Abstract
Breast-feeding infants of human immunodeficiency virus (HIV)-infected women ingest large amounts of HIV, but most escape infection. While the factors affecting transmission risk are poorly understood, HIV-specific cytotoxic T-lymphocyte (CTL) responses play a critical role in controlling HIV levels in blood. We therefore investigated the ability of breast milk cells (BMC) from HIV-infected women from the United States and Zambia to respond to HIV-1 peptides in a gamma interferon enzyme-linked immunospot assay. All (n = 11) HIV-infected women had responses to pools of Gag peptide (range, 105 to 1,400 spot-forming cells/million; mean = 718), 8 of 11 reacted to Pol, 7 reacted to Nef, and 2 of 5 reacted to Env. Conversely, of four HIV-negative women, none responded to any of the tested HIV peptide pools. Depletion and tetramer staining studies demonstrated that CD8+ T cells mediated these responses, and a chromium-release assay showed that these BMC were capable of lysing target cells in an HIV-specific manner. These data demonstrate the presence of HIV-specific major histocompatibility complex class I-restricted CD8+ CTLs in breast milk. Their presence suggests a role in limiting transmission and provides a rationale for vaccine strategies to enhance these responses.
In many parts of sub-Saharan Africa where the human immunodeficiency virus (HIV) epidemic now predominates, breast-feeding is nearly universal and mostly of long duration. An infant of an HIV-positive mother is at risk of acquiring the infection during breast-feeding, and it has been estimated that during the first few months of life infants of HIV-positive mothers may ingest 625,000 virions and 25,000 infected cells daily (27). Despite prolonged exposure to large quantities of virus, most breast-fed infants escape HIV infection. Around 16% of infants breast-fed for up to 24 months acquire HIV infection through breast-feeding (33), and the incidence after the first few months of life may be as low as 3% per year of breast-feeding (26). Moreover, while levels of HIV RNA in breast milk are much lower than those observed in plasma (27, 47), HIV DNA levels are higher in breast milk than in blood (34), suggesting local inhibition of HIV expression. In contrast, levels of both HIV RNA and DNA are much lower in semen than in blood (58). Few factors associated with limiting HIV transmission via breast-feeding have been identified. These are crucially important to identify in order to develop strategies to minimize the risk of postnatal HIV transmission while preserving breast-feeding, since avoidance of all breast-feeding is not a realistic option for many HIV-positive women. Alternatives to breast-feeding can be unsafe, unaffordable, and culturally unacceptable. Knowledge of the factors inhibiting HIV in breast milk is also important for the development of intervention strategies, including vaccines.
In peripheral blood, HIV-specific cytotoxic T lymphocytes (CTL) have been associated with a decrease in the viral load in plasma during primary infection and with a slower course of disease progression (reviewed in 53). Recent studies indicate that mucosal CD8+ T lymphocytes are important mediators of protective immunity (4, 5, 31). HIV-specific CTL have been detected in the cervices of HIV-seropositive women (32) and highly exposed uninfected sex workers in Africa (23). However, these responses do not always correlate with the presence of HIV-specific mucosal immunoglobulin A (IgA) (7). Animal models indicate that mucosal but not systemic CTL are important mediators of protection from HIV infection (4, 5). These data suggest that CTL play an important role in transmission across mucosal surfaces.
Animal studies demonstrate that breast milk lymphocytes can traverse the gastrointestinal tract and function within the neonate (19, 21, 46, 55). While this has not been demonstrated for humans, it has been established that intestinal permeability during the neonatal period is increased in humans (54). Furthermore, human breast milk cells (BMC) have been shown to traverse the neonatal baboon gut (21). Thus, it is plausible that breast milk lymphocytes traverse the human neonatal gut. The transfer of immunologically active cells may be an additional mechanism by which HIV transmission via breast milk is curtailed.
We studied the cellular immune responses of breast milk cells from HIV-positive women. Our studies demonstrated the presence of cytolytic HIV-specific CD8+ T cells in breast milk. These studies have important implications in understanding the pathogenesis of HIV transmission in human milk, as well as in the design of vaccine strategies to modulate these responses. Since BMC are easily accessible, our studies provide a new model for the study of mucosal transmission.
MATERIALS AND METHODS
Subjects and HLA typing.
HIV type 1 (HIV-1)-infected women presenting for clinical care at the University of Alabama at Birmingham (UAB) and the Zambia Exclusive Breastfeeding Study in Lusaka, Zambia, were enrolled in the study after informed consent was obtained. Control subjects were healthy HIV-seronegative women who delivered at UAB and agreed to participate in the study after informed consent. HLA typing was performed at the Tissue Typing Center in the UAB Hospital by using the Micro SSP HLA Typing System (One Lambda, Inc., Canoga Park, Calif.).
Isolation of BMC and IFN-γ ELISPOT assay.
Breast milk was obtained from women at 2 days to 2 months postpartum. Milk samples were centrifuged at 400 × g for 15 min and washed two times with Hank's balanced salt solution (6). Viable BMC were counted by using a hemocytometer and trypan blue stain. A gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay was used to enumerate HIV-specific T cells from breast milk or peripheral blood mononuclear cells (PBMC) of HIV-infected or uninfected women (8). Briefly, 96-well nitrocellulose plates (Milliliter HA, Millipore, Bedford, Mass.) were coated with 5 μg/ml of mouse antihuman IFN-γ monoclonal antibody (clone 1-D1K; Mabtech, Nacka, Sweden) and then incubated overnight at 4°C. Antibody was decanted, and wells were blocked with 200 μl of RPMI containing 10% human AB serum. BMC were added to the plates at a concentration of 2 × 106 cells/ml and incubated with pools of 20-mer peptides (overlapping by 10 amino acids [aa]) spanning the entire sequence of HIV-1 clade B Gag (strain HXB2), Pol (strain HXB2), Env (strain MN), or Nef (strain BRU) (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health [NIAID, NIH]) at 2 μg/ml. Clade C Gag peptides were used for Zambian subjects. Control wells were incubated with tetanus toxoid (Connaught, Ontario, Canada) at 8 limit flocculation (Lft) units, cytomegalovirus (CMV) lysate (Advanced Biotechnologies, Inc., Columbia, Md.) (2 μg/ml), phytohemagglutinin (PHA) (Sigma, St. Louis, Mo.) (5 μg/ml), or medium alone. Responses are considered positive if there were ≥100 spot-forming cells (SFC)/106 cells and twice as many SFC as in the nonstimulated control wells. PBMC were added to the ELISPOT plates at a concentration of 106 cells/ml and incubated with the same peptide pools as the BMC.
Epitope mapping.
The availability of multiple samples of breast milk from volunteers 2 and 5 allowed for epitope mapping. Freshly isolated BMC were stimulated with HIV-1 clade B peptide pools from Gag, Pol, Env, and Nef, as described above. Initial mapping was done using pools of 10 peptides, i.e., 5 pools for Gag, 10 pools for Pol, 8 pools for Env, and 2 pools for Nef. Positive responses were then fine mapped to the individual peptides (20-mers).
Depletion of CD8+ T lymphocytes.
CD8+ BMC were depleted using anti-CD8 monoclonal antibody-coated magnetic beads (Dynal, Lake Success, N.Y.) as described in the manufacturer's instructions (8). Briefly, BMC were incubated with the Dynabeads (7:1 bead-to-cell ratio) for 30 min at 4°C in a rocker and then placed on a magnet for 3 min. The depleted supernatant was removed and added to a fresh tube. Depleted cells were then washed twice with RPMI containing 10% human AB serum, counted, and used directly in the ELISPOT assay.
Stimulation of effector CTL.
Fresh BMC from volunteer 12 were incubated in RPMI supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) and stimulated at a 4:1 effector-to-stimulator ratio with peptide-pulsed BMC. Stimulator breast milk cells were pulsed with pools of 20-mers overlapping by 10 aa (2 μg/ml) from HIV-1 Gag, Pol, and Nef overnight at 37°C under 5% CO2. Effectors were stimulated and cultured for 4 days at 37°C under 5% CO2, with interleukin-2 (50 U/ml) added on day 2.
Preparation of target cells.
Epstein-Barr virus-transformed B-lymphoblastoid cell lines (BLCL) were established from PBMC from volunteer 2 and cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). BLCLs were labeled with 51Cr and infected at a multiplicity of infection of 3 overnight with vaccinia virus recombinant rVV-1291-GDR, which expresses multiple HIV antigens, including Gag, Pol, Env, and Nef, or with the control vaccinia virus recombinant, vSC8 (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, contributed by S. Chakrabarti and B. Moss).
CTL chromium release assay.
Radiolabeled target cells were plated at 5 × 103 cells/well in 96-well round-bottomed plates. Effector cells were added in triplicate to the wells at effector-to-target cell (E/T) ratios of 15:1 and 10:1 in a total volume of 200 μl. After a 5-h incubation (37°C and 5% CO2), 40 μl of supernatant was harvested from each well into Lumaplates (Packard, Meriden, Conn.) and the 51Cr release was measured with a gamma counter (Topcount; Packard). Spontaneous 51Cr release was measured from control wells containing target cells with medium alone. Maximum release values were obtained by lysis of target cells with 1% Triton X-100 (Sigma). The percent specific lysis was calculated as follows: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100%. A positive response was defined as ≥10% specific lysis of the target cells expressing HIV-1 proteins above the control target cells and the presence of ≥10% specific lysis at two or more E:T ratios.
Construction of vP129-GDR vaccinia virus vector.
The parental recombinant vaccinia virus vP1291 (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, contributed by J. Tartaglia) expresses HIV-1MN gp120 linked to the HIV-1 transmembrane anchor sequence (nucleotides 7850 to 7934). In addition, it expresses an HIV-1IIIB Gag/Pro polyprotein with functional protease activity. Additional HIV epitopes from Pol and Nef were added to vP1291 by engineering a nucleotide sequence that encodes a Nef-Pol fusion protein into vaccinia virus recombination plasmid pSC11. For the HIV-1 Nef sequences, plasmid pNL4-3 (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) was used as a PCR template with primers 5′-GCAATACAGCAGCTAGCATGGCTGCTTG (for addition of a NheI restriction site and an ATG start codon with optimal Kozak sequence before nucleotide 8321 of NL4-3 [accession no. M19921]) and 5′-CCCTTGTAGCAAGCTCGATGCCGGCAGT (for disruption of the Nef TGA stop codon and creation of an NgoMIV site). Plasmid pBH10 (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) was used as the source for a fragment of the HIV-1IIIB reverse transcriptase gene. The resulting vP1291-GDR is predicted to express the same HIV genes as vP1291 plus a 573-aa Nef-Pol fusion protein which spans aa 53 to 206 of HIV-1 Nef followed by a single arginine residue before proceeding in frame to aa 157 to 550 of HIV-1IIIB polymerase followed by an additional 23 non-HIV related residues. Radioimmunoprecipitation-polyacrylamide gel electrophoretic analysis using anti-Nef and pooled anti-HIV antibodies confirmed expression of Gag and Env plus an additional band (compared to vP1291) at approximately 68 kDa, which corresponds to the Nef-Pol fusion protein (data not shown).
Quantitation of HIV-1 RNA in plasma and breast milk.
Quantitation of HIV-1 RNA was performed by the Roche Amplicor HIV-1 Monitor test, version 1.5 (Roche Diagnostics Systems, Branchburg, N.J.) according to the manufacturer's specifications.
Enumeration of epitope-specific cells by major histocompatibility complex (MHC) class I tetramer complexes and surface marker staining.
The HLA A3-restricted p17 specific tetramer displaying the 9-mer RLRPGGKKK (RK9) was synthesized by the NIAID Tetramer Facility (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) and conjugated with phycoerythrin (PE). Cells were stained with the HLA A3 RK-9-PE tetramer complex, anti-CD8 peridinin chlorophyll protein (PerCP), and anti-CD3 allophycocyanin (APC) (Becton Dickinson [BD], San Jose, Calif.). Stained cells were acquired using a BD Calibur flow cytometer and analyzed with CellQuest software (BD). Tetramer titration and staining conditions were maximized by using epitope-specific PBMC from both HLA A3 and non-HLA A3 HIV-1-infected volunteers as well as HIV-1-seronegative volunteers as controls. For each analysis dot plot, a minimum of 10,000 events are represented.
RESULTS
Study population.
Breast milk was obtained from HIV-positive women in Birmingham, Ala. (n = 6) and Lusaka (n = 6). All volunteers from UAB received antiretroviral therapy, and all volunteers from Lusaka received nevirapine prophylaxis (16) (Table 1). The median absolute CD4+ cell count in the subjects was 375 (range, 178 to 1,215 cells/μl). The median level of HIV RNA in plasma was 11,461 copies/ml (range, <50 to 169,979), and the median level of HIV RNA in breast milk was 90 copies/ml (range <50 to 6,246). ELISPOT analysis was performed on breast milk from all volunteers except volunteer 12 whose cells were used to assess cytolytic activity.
TABLE 1.
Characteristics of the HIV-infected women donating breast milk
| Subjectf | Age | CD4a | No. of copies of RNA/ml of:
|
|||
|---|---|---|---|---|---|---|
| Plasma | Breast milk | Treatment | ART prior to delivery | |||
| US 1 | 35 | 450 | NPb | NP | ZDV/3TC/NVPc | 18 wk |
| US 2 | 23 | 1,215 | <50 | <50 | ZDV/3TC/NVPc | 18 wk |
| US 3 | 30 | 446 | <50 | <50 | ZDV/3TC/NVPc | 22 wk |
| US 4 | 29 | 265 | <50 | <50 | ZDV/3TC/NVPc,d | 4 wk |
| US 5 | 18 | 290 | 9,400 | <50 | ZDV/3TC/NVPc | 4 wk |
| US 12 | 23 | 419 | 135 | NP | ZDV/3TC/NVPc,d | 11 wk |
| Z 6 | 29 | 178 | 169,979 | 183 | NVPe | None |
| Z 7 | 21 | 316 | 111,929 | 6,246 | NVPe | None |
| Z 8 | 18 | 332 | 37,032 | 120 | NVPe | None |
| Z 9 | 32 | 483 | 11,461 | 382 | NVPe | None |
| Z 10 | 20 | 216 | 33,886 | 258 | NVPe | None |
| Z 11 | 25 | 577 | 27,963 | 61 | NVPe | None |
Most recent CD4 count (range, 4 months to 3 weeks) prior to delivery (cells/μliter).
NP, not performed.
ZDV, zidovudine; 3TC, lamivudine; NVP, nevirapine.
Prior history of antiretroviral therapy (ART).
Two hundred milligrams per os at delivery.
US, United States; Z, Zambian.
IFN-γ-producing HIV-specific T-cell responses in breast milk.
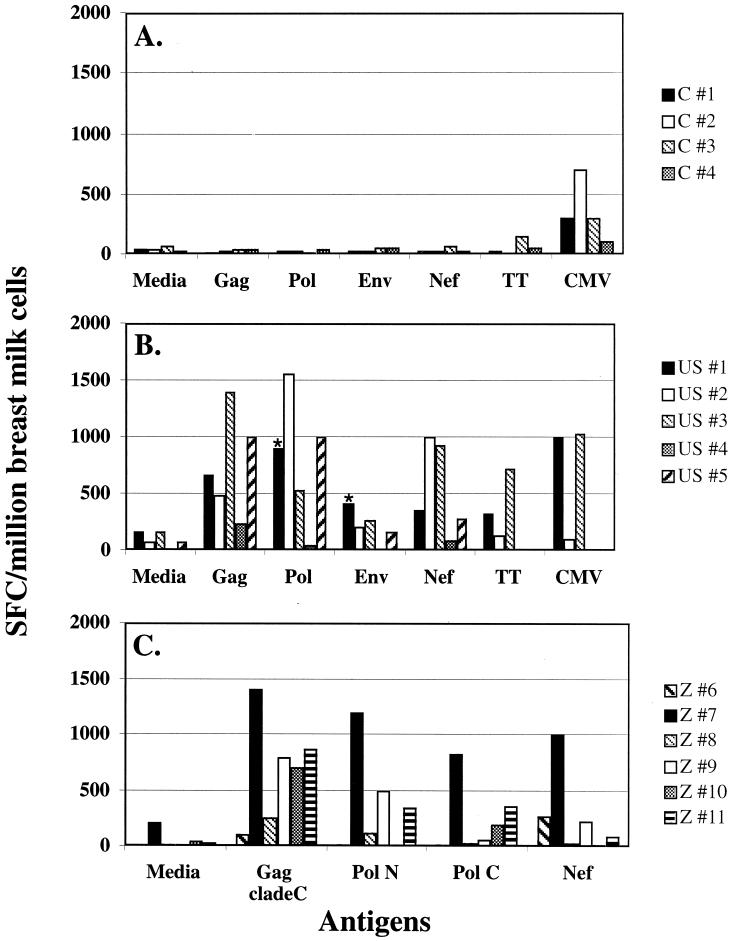
To determine HIV-specific responses in breast milk cells, we employed an ELISPOT assay to measure antigen specific IFN-γ secretion. Breast milk from HIV-infected women was collected, and the cellular immune response was evaluated by using an IFN-γ ELISPOT assay with the following peptide stimuli: Gag, Pol, Env, or Nef HIV peptides, CMV lysate, tetanus toxoid, PHA, or medium only (negative control) (Fig. 1B, 1C). All HIV-infected women from both the United States and Zambia responded to the Gag peptide pool (range, 105 to 1,400 SFC/million cells; mean SFC/million = 719). Eight of 11 women responded to Pol (range, 5 to 1,552 SFC/million cells; mean SFC/million = 450), 7 of 11 responded to Nef (range, 15 to 1,000 SFC/million cells; mean SFC/million = 388), and 2 of 5 responded to Env (range, 5 to 412 SFC/million cells; mean SFC/million = 209) (Fig. 1). Eighty-two percent (9 of 11) of the subjects had responses to two or more of the HIV peptide pools. Responses to baculovirus-derived p24 protein were not detected in two of two subjects tested (data not shown). In contrast, BMC from four HIV-seronegative women did not respond to HIV peptides; however, all four were CMV seropositive and did mount a response to CMV lysate (Fig. 1A). Responses to both HIV and CMV were detected in women with known coinfection but not in one woman who was CMV seronegative (volunteer 2; Fig. 1B). BMC from all HIV-infected and uninfected women responded to PHA (data not shown).
FIG. 1.
HIV-1-specific immune responses to overlapping 20-mer peptide pools from Gag, Pol, Env, and Nef of HIV-1; tetanus toxoid (TT); or CMV lysate as measured by the IFN-γ ELISPOT assay. (A) BMC from HIV-seronegative women. (B) BMC responses from HIV+ women (U.S. cohort). (C) BMC responses from HIV+ women (Zambian cohort). Fifty peptides each, comprising the N terminus (Pol N aa 1 to 510) or the C terminus (Pol C aa 501 to 1003) of Pol were used instead of a pool of 100 peptides (Pol aa 1 to 1003) to stimulate BMC from the Zambian volunteers. Asterisks indicate that for volunteer 1 the responses from Pol N and Pol C were added and plotted as Pol, and the same was done for Env using gp120 and gp41. Responses to CMV and TT were not measured in volunteers 4 and 5. PHA responses were >1,000 SFC/million BMC (data not shown).
Analysis of HIV-specific breast milk T cells: phenotype and epitope specificity.
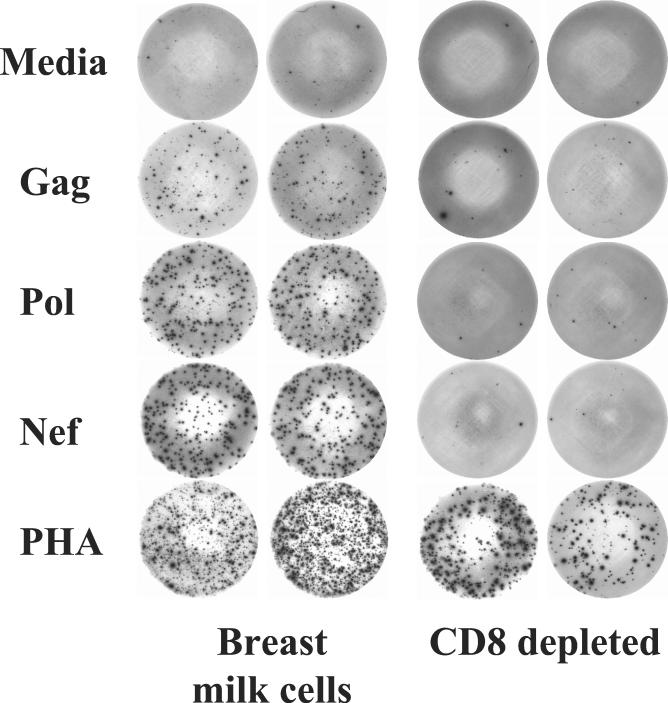
To elucidate the cells responsible for these responses, we repeated these experiments with suspensions depleted of CD8+ cells in volunteer 2. In this instance HIV-stimulated IFN-γ production was reduced by more than 80% in the wells depleted of CD8+ T cells (Fig. 2 and Table 2). Cells in wells stimulated with PHA produced IFN-γ despite CD8 depletion.
FIG. 2.
Depletion of CD8+ T cells markedly diminishes responses to the HIV peptide pools but not that to PHA. BMC or CD8-depleted BMC obtained from volunteer 2 were stimulated with medium or with HIV peptide pools from Gag, Pol, or Nef in an IFN-γ ELISPOT assay.
TABLE 2.
IFN-γ ELISPOT using CD8-depleted BMC
| Antigen | No. of SFC/million
|
|
|---|---|---|
| BMCa | CD8-depleted BMC | |
| Medium | 135 | 15 |
| Gag | 870 | 150 |
| Pol | 1,595 | 75 |
| Nef | 1,405 | 40 |
| PHA | >2,000 | 1,255 |
BMC obtained from volunteer 2.
Additional breast milk samples from volunteer 2 (HLA haplotype, A3, A11, B35, B51) and a second sample from volunteer 5, allowed further characterization of their HIV-specific response. Epitope mapping was performed for volunteer 2 (Table 3). This individual had IFN-γ production to several Pol, Nef, and Gag peptides. All but one of these 20-mers (N18, Nef aa 171 to 190) contained CD8 T-cell epitopes with known restricting MHC class I proteins present for the haplotype expressed by volunteer 2. A second sample of breast milk from volunteer 5 also allowed further epitope characterization that revealed responses to Gag, Pol, and Env epitopes that were also previously reported and had restrictions consistent with her HLA type (data not shown). These results suggest that BMC respond to well-described CD8+ T-cell epitopes presented by MHC class I proteins, consistent with our observation that CD8+ T cells are primarily responsible for the observed HIV-specific responses.
TABLE 3.
HIV epitope mapping of BMC from volunteer 2 (HLA haplotype, A3, A11, B35, B51)
| Peptide (aa) | aa sequencea | No. of SFC/106 cells
|
Haplotype (reference) | |
|---|---|---|---|---|
| BMC | PBMC | |||
| Pol C poolb (201-310) | 760 | 2,150 | ||
| P26 (251-270) | HPAGLKKKKSVTVLDVGDAY | 885 | ND | B35 (57) |
| P27 (261-280) | VTVLDVGDAYFSVPLDEDFR | 1,410 | ND | B35 (57) |
| P28 (271-290) | FSVPLDEDFRKYTAFTIPSI | 950 | ND | B51 (50) |
| P29 (281-300) | KYTAFTIPSINNEETPGIRYQ | 860 | ND | B51 (50) |
| Pol D poolb (301-410) | 1,375 | 2,175 | ||
| P33 (321-340) | KILEPFRKQNPDIVIYQYMD | 1,305 | ND | B35 (42, 49) |
| Nef B poolb (101-205) | 1,580 | 2,330 | ||
| N14 (131-150) | GVRYPLTFGWCYKLVPVEPD | 1,825 | ND | B35 (49) |
| N18 (171-190) | GMDDPEREVLEWRFDSRLAF | 1,595 | ND | A2 (17) |
| Gag A poolb (1-110) | 590 | 355 | ||
| G2 (11-30) | GELDRWEKIRLRPGGKKKYK | 490 | ND | A3 (14) |
| G3 (21-40) | LRPGGKKKYKLKHIVWASRE | 420 | 600 | A3 (14) |
| p17 Gag A3 (20-28) | RLRPGGKKK | ND | 620 | |
| Medium | 175 | 25 | ||
Published epitope for the given HLA is underlined.
Each peptide pool contained 10 20-mers overlapping by 10 aa, covering the indicated region of HIV protein.
Cytolytic response.
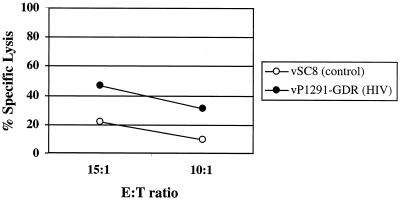
To determine whether these breast milk-derived CD8+ T cells had lytic effector functions, we performed a 51Cr-release assay. HIV-specific lytic activity was measured using BLCL targets infected with vaccinia virus expressing HIV-1 Env, Gag, Pol, and Nef antigens (vP1291-GDR). BMC from volunteer 12 were capable of significantly lysing vaccinia virus-HIV-infected BLCL at two E:T ratios (Fig. 3). These data demonstrated that HIV-specific lytic activity is present in this cell population.
FIG. 3.
Breast milk-derived CTL are capable of lysing infected targets. Fresh BMC from volunteer 12 were stimulated for 3 days with peptide pools from Gag, Pol, and Nef and mixed with autologous BLCL infected with vSC8 (vaccinia virus control) or vP1291-GDR (vaccinia virus expressing Env, Gag, Pol, and Nef epitopes) at the indicated E:T ratios.
MHC class I tetramer analysis.
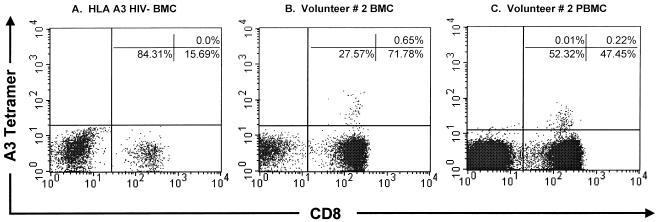
In order to better quantify HIV-1 epitope-specific CD8+ T cells, HLA tetramer analysis of BMC was performed. For this assay, purified HLA molecules are folded around their antigenic virus-derived peptide epitope and linked as tetramers to streptavidin. This reagent allows for the identification and quantitation of antigen-specific CD8+ T cells. Analysis of the BMC ELISPOT responses indicated that volunteer 2 responded to the previously described A∗0301-restricted HIV-1 Gag p17 epitope at aa 17 to 28 (RLRPGGKKK). Flow cytometric analysis using anti-CD3-APC, anti-CD8-PerCP, and the HLA A∗0301 p17 Gag tetramer-PE demonstrated that 0.65% of CD3+/CD8+ BMC and 0.22% of peripheral blood cells were specific for this HIV-1 epitope (Fig. 4). The magnitude of the tetramer responses observed in breast milk to the Gag p17 epitope was similar to those reported in the peripheral blood (range, 0.08% to 2.7%) (10, 15, 36, 37). No tetramer-positive cells were detected in breast milk T cells from an HLA A3 HIV-seronegative woman.
FIG. 4.
Breast milk-derived cells stain with HLA class I tetramer reagents. HIV-1 Gag p17 (aa 17 to 28; RLRPGGKKK) HLA A∗0301-restricted tetramer staining. (A) BMC from an HLA A3+ HIV-seronegative woman. (B) BMC from volunteer 2. (C) PBMC from volunteer 2. For tetramer analysis, cells were gated by using three parameters (forward scatter versus side scatter and CD3+ T cells) in order to focus on the CD3+ T-cell population. The percentage of CD8+ T cells that stained with the tetramer is given in the upper right quadrant of each dot plot.
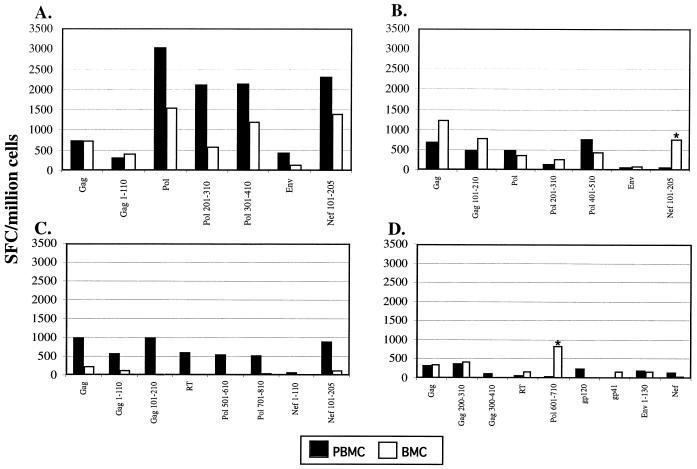
Comparison of breast milk and immune responses in peripheral blood.
Breast milk is recognized as a distinct mucosal compartment, and HIV-specific IgG responses differ in breast milk and blood (3). We compared the specificities of HIV-targeted T-cell responses in breast milk with those in peripheral blood. Although responses to several HIV antigens were detected in both compartments, the frequencies of responding cells in PBMC versus those in BMC varied for most antigens (Fig. 5). Moreover, in two of four women, responses to HIV antigens (Nef aa 101 to 205 and Pol aa 601 to 710) were readily measured in the breast milk but were not detected or weakly present in blood (Fig. 5B and D, asterisks).
FIG. 5.
Comparison of HIV-specific responses between PBMC and BMC. PBMC and BMC were obtained from HIV+ volunteer 2 (A), HIV+ volunteer 3 (B), HIV+ volunteer 4 (C), and HIV+ volunteer 5 (D) and stimulated with peptide pools overnight in an IFN-γ ELISPOT assay. Net spots shown, i.e., cells incubated with medium alone as a negative control antigen, were subtracted from the values shown (control values of 0 to 25 SFC/million PBMC and 0 to 175 SFC/million BMC). Note that PBMC contain ca. 80% T cells and BMC contain ca. 10% T cells and values are not normalized for lymphocyte content in each compartment.
DISCUSSION
These studies present evidence that HIV-specific cytotoxic CD8+ T cells are present in the breast milk of infected women. In our studies, responses were detected in each of the 11 HIV-seropositive women tested, over a wide range of peripheral blood CD4 counts (178 to 1,215 cells/mm3) and viral RNA levels in plasma (<50 to 169,979 copies/ml) or breast milk (<50 to 6,246 copies/ml). Breast milk CTL responses were detected in women receiving highly active antiretroviral therapy or only a single dose of intrapartum nevirapine. HIV-specific responses were detected to all four (Env, Gag, Pol, and Nef) of the HIV proteins tested, and more than 80% of women had responses to two or more HIV proteins. These data show that responses to HIV in breast milk are generally similar in magnitude and breadth to responses published for PBMC (1, 8, 10, 12, 13, 35).
Our data indicate that the majority of the HIV-specific responses detected in breast milk are mediated by cytolytic CD8+ T cells. Depletion of CD8+ BMC diminished HIV-specific responses, BMC were capable of HIV-specific killing of autologous target cells, and tetramer analysis confirmed that CD3+ CD8+ cells were responsible for HIV peptide recognition. Epitope mapping further confirmed that BMC responded to 20-mers containing known MHC class I-restricted HIV epitopes (Table 3). Taken together, these data demonstrate that CD8+ T cells are capable of lytic activity and are a major component of HIV-1-specific responses in BMC. This suggests that breast milk CTL may be critical to the control of HIV within the breast, just as peripheral blood CTL are associated with control of viremia (8, 10, 22, 36, 45).
It is particularly interesting that the magnitude and breadth of the HIV-specific responses observed in BMC are at least similar to those observed in the peripheral blood of HIV-infected persons. This is somewhat surprising, since T cells constitute less than 10% of the cells in breast milk compared to more than 80% of the cells in the peripheral blood of HIV-infected persons (20, 40). Unfortunately, we do not have the number of CD8 cells present in breast milk for these volunteers and are unable to normalize the responses. If this were possible, it is likely that the responses in breast milk would be higher for most antigens tested. Our data would then suggest a greater percentage of HIV-specific CD8+ T cells in breast milk than in blood. This is supported by our observation that 0.65% of CD3+ CD8+ BMC were A3-RK9 tetramer positive, while only 0.22% of simultaneously obtained CD3+ CD8+ PBMC had this specificity (Fig. 4). However, further studies are needed to confirm this finding. These data are consistent with the recent observation that the cervicovaginal compartment of macaques contains a higher frequency of SIV-specific CD8+ T cells than the blood or draining iliac lymph nodes (51).
Although many responses detected in breast milk were also present in blood, in two of four women studied (Fig. 5B and D), responses to one antigen were readily measured in breast milk but were not detected or weakly present in blood. Conversely, in one volunteer (Fig. 5C), most responses were readily detected in blood and not in milk. These findings suggest differences in the specificity of responses between BMC and PBMC, indicating immunologic and/or virologic compartmentalization. This is not unexpected, since the breast is recognized as a distinct mucosal compartment and HIV-specific IgG responses have been found to differ in breast milk and blood (3). Alternatively, these CTL responses could be enriched in breast milk and be present below the level of detection in PBMC.
Data from both rodent and human studies have demonstrated that B cells in the breast originate in the gastrointestinal and respiratory tracts (2, 9, 11, 41, 56). Indeed, after oral immunization with nonpathogenic Escherichia coli, type-specific IgA-producing cells can be detected in breast milk but not in serum or saliva of lactating women (11). There are few data on the origin of breast milk T cells. One study with rats found that breast milk T cells originate from both the gut and the peripheral circulation (29). If this is also the case for humans, the T-cell repertoire in breast milk should include both that of the peripheral circulation and the gut. This would explain our finding that the magnitude and breadth of the responses in breast milk were similar to those of PBMC. Moreover, a dual origin of BMC could explain our observation that most responses detected in milk are also present in blood, with the exception of two antigens only detected in milk.
While we are not aware of other findings which describe HIV-directed T-cell responses, data on in vitro proliferative responses by BMC have been described. Responses to mumps virus (38), rubella virus (28, 38), influenza virus (43), CMV (38), rotavirus (52), E. coli (25), and tuberculin purified protein derivative (24, 30, 39, 44) have been detected. However, questions remain as to the role of these cells in modifying transmission of infectious agents to the infant. Breast milk serves both a critical nutritive and immunologic function essential for the survival of the species. In the natural human state, breast-feeding constitutes the sole source of nutrition for almost the first year of life. The spread of potentially harmful infectious agents via this irreplaceable link would be expected to have an enormous impact on human reproductive fitness. From an evolutionary standpoint, there is every reason to expect that multiple strategies have evolved to protect the infant. Breast milk, unlike most other secretions, contains a relatively large number of viable immune cells, including T cells and macrophages. These cells survive digestion, traverse the intestinal tract, and are functional in the nursing infant (18, 19, 21, 46, 48, 55). This raises the interesting possibility that BMC control pathogens not only within the breast but within the infant as well.
Levels of HIV RNA in breast milk are 1 to 2 log10 lower than those of HIV RNA in plasma, while those of HIV DNA are higher in breast milk than in blood (unpublished data and reference 27). A broader repertoire of T cells or a relatively higher number of HIV-specific T cells in milk versus PBMC could account for the discordance in levels of HIV in breast milk and blood. In addition to effects on local virus production, HIV-specific CTL in breast milk may also be adoptively transferred to the infant. There is ample evidence from animal models that BMC traverse the intestine and function within the neonate. Studies of mice and lambs have shown the direct presence of maternally derived leukocytes in peripheral tissue as well the ability of these cells to transfer T-cell immunity to the nursed offspring (18, 19, 46, 55). Nude mice, which are severely deficient in their plaque-forming cell responses to sheep erythrocytes, suckled by heterozygous normal mothers had a 10-fold increase in the production of plaque-forming cells to sheep erythrocytes than nude mice suckled by their nude mothers (18). Another study showed that 40% of mice susceptible to a transplantable tumor were resistant to tumor challenge if they had been nursed by a resistant mother; however, mice nursed by a susceptible mother developed tumors and died (19). Studies with baboons also suggest that human milk leukocytes may cross from the gastrointestinal tract into the circulation of the neonate (21). Furthermore, there is evidence for humans from some studies of the transfer of maternal T-cell reactivity to tuberculin protein from mother to the neonate via breast-feeding (24, 30, 39, 44). These studies provide evidence that maternally derived T cells make their way into the infant's circulation and potentially protect the infant via adoptive transfer of maternal T cells. In addition to the protection these cells may afford the neonate, we speculate that they may also be acting locally to reduce the viral load in breast milk, lowering the viral burden, and potentially decreasing transmission to newborns from their HIV-infected mothers.
Acknowledgments
We thank the volunteers for their participation, Don Decker for technical assistance, Barbara Corley, Shawn Dillon, and the Children’s Hospital Family Clinic for breast milk procurement, Marion Spell for help with the flow cytometric analysis, and Lynda Tussey for tetramer analysis. In addition, we thank John Sleasman for critically reviewing the manuscript and Allan Zajac for very helpful discussions.
This work was supported by grants from the National Institutes of Health (R01 HD-396110) and the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Addo, M. M., M. Altfeld, E. S. Rosenberg, R. L. Eldridge, M. N. Philips, K. Habeeb, A. Khatri, C. Brander, G. K. Robbins, G. P. Mazzara, P. J. Goulder, and B. D. Walker. 2001. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 98:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allardyce, R. A., D. J. Shearman, D. B. McClelland, K. Marwick, A. J. Simpson, and R. B. Laidlaw. 1974. Appearance of specific colostrum antibodies after clinical infection with Salmonella typhimurium. Br. Med. J. 3:307-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becquart, P., H. Hocini, B. Garin, A. Sepou, M. D. Kazatchkine, and L. Belec. 1999. Compartmentalization of the IgG immune response to HIV-1 in breast milk. AIDS 13:1323-1331. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Investig. 102:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., Z. Hel, B. Kelsall, V. Kuznetsov, J. Ahlers, J. Nacsa, D. Watkins, T. Allen, A. Sette, J. Altman, R. Woodward, P. Markham, J. Clements, G. Franchini, W. Strober, and J. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Immunol. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 6.Crago, S. S., S. J. Prince, T. G. Pretlow, J. R. McGhee, and J. Mestecky. 1979. Human colostral cells. I. Separation and characterization. Clin. Exp. Immunol. 38:585-597. [PMC free article] [PubMed] [Google Scholar]
- 7.Dorrell, L., A. J. Hessell, M. Wang, H. Whittle, S. Sabally, S. Rowland-Jones, D. R. Burton, and P. W. Parren. 2000. Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from The Gambia. AIDS 14:1117-1122. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishaut, M., D. Murphy, M. Neifert, K. McIntosh, and P. L. Ogra. 1981. Bronchomammary axis in the immune response to respiratory syncytial virus. J. Pediatr. 99:186-191. [DOI] [PubMed] [Google Scholar]
- 10.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblum, R. M., S. Ahlstedt, B. Carlsson, L. A. Hanson, U. Jodal, G. Lidin-Janson, and A. Sohl-Akerlund. 1975. Antibody-forming cells in human colostrum after oral immunisation. Nature 257:797-798. [DOI] [PubMed] [Google Scholar]
- 12.Goulder, P. J., M. M. Addo, M. A. Altfeld, E. S. Rosenberg, Y. Tang, U. Govender, N. Mngqundaniso, K. Annamalai, T. U. Vogel, M. Hammond, M. Bunce, H. M. Coovadia, and B. D. Walker. 2001. Rapid definition of five novel HLA-A∗3002-restricted human immunodeficiency virus-specific cytotoxic T-lymphocyte epitopes by ELISPOT and intracellular cytokine staining assays. J. Virol. 75:1339-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulder, P. J., C. Brander, K. Annamalai, N. Mngqundaniso, U. Govender, Y. Tang, S. He, K. E. Hartman, C. A. O'Callaghan, G. S. Ogg, M. A. Altfeld, E. S. Rosenberg, H. Cao, S. A. Kalams, M. Hammond, M. Bunce, S. I. Pelton, S. A. Burchett, K. McIntosh, H. M. Coovadia, and B. D. Walker. 2000. Differential narrow focusing of immunodominant human immunodeficiency virus Gag-specific cytotoxic T-lymphocyte responses in infected African and Caucasoid adults and children. J. Virol. 74:5679-5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulder, P. J., A. K. Sewell, D. G. Lalloo, D. A. Price, J. A. Whelan, J. Evans, G. P. Taylor, G. Luzzi, P. Giangrande, R. E. Phillips, and A. J. McMichael. 1997. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)—identical siblings with HLA-A∗0201 are influenced by epitope mutation. J. Exp. Med. 185:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray, C. M., J. Lawrence, J. M. Schapiro, J. D. Altman, M. A. Winters, M. Crompton, M. Loi, S. K. Kundu, M. M. Davis, and T. C. Merigan. 1999. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J. Immunol. 162:1780-1788. [PubMed] [Google Scholar]
- 16.Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795-802. [DOI] [PubMed] [Google Scholar]
- 17.Haas, G., U. Plikat, P. Debre, M. Lucchiari, C. Katlama, Y. Dudoit, O. Bonduelle, M. Bauer, H. G. Ihlenfeldt, G. Jung, B. Maier, A. Meyerhans, and B. Autran. 1996. Dynamics of viral variants in HIV-1 Nef and specific cytotoxic T lymphocytes in vivo. J. Immunol. 157:4212-4221. [PubMed] [Google Scholar]
- 18.Hale, M. L., E. E. Hanna, and C. T. Hansen. 1976. Nude mice from homozygous nude parents show smaller PFC responses to sheep erythrocytes than nude mice from heterozygous mothers. Nature 260:44-45. [DOI] [PubMed] [Google Scholar]
- 19.Head, J. R., A. E. Beer, and R. E. Billingham. 1977. Significance of the cellular component of the maternal immunologic endowment in milk. Transplant. Proc. 9:1465-1471. [PubMed] [Google Scholar]
- 20.Ho, F. C., R. L. Wong, and J. W. Lawton. 1979. Human colostral and breast milk cells. A light and electron microscopic study. Acta Paediatr. Scand. 68:389-396. [DOI] [PubMed] [Google Scholar]
- 21.Jain, L., D. Vidyasagar, M. Xanthou, V. Ghai, S. Shimada, and M. Blend. 1989. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch. Dis. Child. 64:930-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 24.Keller, M. A., A. L. Rodgriguez, S. Alvarez, N. C. Wheeler, and D. Reisinger. 1987. Transfer of tuberculin immunity from mother to infant. Pediatr. Res. 22:277-281. [DOI] [PubMed] [Google Scholar]
- 25.Keller, M. A., J. L. Turner, J. A. Stratton, and M. E. Miller. 1980. Breast milk lymphocyte response to K1 antigen of Escherichia coli. Infect. Immun. 27:903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leroy, V., M. L. Newell, F. Dabis, C. Peckham, P. Van de Perre, M. Bulterys, C. Kind, R. J. Simonds, S. Wiktor, P. Msellati, and Ghent International Working Group on Mother-to-Child Transmission of HIV. 1998. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Lancet 352:597-600. [DOI] [PubMed] [Google Scholar]
- 27.Lewis, P., R. Nduati, J. K. Kreiss, G. C. John, B. A. Richardson, D. Mbori-Ngacha, J. Ndinya-Achola, and J. Overbaugh. 1998. Cell-free human immunodeficiency virus type 1 in breast milk. J. Infect. Dis. 177:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losonsky, G. A., J. M. Fishaut, J. Strussenberg, and P. L. Ogra. 1982. Effect of immunization against rubella on lactation products. I. Development and characterization of specific immunologic reactivity in breast milk. J. Infect. Dis. 145:654-660. [DOI] [PubMed] [Google Scholar]
- 29.Manning, L., and M. Parmely. 1980. Cellular determinants of mammary cell-mediated immunity in the rat. I. The migration of radioisotopically labeled T lymphocytes. J. Immunol. 125:2508-2514. [PubMed] [Google Scholar]
- 30.Mohr, J. A. 1972. Lymphocyte sensitisation passed to the child from the mother. Lancet i:688. [DOI] [PubMed]
- 31.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540-549. [PubMed] [Google Scholar]
- 32.Musey, L., Y. Hu, L. Eckert, M. Christensen, T. Karchmer, and M. J. McElrath. 1997. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J. Exp. Med. 185:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nduati, R., G. John, D. Mbori-Ngacha, B. Richardson, J. Overbaugh, A. Mwatha, J. Ndinya-Achola, J. Bwayo, F. E. Onyango, J. Hughes, and J. Kreiss. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167-1174. [DOI] [PubMed] [Google Scholar]
- 34.Nduati, R. W., G. C. John, B. A. Richardson, J. Overbaugh, M. Welch, J. Ndinya-Achola, S. Moses, K. Holmes, F. Onyango, and J. K. Kreiss. 1995. Human immunodeficiency virus type 1-infected cells in breast milk: association with immunosuppression and vitamin A deficiency. J. Infect. Dis. 172:1461-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novitsky, V., N. Rybak, M. F. McLane, P. Gilbert, P. Chigwedere, I. Klein, S. Gaolekwe, S. Y. Chang, T. Peter, I. Thior, T. Ndung'u, F. Vannberg, B. T. Foley, R. Marlink, T. H. Lee, and M. Essex. 2001. Identification of human immunodeficiency virus type 1 subtype C Gag-, Tat-, Rev-, and Nef-specific ELISPOT-based cytotoxic T-lymphocyte responses for AIDS vaccine design. J. Virol. 75:9210-9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 37.Ogg, G. S., S. Kostense, M. R. Klein, S. Jurriaans, D. Hamann, A. J. McMichael, and F. Miedema. 1999. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J. Virol. 73:9153-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogra, S. S., and P. L. Ogra. 1978. Immunologic aspects of human colostrum and milk. II. Characteristics of lymphocyte reactivity and distribution of E-rosette forming cells at different times after the onset of lactation. J. Pediatr. 92:550-555. [DOI] [PubMed] [Google Scholar]
- 39.Ogra, S. S., D. Weintraub, and P. L. Ogra. 1977. Immunologic aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J. Immunol. 119:245-248. [PubMed] [Google Scholar]
- 40.Parmely, M. J., A. E. Beer, and R. E. Billingham. 1976. In vitro studies on the T-lymphocyte population of human milk. J. Exp. Med. 144:358-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roux, M. E., M. McWilliams, J. M. Phillips-Quagliata, P. Weisz-Carrington, and M. E. Lamm. 1977. Origin of IgA-secreting plasma cells in the mammary gland. J. Exp. Med. 146:1311-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowland-Jones, S. L., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi, J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruben, F. L., I. R. Holzman, and P. Fireman. 1982. Responses of lymphocytes from human colostrum or milk to influenza antigens. Am. J. Obstet. Gynecol. 143:518-522. [DOI] [PubMed] [Google Scholar]
- 44.Schlesinger, J. J., and H. D. Covelli. 1977. Evidence for transmission of lymphocyte responses to tuberculin by breast-feeding. Lancet ii:529-532. [DOI] [PubMed]
- 45.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 46.Schnorr, K. L., and L. D. Pearson. 1984. Intestinal absorption of maternal leucocytes by newborn lambs. J. Reprod. Immunol. 6:329-337. [DOI] [PubMed] [Google Scholar]
- 47.Semba, R. D., N. Kumwenda, D. R. Hoover, T. E. Taha, T. C. Quinn, L. Mtimavalye, R. J. Biggar, R. Broadhead, P. G. Miotti, L. J. Sokoll, L. van der Hoeven, and J. D. Chiphangwi. 1999. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J. Infect. Dis. 180:93-98. [DOI] [PubMed] [Google Scholar]
- 48.Sheldrake, R. F., and A. J. Husband. 1985. Intestinal uptake of intact maternal lymphocytes by neonatal rats and lambs. Res. Vet. Sci. 39:10-15. [PubMed] [Google Scholar]
- 49.Shiga, H., T. Shioda, H. Tomiyama, Y. Takamiya, S. Oka, S. Kimura, Y. Yamaguchi, T. Gojoubori, H. G. Rammensee, K. Miwa, and M. Takiguchi. 1996. Identification of multiple HIV-1 cytotoxic T-cell epitopes presented by human leukocyte antigen B35 molecules. AIDS 10:1075-1083. [PubMed] [Google Scholar]
- 50.Sipsas, N. V., S. A. Kalams, A. Trocha, S. He, W. A. Blattner, B. D. Walker, and R. P. Johnson. 1997. Identification of type-specific cytotoxic T lymphocyte responses to homologous viral proteins in laboratory workers accidentally infected with HIV-1. J. Clin. Investig. 99:752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevceva, L., B. Kelsall, J. Nacsa, M. Moniuszko, Z. Hel, E. Tryniszewska, and G. Franchini. 2002. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J. Virol. 76:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Totterdell, B. M., S. Patel, J. E. Banatvala, and I. L. Chrystie. 1988. Development of a lymphocyte transformation assay for rotavirus in whole blood and breast milk. J. Med. Virol. 25:27-36. [DOI] [PubMed] [Google Scholar]
- 53.Walker, B. D., and P. J. Goulder. 2000. AIDS. Escape from the immune system. Nature 407:313-314. [DOI] [PubMed] [Google Scholar]
- 54.Walker, W. A. 1975. Antigen absorption from the small intestine and gastrointestinal disease. Pediatr. Clin. N. Am. 22:731-746. [DOI] [PubMed] [Google Scholar]
- 55.Weiler, I. J., W. Hickler, and R. Sprenger. 1983. Demonstration that milk cells invade the suckling neonatal mouse. Am. J. Reprod. Immunol. 4:95-98. [DOI] [PubMed] [Google Scholar]
- 56.Weisz-Carrington, P., M. E. Roux, M. McWilliams, J. M. Phillips-Quagliata, and M. E. Lamm. 1979. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J. Immunol. 123:1705-1708. [PubMed] [Google Scholar]
- 57.Wilson, C. C., R. C. Brown, B. T. Korber, B. M. Wilkes, D. J. Ruhl, D. Sakamoto, K. Kunstman, K. Luzuriaga, I. C. Hanson, S. M. Widmayer, A. Wiznia, S. Clapp, A. J. Ammann, R. A. Koup, S. M. Wolinsky, and B. D. Walker. 1999. Frequent detection of escape from cytotoxic T-lymphocyte recognition in perinatal human immunodeficiency virus (HIV) type 1 transmission: the Ariel Project for the Prevention of Transmission of HIV from Mother to Infant. J. Virol. 73:3975-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu, C., J. A. Politch, L. Tucker, K. H. Mayer, G. R. Seage, and D. J. Anderson. 1997. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J. Infect. Dis. 176:941-947. [DOI] [PubMed] [Google Scholar]