Abstract
Whey protein concentrate (WPC 80) and sodium caseinate were hydrolyzed by Protamex to 5%, 10%, 15%, and 20% degree of hydrolysis (DH). WPC 80, sodium caseinate and their hydrolysates were then analyzed, compared and evaluated for their nutritional qualities. Their chemical composition, protein solubility, amino acid composition, essential amino acid index (EAA index), biological value (BV), nutritional index (NI), chemical score, enzymic protein efficiency ratio (E-PER) and in vitro protein digestibility (IVPD) were determined. The results indicated that the enzymatic hydrolysis of WPC 80 and sodium caseinate by Protamex improved the solubility and IVPD of their hydrolysates. WPC 80, sodium caseinate and their hydrolysates were high-quality proteins and had a surplus of essential amino acids compared with the FAO/WHO/UNU (1985) reference standard. The nutritive value of WPC 80 and its hydrolysates was superior to that of sodium caseinate and its hydrolysates as indicated by some nutritional parameters such as the amino acid composition, chemical score, EAA index and predicted BV. However, the E-PER was lower for the WPC hydrolysates as compared to unhydrolyzed WPC 80 but sodium caseinate and its hydrolysates did not differ significantly. The nutritional qualities of WPC 80, sodium caseinate and their hydrolysates were good and make them appropriate for food formulations or as nutritional supplements.
Keywords: Nutritional qualities, Enzymatic hydrolysis, Hydrolysates, WPC 80, Sodium caseinate, Protamex
INTRODUCTION
Milk proteins have long been known for their nutritional and technological value. Proteins are important constituents of the human diet, since they comprise a principal source of nitrogen and essential amino acids. Milk proteins have high nutritional value compared to other proteins because of their relatively high content of essential amino acids and good digestibility (Hambraeus, 1992).
Caseins and whey proteins are the two main protein groups in milk. Caseins, representing about 80% of the protein content in bovine milk, are isolated from milk by acid or by rennet precipitation. The acid, or isoelectric, precipitation is performed at pH 4.6, where the caseins precipitate and the whey proteins remain soluble. Caseins are flexible and heat stable proteins.
Whey proteins comprise approximately 20% of the total milk proteins. Whey proteins (or milk serum proteins) are defined as proteins in milk that remain soluble after acid (Walstra and Jenness, 1984) or after rennet casein precipitation (Barth and Behnke, 1997). The former whey protein source is known as acid whey, the latter is referred to as sweet or rennet whey (Morr, 1989). Whey proteins are globular proteins that are soluble over a broad pH range (Mulvihill, 1992).
From the nutritional point of view, milk whey proteins have been considered superior to casein in various aspects. They present amino acid profile superior to casein, being similar to human milk, is what recommends whey proteins for the formulation of humanized milk products for replacement of bovine milk in infant nutrition (Hambraeus, 1982). Some publications (Boirie et al., 1997; Frühbeck, 1998) reported on important differential properties between caseins and the milk whey proteins. It was observed that the caseins undergo much lower digestion and absorption than the whey proteins.
The amino acid composition is the most important factor in defining food protein quality, followed by the digestibility of the protein and the bioavailability of its amino acids. Because of their amino acid composition the main bovine milk proteins, caseins and whey proteins, can be regarded as a complete source of amino acids. Milk proteins are currently the main source of a range of biologically active peptides and the occurrence of their specific physiological properties which might have nutritional implication is another aspect of their nutritive value.
This work aimed at evaluating and comparing some nutritional qualities of caseins and whey proteins and their hydrolysates from Protamex.
MATERIALS AND METHODS
Materials
Whey protein concentrate (WPC 80, 80% protein based on dry weight) and sodium caseinate (88.03% protein based on dry weight) were obtained from New Zealand Milk Products (Wellington, New Zealand). Protamex, a commercial Bacillus proteinase complex, was obtained from Novo Nordisk’s Enzyme Business (Wuxi, China). All the other chemicals were of analytical grade available from the Southern Yangtze University Chemical Store (Wuxi, China).
Preparation of hydrolysates
Whey protein concentrate (WPC 80) and sodium caseinate were reconstituted at 50 °C in distilled water to give a starting protein concentration of 5% (w/V). Prior to enzymatic treatment the aqueous solution of WPC 80 was allowed to hydrate for 1 h at room temperature with gentle mixing, adjusted to pH 4.6 with 2 mol/L HCl and heated to 85 °C for 30 min to denature WPC 80.
The protein solution was then equilibrated at 50 °C and the pH for Protamex hydrolysis was adjusted to 8.0 with 1 mol/L NaOH before addition of enzyme.
Protamex (1.5 AU/g) was added at a rate of 0.40 AU per 1 g of WPC 80 or sodium caseinate. Hydrolysis experiments were carried out in a 1500 L reaction vessel maintained at 50 °C with the solution being agitated by an over-head stirrer (Kika Labortechnik RW 20.n, Germany) throughout the hydrolysis process adequately controlled by monitoring the degree of hydrolysis (DH) using the pH-stat technique (Adler-Nissen, 1986). Aliquots (250 ml) were taken at 5%, 10%, 15% and 20% DH.
Protamex was inactivated by heating at 90 °C for 15 min. The hydrolysates were centrifuged (3 000 g per 30 min) at room temperature and the pH of hydrolysis. Supernates were then frozen and later freeze dried with a FD-5 freeze dryer (Shanghai, China).
Calculation of the degree of hydrolysis (DH)
The hydrolysis was carried out using the pH-stat method described by Adler-Nissen (1986) and the DH (%) was calculated from the volume and the molarity of alkali used to maintain constant pH.
Moisture, ash and protein determination
Moisture and ash contents were determined according to AOAC (1984) and the crude protein content was measured with Kjeldahl method as described by Ceirwyn (1995).
Amino acid composition
A modified method of AOAC 982.30a (AOAC, 1990) was used for amino acid analysis. Sixty milligrams of freeze-dried sample were hydrolyzed with 8 ml of 6 mol/L HCl under vacuum at 110 °C for 24 h. After cooling, the hydrolysate was washed with distilled water, filtered (Whatman No. 2) and dried at 60 °C (also under vacuum) in a rotary evaporator. The dried sample was then dissolved in 0.01 mol/L HCl. The amino acids in the hydrolysate were separated and quantified by injecting 50 μl into a Hitachi 835-50 amino acid analyzer equipped with a 2.6 mm×150 mm ion exchange column coated with resin 2619#. The column temperature was 53 °C. Sodium citrate buffers (pH 3.3, 4.3, and 6.3) were used as eluents with a flow rate of 0.225 ml/min. The light absorbance of the amino acids was detected with a 166 Detector (Beckman Instruments) at 570 nm and the amino acids were quantified by comparing them with amino acid profiles from external amino acid standard.
Determination of tryptophan
Tryptophan was estimated by the ninhydrin method of Pintér-Szakács and Molnán-Perl (1990). One gram of sample was introduced into a 25 ml polypylene test tube with caps and then 10 ml of 0.075 mol/L NaOH was added and mixed until there were no lumps. The dispersion was shaken for 30 min and centrifuged at 5000 r/min for 10 min and the supernate was transferred to a clean test tube. To 0.5 ml of supernate, 5 ml of ninhydrin reagent (1.0 g of ninhydrin in 100 ml mixture of 37% HCl and 96% HCOOH at a ratio of 2:3) was added and then solution was incubated at 35 °C for 2 h, and then cooled to room temperature after which the volume was made up to 10 ml with diethyl ether, thoroughly mixed with a Vortex mixer, filtrated and the clear filtrate was read at 380 nm. A standard tryptophan curve was prepared using 0~100 μg tryptophan. From the standard graph, the concentration of tryptophan was calculated and expressed as g/100 g protein.
Protein solubility
Protein solubility (PS) was determined by the method of Bera and Mukherjee (1989). Two hundred milligrams of proteins were dispersed in 10 ml of deionized water. The pH of suspensions was adjusted to different levels (2.0 to 10.0) by using 1 mol/L HCl or 1 mol/L NaOH. The suspensions were stirred at room temperature for 30 min and then centrifuged at 10000×g for 30 min (Kika Ultra Turrax T18 basic, Germany). Protein contents in supernates were determined by Kjeldahl method (Ceirwyn, 1995). The percentage of protein solubility in each suspension was calculated by the ratio of protein in the supernate to protein in 200 mg sample.
Determination of in vitro protein digestibility (IVPD)
The method of Saunders et al.(1973) was applied with slight modifications to determine the in vitro protein digestibility. The sample (0.2 g) was placed in a 50 ml centrifuge tube, to which 15 ml of 0.1 mol/L HCl containing 1.5 mg pepsin was added and then the tube was incubated at 37 °C for 3 h. The suspension was then neutralized with 0.5 mol/L NaOH (ca. 3.3 ml), then treated with 4 milligrams of pancreatin in 7.5 ml of 0.2 mol/L phosphate buffer (pH 8.0), containing 0.005 mol/L sodium azide. One milliliter of toluene was added to prevent microbial growth and the mixture was then gently shaken and incubated for additional 24 h at 37 °C. After incubation, the sample was treated with 10 ml of 10% trichloroacetic acid (TCA) and centrifuged at 50 000 g for 20 min at room temperature. Protein in the supernate was estimated using the Kjeldahl method (Ceirwyn, 1995). The percentage of protein digestibility was calculated by the ratio of protein in supernate to protein in sample.
Determination of in vitro enzymic protein efficiency ratio (E-PER)
The computed protein efficiency ratio (E-PER) was determined using the model developed by Ihekoronye (1981). This model provides estimates of protein quality based on amino acid profiles after enzymic proteolysis.
Calculation of chemical score, essential amino acid index, biological value and nutritional index
Chemical score which is based on the amount of most limiting amino acid present in the test protein relative to the amount of that amino acid in reference egg protein was calculated using the equation given by Block and Mitchel (1946).
Essential amino acid index (EAA index) was calculated according to the procedure of Oser (1951) taking into account the ratio of EAA in the test protein relative to their respective amounts in whole egg protein.
The biological value (BV) was calculated using the formula of Oser (1959).
The nutritional index (NI) was calculated using the equation of Crisan and Sands (1978).
Statistical analysis
Except for chromatographic analysis, all other experiments were replicated thrice and all measurements were carried out at least twice. Data were analyzed using analysis of variance (ANOVA) and the differences between treatment means were determined by the multiple range test (Duncan, 1955). Differences were considered to be significant at P<0.05 throughout this study.
RESULTS AND DISCUSSION
Proximate composition of WPC 80, sodium caseinate and their hydrolysates
The protein, moisture and ash content of WPC 80, sodium caseinate and their freeze-dried hydrolysates at four different DH levels were compared (Tables 1 and 2).
Table 1.
Proximate composition of WPC 80 and its hydrolysates
| Sample | Protein* (%) | Moisture* (%) | Ash* (%) |
| WPC 80 | 79.54±0.11 | 4.01±0.04 | 3.56±0.11 |
| Hydrolysates | |||
| 5% DH | 63.78±0.12 | 6.51±0.52 | 9.18±0.05 |
| 10% DH | 66.03±0.43 | 6.10±0.29 | 10.55±0.19 |
| 15% DH | 64.89±0.37 | 6.20±0.01 | 12.14±0.29 |
| 20% DH | 63.18±0.11 | 7.45±0.84 | 12.97±0.11 |
Values represent means of three replicates±standard deviation
Table 2.
Proximate composition of sodium caseinate and its hydrolysates
| Sample | Protein* (%) | Moisture* (%) | Ash* (%) |
| Sodium caseinate | 88.03±0.14 | 4.83±0.03 | 5.22±0.06 |
| Hydrolysates | |||
| 5% DH | 85.89±0.14 | 5.39±0.31 | 8.87±0.08 |
| 10% DH | 82.35±0.15 | 6.41±0.11 | 10.36±0.01 |
| 15% DH | 76.46±0.29 | 6.37±0.13 | 11.39±0.14 |
| 20% DH | 76.67±0.31 | 7.11±0.44 | 13.39±0.09 |
Values represent means of three replicates±standard deviation
All hydrolysates produced at various DH were different from unhydrolyzed protein (WPC 80 or sodium caseinate) in protein, moisture and ash content. In general, protein content decreased after hydrolysis but no relationship between DH and protein content was observed. The differences in protein content of hydrolysates at different DH were due to the corresponding difference in non-protein nitrogen (NPN). The produced peptides can interact with unhydrolyzed protein (WPC 80 or sodium caseinate) via hydrophobic interactions resulting in increase of the insoluble protein fraction. Also some protein/peptides were lost during the centrifugation prior to freeze-drying with the apparent decrease in protein content of freeze-dried hydrolysates being also related to higher ash levels in the samples. In general protein was lost and moisture increased with increasing hydrolysis. The differences in moisture could arise from varying efficiency of freeze drying or storage conditions. Ash content of the hydrolysates increased with increasing DH with all hydrolysates having higher ash content than the unhydrolyzed protein (WPC 80 or sodium caseinate). Increase in ash content corresponded to the increase in base (NaOH) consumption with DH and the adjustment of pH before enzymatic hydrolysis.
Protein solubility
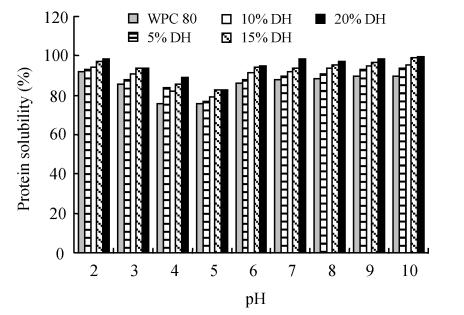
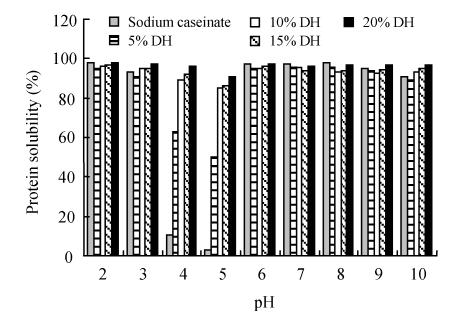
The protein solubility was measured in the pH range of 2 to 10 (the pH-protein solubility profiles of WPC 80, sodium caseinate and their hydrolysates are shown in Fig.1). WPC 80, sodium caseinate and their hydrolysates had minimum solubility at pH 4.0~5.0. They had the highest solubility values at alkaline pH and in the pH range between 2.0 and 3.0. Solubility in the pI range increased from 75.5% to 77.8%, 79.3%, 81.3% and 86.5% for WPC 80 and its hydrolysates at 5%, 10%, 15% and 20% DH, respectively. At 5%, 10%, 15% and 20% DH, the sodium caseinate hydrolysates were 50%, 84.8%, 86% and 91% soluble at pH=5.0, respectively. They were from 84.8%~98% soluble between pH 4.0~5.0 at 10%~20% DH. These results accord with those of Slattery and Fitzgerald (1998), Chobert et al.(1988a; 1988b).
Fig. 1.
Protein solubility of (a) WPC 80 and (b) sodium caseinate, and their hydrolysates
The results indicated that the enzymatic hydrolysis of WPC 80 and sodium caseinate by Protamex improved the solubility of their hydrolysates. This enzymatic hydrolysis of WPC 80 and sodium caseinate increased the number of ionizable groups NH4 +, COO−) with concomitant increase in hydrophilicity and net charge of the resulting hydrolysates, promoting hydrolysate-water interaction and enhancing their solubility. It altered their structure and exposed previously buried hydrophobic regions to the aqueous environment. The enhanced solubility of sodium caseinate and WPC hydrolysates was also due to their smaller molecular size (data not shown) as confirmed by Chobert et al.(1988b) and Mutilangi et al.(1996). Although Protamex is an endopeptidase with a broad specificity to hydrophobic amino acids, it attacked WPC 80 and sodium caseinate at specific sites. Therefore, they produced hydrolysates with different M w distributions (data not shown) and different solubility profiles (Fig.1).
The results showed also that changes in solubility with DH were small at 5% and 10% DH and became more noticeable up to 15% and 20% DH for WPC 80. The statistical analysis revealed that WPC 80 and its hydrolysates at 5% and 10% were not significantly different (P<0.05) but they were significantly different to those at 15% and 20% DH (data not shown). It was postulated that the heat treatment of hydrolysates for enzyme inactivation lowered their solubility by promoting hydrophobic interactions (peptide-peptide or peptide-protein interactions).
Amino acid composition
Amino acid profile of WPC 80, sodium caseinate and their hydrolysates; human milk (Hambraeus, 1982) and the FAO/WHO/UNU (1985) reference standard are shown in Tables 3 and 4.
Table 3.
Amino acid pattern (mg/g protein) of WPC 80 and its hydrolysates, human milk and the FAO/WHO/UNU reference standard
| Amino acid | WPC 80 | WPC hydrolysates |
Human milka | FAO/WHO/UNU (1985) reference standardb | |||
| 5% DH | 10% DH | 15% DH | 20% DH | ||||
| Essential | |||||||
| Ile | 49.7 | 54.1 | 52.4 | 54.6 | 49.1 | 49 | 28 |
| Leu | 106.6 | 105.7 | 110.1 | 108.4 | 106.5 | 91 | 66 |
| Lys | 88.1 | 91.5 | 93.2 | 92.5 | 91.9 | 65 | 58 |
| Met+Cys | 79.7 | 40.7 | 43.5 | 41.1 | 42.2 | 37 | 25 |
| Phe+Tyr | 58.2 | 61.0 | 63.2 | 61.6 | 60.6 | 76 | 63 |
| Thr | 68.7 | 72.9 | 71.9 | 70.5 | 71.8 | 44 | 34 |
| Val | 18.4 | 53.5 | 51.7 | 54.6 | 47.8 | 52 | 35 |
| Trp | 17.3 | 21.4 | 17.9 | 19.7 | 17.1 | NAc | 11 |
| Non-essential | |||||||
| His | 7.8 | 17.8 | 18.4 | 17.4 | 16.7 | ||
| Ala | 55.5 | 54.1 | 54.8 | 54.0 | 54.7 | ||
| Arg | 27.1 | 29.0 | 29.0 | 29.4 | 28.2 | ||
| Asp | 91.8 | 91.1 | 93.0 | 90.4 | 93.4 | ||
| Glu | 158.4 | 172.5 | 170.8 | 167.6 | 169.4 | ||
| Gly | 53.2 | 19.1 | 19.6 | 19.6 | 19.9 | ||
| Pro | 66.6 | 60.5 | 55.1 | 64.5 | 74.8 | ||
| Ser | 53.0 | 55.1 | 55.3 | 54.0 | 55.6 | ||
From Hambraeus (1982)
FAO/WHO/UNU amino acid requirement pattern based on amino acid requirements of preschool-age child
Not analyzed
Table 4.
Amino acid pattern (mg/g protein) of sodium caseinate and its hydrolysates, human milk and the FAO/WHO/UNU reference standard
| Amino acid | SCa | SC hydrolysates |
Human milkb | FAO/WHO/UNU (1985) reference standardc | |||
| 5% DH | 10% DH | 15% DH | 20% DH | ||||
| Essential | |||||||
| Ile | 45.9 | 47.5 | 44.6 | 41.0 | 41.1 | 49 | 28 |
| Leu | 88.9 | 85.0 | 88.5 | 89.2 | 88.3 | 91 | 66 |
| Lys | 77.5 | 79.6 | 79.7 | 77.1 | 74.4 | 65 | 58 |
| Met+Cys | 32.0 | 30.3 | 31.8 | 32.3 | 31.9 | 37 | 25 |
| Phe+Tyr | 101.4 | 90.6 | 97.2 | 102.1 | 101.6 | 76 | 63 |
| Thr | 40.5 | 42.5 | 42.1 | 41.7 | 41.4 | 44 | 34 |
| Val | 56.4 | 52.3 | 54.1 | 49.7 | 49.8 | 52 | 35 |
| Trp | 10.4 | 10.0 | 9.6 | 11.2 | 9.9 | NAd | 11 |
| Non-essential | |||||||
| His | 25.4 | 24.4 | 25.0 | 24.7 | 24.1 | ||
| Ala | 27.6 | 25.9 | 26.9 | 28.4 | 28.2 | ||
| Arg | 33.5 | 38.5 | 33.1 | 32.9 | 32.7 | ||
| Asp | 75.7 | 82.4 | 78.4 | 77.6 | 77.5 | ||
| Glu | 218.4 | 230.3 | 223.9 | 223.3 | 220.7 | ||
| Gly | 17.3 | 17.1 | 17.5 | 17.6 | 17.3 | ||
| Pro | 93.3 | 85.9 | 90.7 | 94.5 | 104.7 | ||
| Ser | 55.4 | 57.6 | 57.0 | 56.7 | 56.2 | ||
Sodium caseinate
From Hambraeus (1982)
FAO/WHO/UNU amino acid requirement pattern based on amino acid requirements of preschool-age child
Not analyzed
The global amino acid compositions did not differ significantly between the sodium caseinate hydrolysates and they were very close among sodium caseinate and its hydrolysates (Table 4). The same trend was observed for WPC 80 and its hydrolysates except for Met, Gly, His and Val (Table 3).
Some important protein characteristics of WPC 80 and its hydrolysates were the high content of sulfur-containing amino acids (Met and Cys); the high concentration of Leu, Ile, Lys, Thr; and the relatively low content of the aromatic amino acids Phe and Tyr. On the contrary, sodium caseinate and its hydrolysates showed low contents of Ile, Leu, Lys, Thr and Cys; higher contents of Phe and Tyr than WPC 80 and its hydrolysates. By comparing essential amino acid pattern of human milk with WPC 80, sodium caseinate and their hydrolysates; it is apparent that WPC 80 and its hydrolysates are more similar to human milk than the sodium caseinate and its hydrolysates. These results accord with those of Hambraeus (1982), Valdemiro et al.(2000). This is why WPC 80 and its hydrolysates have been used with success in the formulation of infant formulas and humanized milk products. WPC 80, sodium caseinate and their hydrolysates had a surplus of essential amino acids compared with the FAO/WHO/UNU (1985) reference standard. Tables 3 and 4 show that WPC 80, sodium caseinate and their hydrolysates are high-quality proteins although the nutritive value of sodium caseinate and its hydrolysates is inferior to that of WPC 80 and its hydrolysates.
In vitro protein digestibility (IVPD) and some nutritional parameters of WPC 80, sodium caseinate and their hydrolysates
Chemical scores for essential amino acids of WPC 80, sodium caseinate and their hydrolysates were calculated and results are reported in Table 5. They were calculated with comparison to the FAO egg reference pattern (FAO/WHO, 1965).
Table 5.
Chemical score of WPC 80 and sodium caseinate and their hydrolysates
| EAA | WPC 80 | WPC hydrolysates |
SC | SC hydrolysates |
||||||
| 5% DH | 10% DH | 15% DH | 20% DH | 5% DH | 10% DH | 15% DH | 20% DH | |||
| Ile | 79.37 | 83.97 | 80.83 | 84.37 | 78.37 | 78.76 | 84.33 | 77.45 | 71.73 | 72.87 |
| Leu | 127.68 | 123.04 | 127.37 | 125.63 | 127.48 | 114.40 | 113.18 | 115.26 | 117.04 | 117.42 |
| Lys | 145.09 | 146.45 | 148.25 | 147.40 | 151.26 | 137.13 | 145.74 | 142.73 | 139.10 | 136.03 |
| Met+Cys | 152.74 | 75.80 | 80.52 | 76.21 | 80.82 | 65.89 | 64.55 | 66.27 | 67.81 | 67.87 |
| Phe+Tyr | 61.34 | 62.49 | 64.34 | 62.82 | 63.84 | 114.83 | 106.16 | 111.40 | 117.89 | 118.89 |
| Thr | 141.99 | 146.42 | 143.53 | 140.98 | 148.30 | 89.93 | 97.65 | 94.61 | 94.41 | 94.99 |
| Val | 26.57 | 75.07 | 72.10 | 76.28 | 68.98 | 87.49 | 83.95 | 84.94 | 78.61 | 79.83 |
| Trp | 113.97 | 137.01 | 113.90 | 125.57 | 112.58 | 73.61 | 73.24 | 68.77 | 80.82 | 72.40 |
| 1st LAA | Val | Phe+Tyr | Phe+Tyr | Phe+Tyr | Phe+Tyr | Met+Cys | Met+Cys | Met+Cys | Met+Cys | Met+Cys |
| 2nd LAA | Phe+Tyr | Val | Val | Met+Cys | Val | Trp | Trp | Trp | Ile | Trp |
| 3rd LAA | Ile | Met+Cys | Met+Cys | Val | Ile | Ile | Val | Ile | Val | Ile |
EAA: Essential amino acids; SC: Sodium caseinate; LAA: Limiting amino acids
Val and Phe+Tyr were found to be respectively the first and second limiting amino acids in WPC 80 while Phe+Tyr and Val (or Met+Cys) were respectively the first and second limiting amino acids in all WPC hydrolysates (Table 5).
Met+Cys and Trp (or Ile) were found to be respectively the first and second limiting amino acids in sodium caseinate and its hydrolysates (Table 5). These results confirmed those of Block and Mitchel (1946). From the results, it was obvious that the differences in the score were due to amino acid composition, which depends on the type of protein. Table 5 shows that the chemical scores for many essential amino acids of WPC 80 and its hydrolysates exceeded those of sodium caseinate and its hydrolysates.
The IVPD along with some nutritional parameters are shown in Table 6.
Table 6.
IVPD and some nutritional indices (%) of WPC 80, sodium caseinate (SC) and their hydrolysates
| Parameters | WPC 80 | WPC hydrolysates |
SC | SC hydrolysates |
||||||
| 5% DH | 10% DH | 15% DH | 20% DH | 5% DH | 10% DH | 15% DH | 20% DH | |||
| EAA index | 72.28 | 85.90 | 85.31 | 85.70 | 81.66 | 77.80 | 77.09 | 76.74 | 76.74 | 74.99 |
| Predicted BV | 67.09 | 81.93 | 81.29 | 81.71 | 77.31 | 73.10 | 72.33 | 71.95 | 71.95 | 70.04 |
| NI | 58.42 | 56.07 | 57.10 | 56.50 | 52.43 | 69.34 | 66.91 | 63.85 | 59.46 | 58.16 |
| E-PER | 4.85 | 3.01 | 3.37 | 2.98 | 2.95 | 3.06 | 3.45 | 3.28 | 3.13 | 2.85 |
| IVPD | 73.22 | 77.18 | 78.59 | 79.18 | 79.50 | 76.25 | 79.34 | 79.65 | 80.86 | 81.20 |
The results indicated that enzymatic hydrolysis of WPC 80 and sodium caseinate by Protamex improved the in vitro protein digestibility of their hydrolysates. The improvement was due to the increase in solubility and the denaturation of these protein molecules making them more accessible to proteolytic enzymes. These results accord with those of Lahl and Braum (1994), Clemente (2000). In vitro studies showed that WPC and sodium caseinate hydrolysates had higher digestibility compared to unhydrolyzed WPC 80 and sodium caseinate. It is apparent that enzymatic hydrolysis affected amino acid availability. The IVPD values of sodium caseinate and its hydrolysates were higher than those of WPC 80 and its hydrolysates. These differences in IVPD values should be explained by the fact that WPC 80 and its hydrolysates were more resistant to enzymatic hydrolysis than sodium caseinate and its hydrolysates; it was postulated that the protein structure played an important role here. The computed E-PER values were lower for the WPC hydrolysates as compared to the original WPC 80. However, there were no significant differences in E-PER between sodium caseinate and its hydrolysates (Table 6). These results confirmed those of Valdemiro et al.(2000), Boza et al.(1995). These results suggested also that the differences in E-PER were related to their nutritional quality and protein content. The NI values of sodium caseinate and its hydrolysates were higher than those of WPC 80 and its hydrolysates, and only the protein content could explain these discrepancies. WPC hydrolysates showed higher EAA index and BV than the unhydrolyzed WPC 80. However, they were slightly lower for the sodium caseinate hydrolysates as compared to the parental sodium caseinate. The EAA index and BV of WPC 80, sodium caseinate and their hydrolysates were normally higher indicating their good nutritional quality. However, the EAA index and BV of WPC hydrolysates were much higher than those of sodium caseinate suggesting their superiority in nutritional quality.
CONCLUSION
Milk proteins, caseins and whey proteins, have high nutritional value compared to other proteins because of their relatively high content of essential amino acids and their good digestibility. Sodium caseinate and WPC 80 are low-lactose milk products so lactose-intolerant people can tolerate them. In fact, lactose intolerance is the inability to digest significant amounts of lactose (the predominant sugar of milk) and this inability results from a shortage of the enzyme lactase which hydrolyzes much of the lactose in milk to glucose and galactose, which lactose-intolerant people can absorb without ill effects. A possible solution to this problem is the use of low-lactose or reduced-lactose milk products such as cheese, yogurt, sodium caseinate and WPC.
In this study, WPC 80, sodium caseinate and their hydrolysates from Protamex at four different DH levels were analyzed, compared and evaluated for their nutritional qualities. The results indicated that the enzymatic hydrolysis of WPC 80 and sodium caseinate by Protamex improved the solubility and IVPD of their hydrolysates. WPC 80, sodium caseinate and their hydrolysates are high-quality proteins and have a surplus of essential amino acids compared with the FAO/WHO/UNU (1985) reference standard. Nevertheless, the nutritive value of WPC 80 and its hydrolysates is superior to that of sodium caseinate and its hydrolysates as indicated by some nutritional parameters such as the amino acid composition, chemical score, EAA index and predicted BV. The nutritional qualities of WPC 80, sodium caseinate and their hydrolysates are good and make them appropriate for food formulations or as nutritional supplements.
Footnotes
Project (No. 1997-17) supported by the National Innovation of Technology Program, the State Economy and Trade Commission, China
References
- 1.Adler-Nissen J. Enzymic Hydrolysis of Food Proteins. London: Elsevier Applied Science Publishers; 1986. pp. 116–124. [Google Scholar]
- 2.AOAC (Association of Official Analytical Chemists) Dairy Products. In: Williams S, editor. Official Methods of Analysis. 14th Ed. Arlington: Association of Official Analytical Chemists Inc; 1984. pp. 276–317. [Google Scholar]
- 3.AOAC (Association of Official Analytical Chemists) Dairy Products. In: Cunniff P, editor. Official Methods of Analysis. 15th Ed. Arlington: Association of Official Analytical Chemists Inc; 1990. pp. 1096–1097. [Google Scholar]
- 4.Barth CA, Behnke U. Nutritional significance of whey and whey components. Nahrung. 1997;41:2–12. doi: 10.1002/food.19970410103. [DOI] [PubMed] [Google Scholar]
- 5.Bera MB, Mukherjee RK. Solubility, emulsifying, and foaming properties of rice bran protein concentrates. Journal of Food Science. 1989;54:142–145. [Google Scholar]
- 6.Block RJ, Mitchel HH. The correlation of the amino acid composition of proteins with their nutritive value. Nutr Abst Review. 1946;16:249–278. [Google Scholar]
- 7.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL. Slow and fast dietary proteins differently modulate post prandial protein accretion. Proceedings of the National Academy of Science, USA. 1997;94(26):14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boza JJ, Martínez-Augustin O, Gil A. Nutritional and antigenic characterization of an enzymatic whey protein hydrolysate. J Agric Food Chem. 1995;43(4):872–875. doi: 10.1021/jf00052a005. [DOI] [Google Scholar]
- 9.Ceirwyn SJ. Analytical Chemistry of Foods. London: Chapman & Hall; 1995. pp. 88–89. [Google Scholar]
- 10.Chobert JM, Sitohy MZ, Whitaker JR. Solubility and emulsifying properties of caseins modified enzymatically by Staphylococcus aureus V8 protease. Journal of Agricultural and Food Chemistry. 1988;36(1):220–224. doi: 10.1021/jf00079a055. [DOI] [Google Scholar]
- 11.Chobert JM, Bertrand HC, Nicolas MG. Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin. Journal of Agricultural and Food Chemistry. 1988;36(5):883–892. doi: 10.1021/jf00083a002. [DOI] [Google Scholar]
- 12.Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends in Food Science & Technology. 2000;11(7):254–262. doi: 10.1016/S0924-2244(01)00007-3. [DOI] [Google Scholar]
- 13.Crisan EV, Sands A. Biology and Cultivation of Edible Mushrooms. New York: Hangeri Academic Press; 1978. pp. 137–142. [Google Scholar]
- 14.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 15.FAO/WHO. Geneva: WHO; 1965. Protein Requirements. WHO Technical Report Series, No. 301. [Google Scholar]
- 16.FAO/WHO/UNU. Geneva: WHO; 1985. Energy and Protein Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation, WHO Technical Report, No. 724. [Google Scholar]
- 17.Frühbeck G. Slow and fast dietary protein. Nature. 1998;391(6670):843–945. doi: 10.1038/35993. [DOI] [PubMed] [Google Scholar]
- 18.Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Developments in Dairy Chemistry–1: Proteins. London: Applied Science Publishers; 1982. pp. 289–313. [Google Scholar]
- 19.Hambraeus L. Nutritional Aspects of Milk Proteins. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Applied Science; 1992. pp. 457–490. [Google Scholar]
- 20.Ihekoronye AI. A Rapid Enzymatic and Chromatographic Predictive Model for the in-vivo Rat-Based Protein Efficiency Ratio. Columbia: University of Missouri; 1981. Ph.D. Thesis. [DOI] [PubMed] [Google Scholar]
- 21.Lahl W, Braum S. Enzymic production of protein hydrolysates for food use. Food Technology. 1994;48:68–71. [Google Scholar]
- 22.Morr CV. Whey Proteins: Manufacture. In: Fox PF, editor. Developments in Dairy Chemistry–4: Proteins. London: Elsevier Science Publishers; 1989. pp. 245–248. [Google Scholar]
- 23.Mulvihill DM. Production, Functional Properties and Utilization of Milk Protein Products. In: Fox PF, editor. Advanced Dairy Chemistry–1: Proteins. London: Elsevier Science Publishers; 1992. pp. 369–404. [Google Scholar]
- 24.Mutilangi WAM, Panyam D, Kilara A. Functional properties of hydrolyzates from proteolysis of heat denatured whey protein isolate. Journal of Food Science. 1996;61:270–274. 303. [Google Scholar]
- 25.Oser BL. Methods for integrating essential amino acid content in the nutritional evaluation of protein. Journal of the American Dietetic Association. 1951;27:396–402. [PubMed] [Google Scholar]
- 26.Oser BL. Protein and Amino Acid Nutrition. New York: Albanese Academic Press; 1959. pp. 281–291. [Google Scholar]
- 27.Pintér-Szakács M, Molnán-Perl I. Determination of tryptophan in unhydrolysed food and feed stuff by the acid ninhydrin method. J Agric Food Chem. 1990;38(3):720–726. doi: 10.1021/jf00093a028. [DOI] [Google Scholar]
- 28.Saunders RM, Connor MA, Booth AN, Bickoff EN, Kohier CO. Measurement of digestibility of alfalfa protein concentrate by in vitro and in vivo methods. Journal of Nutrition. 1973;103:530–535. doi: 10.1093/jn/103.4.530. [DOI] [PubMed] [Google Scholar]
- 29.Slattery H, Fitzgerald RJ. Functional properties and bitterness of sodium caseinate hydrolysates prepared with a bacillus proteinase. Journal of Food Science. 1998;63(3):418–422. [Google Scholar]
- 30.Valdemiro CS, Humberto AR, Zinsly PF, et al. 4th International Conference of Food Science and Technology. Wuxi: China Light Industry Press; 2000. Novel Nutritional and Physiological Functions of Milk Proteins; pp. 196–210. [Google Scholar]
- 31.Walstra P, Jenness R. Dairy Chemistry and Physics. New York: John Willey & Sons Inc; 1984. pp. 114–122. [Google Scholar]