in the CA-hypo group (Fig. 6). This level of damage is similar to that previously documented in rats following cardiac arrest (Ooboshi et al., 2000; Sadowski et al., 1999) as well as in mice.
CONCLUSION
This study demonstrates that cardiac arrest/CPR results in increased anxiety and decreased social interaction and that these behavioral changes can be attributed to CNS damage. Future studies will use this global ischemia model to study how cardiac arrest/CPR-induced changes in neurotransmitter systems and inflammatory pathways can affect behavioral recovery.
Acknowledgments
The authors thank Stephanie L. Bowers, Tara K.S. Craft, and Jim Power for technical support, Tricia Uhor for animal care, and Erica R. Glasper for helpful comments on this manuscript.
Footnotes
Supported by National Institutes of Health Grants NS 40267 and NS20020 and American Heart Association EIA (A.C.D.). Also supported by grants J1864-MED and J2106 from the Austrian Science Fund (Fond zur Förderung der Wissenschaftlichen Forschung) (J. Kofler).
References
- American Heart Association (2002) Heart disease and stroke statistics: 2003 update, Dallas, TX: American Heart Association. Available at: www.americanheart.org/presenter:jhtml?identifier-1928
- Bachevalier J, Alvarado MC, Malkova L. Memory and socio-emotional behavior in monkeys after hippocampal damage incurred in infancy or in adulthood. Biol Psychiatry. 1999;46:329–339. doi: 10.1016/s0006-3223(99)00123-7. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, Motsch J, Martin E, Gass P. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Brain Res Mol Brain Res. 1999;65:135–142. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- Bunch TJ, White RD, Gersh BJ, Meverden RA, Hodge DO, Ballman KV, Hammill SC, Shen WK, Packer DL. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N Engl J Med. 2003;348:2626–2633. doi: 10.1056/NEJMoa023053. [DOI] [PubMed] [Google Scholar]
- Calle PA, Bogaert MG, Van Reempts JL, Buylaert WA. Neurological damage in a cardiopulmonary arrest model in the rat. J Pharmacol Methods. 1989;22:185–195. doi: 10.1016/0160-5402(89)90013-2. [DOI] [PubMed] [Google Scholar]
- Carson FL (1997) Histotechnology: a self-instructional text Chicago, IL: American Society of Clinical Pathologists
- Cerchiari EL, Safar P, Klein E, Cantadore R, Pinsky M. Cardiovascular function and neurologic outcome after cardiac arrest in dogs. The cardiovascular post-resuscitation syndrome. Resuscitation. 1993;25:9–33. doi: 10.1016/0300-9572(93)90003-9. [DOI] [PubMed] [Google Scholar]
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358:1766–1771. doi: 10.1016/S0140-6736(01)06803-9. [DOI] [PubMed] [Google Scholar]
- Cowan MJ, Pike KC, Budzynski HK. Psychosocial nursing therapy following sudden cardiac arrest: impact on two-year survival. Nurs Res. 2001;50:68–76. doi: 10.1097/00006199-200103000-00002. [DOI] [PubMed] [Google Scholar]
- de Vos R, de Haes HC, Koster RW, de Haan RJ. Quality of survival after cardiopulmonary resuscitation. Arch Intern Med. 1999;159:249–254. doi: 10.1001/archinte.159.3.249. [DOI] [PubMed] [Google Scholar]
- Dhooper A, Young C, Reid KH. Ischemia-induced anxiety following cardiac arrest in the rat. Behav Brain Res. 1997;84:57–62. doi: 10.1016/s0166-4328(96)00133-7. [DOI] [PubMed] [Google Scholar]
- Drysdale EE, Grubb NR, Fox KA, O’Carroll RE. Chronicity of memory impairment in long-term out-of-hospital cardiac arrest survivors. Resuscitation. 2000;47:27–32. doi: 10.1016/s0300-9572(00)00194-5. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Graves JR, Herlitz J, Bang A, Axelsson A, Ekstrom L, Holmberg M, Lindqvist J, Sunnerhagen K, Holmberg S. Survivors of out of hospital cardiac arrest: their prognosis, longevity and functional status. Resuscitation. 1997;35:117–121. doi: 10.1016/s0300-9572(97)00035-x. [DOI] [PubMed] [Google Scholar]
- Hachimi-Idrissi S, Corne L, Huyghens L. The effect of mild hypothermia and induced hypertension on long term survival rate and neurological outcome after asphyxial cardiac arrest in rats. Resuscitation. 2001;49:73–82. doi: 10.1016/s0300-9572(00)00268-9. [DOI] [PubMed] [Google Scholar]
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Hickey RW, Kochanek PM, Ferimer H, Alexander HL, Garman RH, Graham SH. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Crit Care Med. 2003;31:531–535. doi: 10.1097/01.CCM.0000050323.84293.11. [DOI] [PubMed] [Google Scholar]
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Irvine J, Basinski A, Baker B, Jandciu S, Paquette M, Cairns J, Connolly S, Roberts R, Gent M, Dorian P. Depression and risk of sudden cardiac death after acute myocardial infarction: testing for the confounding effects of fatigue. Psychosom Med. 1999;61:729–737. doi: 10.1097/00006842-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Jiang JY, Lyeth BG, Clifton GL, Jenkins LW, Hamm RJ, Hayes RL. Relationship between body and brain temperature in traumatically brain-injured rodents. J Neurosurg. 1991;74:492–496. doi: 10.3171/jns.1991.74.3.0492. [DOI] [PubMed] [Google Scholar]
- Kato A, Singh S, McLeish KR, Edwards MJ, Lentsch AB. Mechanisms of hypothermic protection against ischemic liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G608–G616. doi: 10.1152/ajpgi.00454.2001. [DOI] [PubMed] [Google Scholar]
- Katz LM, Lotocki G, Wang Y, Kraydieh S, Dietrich WD, Keane RW. Regulation of caspases and XIAP in the brain after asphyxial cardiac arrest in rats. Neuroreport. 2001;12:3751–3754. doi: 10.1097/00001756-200112040-00029. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ladwig KH, Schoefinius A, Dammann G, Danner R, Gurtler R, Herrmann R. Long-acting psychotraumatic properties of a cardiac arrest experience. Am J Psychiatry. 1999;156:912–919. doi: 10.1176/ajp.156.6.912. [DOI] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Miranda DR. Quality of life after cardiopulmonary resuscitation. Chest. 1994;106:524–530. doi: 10.1378/chest.106.2.524. [DOI] [PubMed] [Google Scholar]
- Mizushima H, Zhou CJ, Dohi K, Horai R, Asano M, Iwakura Y, Hirabayashi T, Arata S, Nakajo S, Takaki A, Ohtaki H, Shioda S. Reduced postischemic apoptosis in the hippocampus of mice deficient in interleukin-1. J Comp Neurol. 2002;448:203–216. doi: 10.1002/cne.10262. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Ibayashi S, Takano K, Sadoshima S, Kondo A, Uchimura H, Fujishima M. Hypothermia inhibits ischemia-induced efflux of amino acids and neuronal damage in the hippocampus of aged rats. Brain Res. 2000;884:23–30. doi: 10.1016/s0006-8993(00)02861-4. [DOI] [PubMed] [Google Scholar]
- Paradis NA, Wenzel V, Southall J. Pressor drugs in the treatment of cardiac arrest. Cardiol Clin. 2002;20:61–78. doi: 10.1016/s0733-8651(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pusswald G, Fertl E, Faltl M, Auff E. Neurological rehabilitation of severely disabled cardiac arrest survivors. Part II. Life situation of patients and families after treatment. Resuscitation. 2000;47:241–248. doi: 10.1016/s0300-9572(00)00240-9. [DOI] [PubMed] [Google Scholar]
- Reich P, Regestein QR, Murawski BJ, DeSilva RA, Lown B. Unrecognized organic mental disorders in survivors of cardiac arrest. Am J Psychiatry. 1983;140:1194–1197. doi: 10.1176/ajp.140.9.1194. [DOI] [PubMed] [Google Scholar]
- Robson MG, Cook HT, Pusey CD, Walport MJ, Davies KA. Antibody-mediated glomerulonephritis in mice: the role of endotoxin, complement and genetic background. Clin Exp Immunol. 2003;133:326–333. doi: 10.1046/j.1365-2249.2003.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RE, Misra M, Diwan BA, Riggs CW, Kasprzak KS. Relative susceptibilities of C57BL/6, (C57BL/6 x C3H/He)F1, and C3H/He mice to acute toxicity and carcinogenicity of nickel sub-sulfide. Toxicology. 1996;107:131–140. doi: 10.1016/0300-483x(95)03251-a. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Lazarewicz JW, Jakubowska-Sadowska K, Wisniewski HM, Mossakowski MJ, Brown WT. Long-term changes in calbindin D(28K) immunoreactivity in the rat hippocampus after cardiac arrest. Neurosci Lett. 2002;321:90–94. doi: 10.1016/s0304-3940(01)02426-0. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Wisniewski HM, Jakubowska-Sadowska K, Tarnawski M, Lazarewicz JW, Mossakowski MJ. Pattern of neuronal loss in the rat hippocampus following experimental cardiac arrest-induced ischemia. J Neurol Sci. 1999;168:13–20. doi: 10.1016/s0022-510x(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Sheps DS, Sheffield D. Depression, anxiety, and the cardiovascular system: the cardiologist’s perspective. J Clin Psychiatry. 2001;62(Suppl 8):12–16. discussion 17–18. [PubMed] [Google Scholar]
- Smith TL, Bleck TP. Hypothermia and neurologic outcome in patients following cardiac arrest: should we be hot to cool off our patients? Crit Care. 2002;6:377–380. doi: 10.1186/cc1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnerhagen KS, Johansson O, Herlitz J, Grimby G. Life after cardiac arrest; a retrospective study. Resuscitation. 1996;31:135–140. doi: 10.1016/0300-9572(95)00903-5. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12:163–173. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]


