Abstract
Increasing attention is being paid to the scientific evaluation of traditional Chinese medicine (TCM). As many TCMs are capable of biotransformation in the gastrointestinal tract, attention to biotransformation of TCM in the gastrointestinal tract may lead to discovery of the active components and active mechanisms. In this article, we review reports that host metabolic enzymes and intestinal bacteria may be responsible for the metabolism of TCM. Good understanding of the in vivo course of TCM will help us to know how to conduct metabolism evaluation of TCM by using in vitro human-derived system. This evaluation system will create new views on TCM as effective and safe therapeutic agents.
Keywords: Traditional Chinese medicine (TCM), Metabolism, Gastrointestinal biotransformation
INTRODUCTION
There are abundant resources of traditional Chinese medicines (TCMs), which have been clinically used for more than 5 000 years, in China and Asia. As TCM is attractive as a pool chemical compounds and natural products for medicinal use, increasing attention is being paid to the scientific evaluation of TCM. However, the study is full of various challenges: varied resource, unknown active ingredients, difficulties in quality control, lack of safety evaluation, or unclear biological mechanism, etc. Due to historical or cultural reasons, most of TCMs have been used as health food or drug without strict experimental evidences or scientific instruction (de Smet, 2002). The US Food and Drug Administration (FDA) has regulations on botanic medicines examination and approval for use in the United States. Although study is dramatically being developed at the source of TCM, the lack of quality standards together with scarcity of mechanism and safety research has seriously limited further development of TCM in China.
Different from single component of western drug, TCM is a complex system consisting of multiple compounds, which makes scientific evaluation of TCM more difficult. Traditional pharmacokinetics methods cannot lead to discovery of the pharmacokinetics properties of TCM, because of the lack of knowledge on their active components. So minimal effective dose and minimal toxic dose of a certain TCM are completely derived from clinical experiences or ancient books, which directly resulted in a number of adverse reactions associated with the use of TCM (Ko, 2004; Siow et al., 2005). In addition, traditional pharmacokinetics methods using animal models in drug discovery and development may not be suitable for TCM, although TCM has been clinically used for thousands of years. Species difference cannot be excluded by these methods. Therefore, a human-derived evaluation system is urgently needed in the development of TCM, by which quantitive and accurate evaluation of pharmacokinetics properties of TCM can be achieved.
In the last decades, major advances in chemistry, molecular biology, and high-throughput technology have provided rapid and efficient identification of leads in western drug discovery (Kennedy, 1997), but the high attrition rate of candidates (about 90%) in clinical studies has made the pharmaceutical industry realize that they urgently need to increase productivity and development efficiency, and also implement strategies to avoid predictable failure (Eddershaw et al., 2000; Prentis et al., 1988). Since the 1990’s, computer techniques and in vitro techniques using human-derived system, which can characterize chemicals’ absorption, distribution, metabolism, and elimination (ADME) properties in the early stage of drug discovery, have dramatically reduced the attrition rate of drug candidates (Kola and Landis, 2004). Therefore, following successful experiences in western and modern drug discovery and development, introducing ADME in vitro techniques using human-derived system into TCM scientific evaluation will enormously accelerate this process. In addition, many TCMs can undergo biotransformation in the gastrointestinal tract. Integration of biotransformation in the gastrointestinal tract into ADME evaluation of TCM will favorably enable discovery of the active components and active mechanisms, and can predict the role of their metabolites in the early stage of TCM evaluation. In the present article, we review reports on how to conduct early metabolism evaluation of TCM by using in vitro ADME-based human-derived systems.
IN VIVO COURSE OF TCM
Different from the single component of western drugs, TCM is a complex system consisting of multiple compounds. Degradation of TCM in the gastrointestinal tract is often observed (Kobashi et al., 1992; Kobashi and Akao, 1997; Hasegawa, 2004; Dreessen et al., 1981; Shu et al., 1987). Therefore, good understanding of TCM metabolism is based on a good understanding of the in vivo course of TCM.
As TCMs are orally administered in most cases, the gastrointestinal tract serves principally as absorption site for absorption and first biotransformation site. TCM biotransformation in the human gastrointestinal tract includes degradation by acid or intestinal bacteria in gastrointestinal tract, and the biotransformation by metabolic enzymes in the intestinal epithelium. Both Phase I and Phase II metabolic enzymes are expressed in the human gastrointestinal tract, together with associated transporters (Kaminsky and Zhang, 2003). The transporters, despite their importance for the fate of enterocyte-absorbed xenobiotics, are beyond the scope of this mini review.
The naturally occurring components of TCM and/or their degradation products in the gastrointestinal tract might be metabolized by metabolic enzymes in the intestinal epithelium, and/or influence their activities. After the TCM components are absorbed into the bloodstream through the intestinal epithelium, they are first delivered to the liver via the portal vein. A substrate of metabolic enzymes can be effectively cleared by intestinal or hepatic metabolism before it reaches the systemic circulation, a process known as first-pass metabolism. In liver, most absorbed components lose their activities, although their metabolism can also sometimes lead to the generation of active metabolites, which can be solely or partially responsible for the pharmacological response. In some instances, metabolism can also produce reactive or toxic intermediates or metabolites, with potential toxicological implications. The components reaching the liver and their metabolites might also possibly influence the activities of metabolic enzymes.
So good understanding of TCM metabolism needs good understanding of the following information (Fig.1):
Fig. 1.
The in vitro characterization of TCM metabolism
Quantitative content of main components;
Degradation course in human gastrointestinal tract (intestinal metabolite profile);
Role of metabolic enzyme in intestinal epithelium responsible for their biotransformation, as well as metabolic stability and metabolite profile;
Influence naturally occurring TCM components and/or their degradation products on intestinal metabolic enzymes;
Identification of components that can be absorbed from the gastrointestinal tract;
Biotransformation in the liver of the components that can be absorbed into the bloodstream;
TCM components’ influence on hepatic metabolic enzymes that can be absorbed into the bloodstream;
Biotransformation potential in gastrointestinal tract (intestinal bacteria, acid, or intestinal metabolic enzymes) and liver (hepatic metabolic enzymes).
IN VITRO CHARACTERIZATION OF TCM METABOLISM
Intestinal biotransformation of TCM
The gastrointestinal tract is the first site of biotransformation for orally administered xenobiotics or TCM; although the role of gastrointestinal biotransformation is still not being paid its deserved attention to current studies. Gastrointestinal microorganisms comprise an important component of the diverse and dynamic intestinal ecosystem. There are approximately 1012 parenchymal cells in an average human (excluding blood cells and neurons), but about 1012 bacteria on the skin, another 1010 in the mouth, and the gut contains 1014 microorganisms (weighing>1 kg) (Mackie et al., 1999; Hooper et al., 2001). The main human intestinal bacteria are predominantly obligate anaerobes and include species of the genera Bacteroides, Clostridium, Lactobacillus, Escherichia and Bifidobacterium, together with various yeasts and microorganisms coexisting in dynamic ecological equilibrium. The estimated more than 1 000 species of gut microorganisms presents an important challenge to understanding the microbiotal contribution to drug/xenobiotic metabolism and the development of human disease (Nicholson et al., 2005).
TCM degradation in gastrointestinal tract is often observed. Kobashi et al. proposed the concept of plant glycoside acting as a “Bioprodrug” metabolized to the active form by intestinal bacterial deglycosylation (Kobashi et al., 1992; Kobashi and Akao, 1997).
The main metabolic pathways of naturally occurring ginsenosides in gastrointestinal tract are supposed to be as follows: protopanaxadiol-type, Rb1→Rd→F2/M9→M13→C-K (M1), Rb2→M6→M2→C-K, Rc→M7→M3→C-K, (C-K is gradually hydrolyzed to M12 (Ppd)); protopanaxatriol-type, Re→Rg1→F1/Rh1→Ppt (M4), Rg1→F1/Rh1→Ppt (Hasegawa, 2004). There are also instances of TCM biotransformation by intestinal bacteria, such as sennosides (Dreessen et al., 1981), paeoniflorin (Shu et al., 1987), baicalin, glycyrrhizin, geniposide (Yim et al., 2004), and by flavenoids (Kim et al., 1998).
Clarifying the active ingredients of TCM
Knowledge of the in vivo course of TCM is essential for clarifying the active mechanism. According to classic theory of drug action, the concentration of a drug available to bind a target receptor is that which is free in solution within the target organ. So the active ingredients of TCM should be those that can enter the systemic circulation, and include the naturally occurring components and their gastrointestinal metabolites produced by intestinal bacteria or other gastrointestinal factors. The same theory should hold true for the effect of TCM components on metabolic enzymes (Margolis and Obach, 2003). In addition, TCM components which cannot be absorbed from the gastrointestinal tract, could also possibly act solely or cooperatively on some targets in the gastrointestinal tract, such as enzymes or transporters. Therefore, clarifying the active ingredients of TCM is the primary issue in TCM metabolism research.
Although sennosides have no jalap activity, their metabolites, particularly rheinanthron in the intestinal microflora exhibit potent function of promoting fecal excretion (Dreessen et al., 1981). Yang et al.(1996) purified and characterized the sennoside-hydrolyzing β-glucosidase from Bifidobacterium sp. strain SEN, a human intestinal anaerobe. Oral absorption of paeoniflorin is poor, but paeonimetaboline I formed by human intestinal bacteria exhibited potent anticonvulsant activity (Shu et al., 1987; Abdel-Hafez et al., 1998). Most naturally occurring ginsenosides including Rb1, Rb2, and Rg1 are poorly absorbed, although their intestinal metabolites including Compound K (C-K), protopanaxadiol (Ppd), and protopanaxadiol (Ppt), are easily absorbed and appear in the plasma of rat or human after oral administration of Rb1, Re, or Rg1 (Hasegawa, 2004).
Solubility and permeability assays of components, together with ex vivo culture of intestinal bacteria, purification or in vitro recombination of their metabolic enzymes have become a powerful tool for identifying active ingredients of TCM (Holzapfel et al., 1998).
Metabolism of TCM in intestine and liver
Metabolism of drugs represents a key process by which drugs are cleared from the body. They are mainly eliminated by cytochrome P450 (CYPs or P450) enzymes (Phase I metabolism) and, after that, by conjugating enzymes (Phase II metabolism), such as UDP-glucuronosyl transferases and N-acetyl transferases (Delaforge, 1998; Wrighton and Stevens, 1992; Kaminsky and Zhang, 2003). These enzymes add functional groups to make lipophilic molecules more hydrophilic and hence easier to eliminate.
Among hepatic enzymes, the superfamily of CYPs plays a key role in the metabolism of a wide variety of xenobiotics, such as drugs, pesticides and (pre)carcinogens (Wrighton and Stevens, 1992; Gonzalez, 1989). In liver, the most important CYPs from the viewpoint of drug metabolism are CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4, which represent about 70% of the total CYPs enzymes and are responsible for the oxidation of more than 90% clinical drugs (Shimada et al., 1994; Lasker et al., 1998). In intestine, CYP3A4 is the predominant CYP, while only a very limited number of other CYPs including CYP2C9, CYP2C19, and CYP1A1 are expressed (Zhang et al., 1999; Obach et al., 2001). Intestinal CYPs also have been shown to contribute significantly to the metabolism of several drugs, including nifedipine and midazolam (Holtbecker et al., 1996; Paine et al., 1996).
Using in vitro experimental system, such as cDNA-expressed metabolic enzymes, microsomes, tissue slice, hepatocyte, or S9, etc., the methods for characterizing the metabolic stability, metabolite profile, metabolic enzyme profile, inhibition or induction of metabolic enzymes have been well established (Gunaratna, 2000).
Metabolic stability
Metabolic stability is one of several major determinants in defining the oral bioavailability and systemic clearance of a drug. The metabolism of both host and intestinal bacteria play an important role in a number of TCMs.
The stability of active TCM ingredients in metabolism by liver and extrahepatic tissues as well as intestinal bacteria will ultimately determine the concentration of the compound found in the systemic circulation and affect its half-life and residence time within the body (Masimirembwa et al., 2001; Yan and Caldwell, 2001). By determining the time and concentration dependence of metabolite formation from an active ingredient in vitro and the kinetics properties in an appropriate system, such as intestinal bacteria, microsomes, hepatocytes, or cDNA-expressed metabolizing enzymes, the active ingredient’s metabolic fate and half-life in vivo can be predicted. Similar studies on human and test species give valuable information for selecting test species for pharmacokinetics in vivo studies.
Metabolite profile
Metabolite profile is important for the identification of TCM active ingredients. The components that can enter the systemic circulation after oral administration of TCM might be parent compound, metabolic enzymes-based, or gastrointestinal degradation-based metabolites. Today, there are effective methods for metabolite identification and subsequent construction of metabolic routes. Metabolite identification can be developed from incubations with human liver or intestine preparations (Rodrigues and Wong, 1997; Ferrini et al., 1997; Lin and Lu, 1998; Crespi and Miller, 1999). The rapid development of analytical methods, such as liquid chromatography-mass spectrometry (LC-MS), nuclear magnetic resonance (NMR), etc., makes it possible to determine with high accuracy the exact molecular masses and metabolite structures (Ansede and Thakker, 2004). Similar studies on human and test species yielded valuable information for the selection of test species for toxicological in vivo studies (Chauret et al., 1997). In addition, for prodrug needing the activation of metabolic enzymes or gastrointestinal degradation, the research on metabolite profile is also vital (Delaforge, 1998).
Metabolic enzyme profile
Metabolic enzyme profile includes both the enzymes of host and those excreted by intestinal bacteria, which are involved in the metabolism of TCM. The content and activity of CYPs exhibit a high degree of inter- and intra-individual variability (Shimada et al., 1994; Iyer and Sinz, 1999). The genetic polymorphism of CYPs is extensive, and the rate of metabolism for a certain drug can even differ 1000-fold between phenotypes (Ingelman-Sundberg, 2004). In addition, human intestinal bacteria exhibit a high degree of intra-individual variability, which is very changeable depending on host conditions, including diet, health, and even stress. Bacterial ginsenoside-hydrolyzing potentials also exhibit a high degree of inter-individual variability in humans and experimental mice (Hasegawa, 2004). There might be tremendous difference in pharmaceutical activities and adverse reactions among patients administered the same dose of TCM (Schaeffeler et al., 2003). Therefore, the identification of the metabolizing enzyme profile will be of help to clarify the active mechanism and determine the direction of individual medicine.
Inhibition and induction of metabolic enzyme
It is likely that some of these components share metabolic pathways catalyzed by the same enzymes. In addition, it is most likely that coadministered prescription drugs can be affected by the components of TCM, leading to alterations in their pharmacokinetic profile. This would lead to unwarranted adverse interactions and/or therapeutic failure (Kedderis, 1997). The metabolic enzymes involved include both the enzymes of host and those excreted by intestinal bacteria, which are involved in the metabolism of TCM.
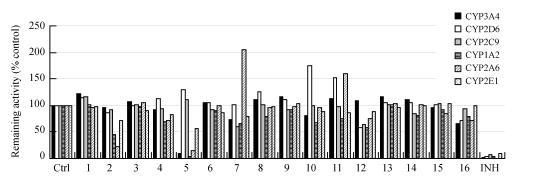
There are a number of reported herb-prescription drug interactions via the influence of metabolizing enzymes, such as St. John’s Wort, ginseng, ginkgo, garlic, etc. (Barone et al., 2000; Piscitelli et al., 2000; Rosenblatt and Mindel, 1997; Sunter, 1991; Yue et al., 2000). Up to now, most studies on the influence of TCM on metabolic enzymes focused on their naturally occurring components. However, Fig.2 (in the preceding page) shows that the degradation products of TCM by gastrointestinal factors may exhibit distinct effects on metabolic enzymes compared to naturally occurring components. Liu et al.(2004) reported that naturally occurring ginsenosides including Rb1, Rb2, Rc, Re, Rg1 and one of the intestinal metabolites of ginsenosides, C-K, had no inhibitory effect, whereas another intestinal metabolite, Ppt, exhibited potent inhibition against rat CYP3A activity.
Fig. 2.
The contrast of inhibition on main cytochrome P450 isoforms of TCM extracts (a) and their degradation products by snailase (b) in human liver microsomes
1: Rizome of Chuanxiong Ligusticum; 2: Dan-shen; 3: Safflower; 4: Radix Paeoniae Rubra; 5: Dalbergia wood; 6: Siberian Thorowax; 7: Fruit of Wilson Citron; 8: Sanchi; 9: Rhizome of grassleaf sweetflag; 10: Immature fruit of Trifoliate-orange; 11: Cassia twig; 12: Spine of Chinese Honeylocust; 13: Seed of Spine Date; 14: Xuefu; 15: Guanxin; 16: Corydalis glaucescens Regel; Ctrl: Negative Control; INH: Positive Control; # P<0.05 versus parent TCM extracts
The biotransformation of naturally occurring TCM components in the gastrointestinal tract may play an important role in TCM-associated drug interactions via CYPs inhibition.
CONCLUSION
In summary, host metabolic enzymes and intestinal bacteria may be responsible for TCM metabolism. The characterization of TCM metabolism needs a good understanding of the in vivo course of TCM, which will enormously accelerate the process of scientific evaluation of TCM as effective and safe therapeutics.
Footnotes
Project supported by the National Basic Research Program (973) of China (Nos. 2003CCA03400 and 2003CB716005), and the Leading Program of the Chinese Academy of Sciences (No. KGCXZ-SW-213-04), China
References
- 1.Abdel-Hafez AA, Meselhy MR, Nakamura N, Hattori M, Watanabe H, Mohamed TA, Mahfouz NM, el-Gendy MA. Potent anticonvulsant paeonimetabolin-I derivatives obtained by incubation of paeoniflorin and thiol compounds with Lactobacillus brevis . Chem Pharm Bull. 1998;46:1486–1487. doi: 10.1248/cpb.46.1486. [DOI] [PubMed] [Google Scholar]
- 2.Ansede JH, Thakker DR. High-throughput screening for stability and inhibitory activity of compounds toward cytochrome P450-mediated metabolism. J Pharm Sci. 2004;93(2):239–255. doi: 10.1002/jps.10545. [DOI] [PubMed] [Google Scholar]
- 3.Barone GW, Gurley BJ, Ketel BL, Lightfoot ML, Abul-Ezz SR. Drug interaction between St. John’s Wort and cyclosporin. Ann Pharmacother. 2000;34(9):1013–1016. doi: 10.1345/aph.10088. [DOI] [PubMed] [Google Scholar]
- 4.Chauret N, Gauthier A, Martin J, Nicoll-Griffith DA. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat, and horse. Drug Metab Dispos. 1997;25:1130–1136. [PubMed] [Google Scholar]
- 5.Crespi CL, Miller VP. The use of heterologously expressed drug metabolizing enzymes–state of the art and prospects for the future. Pharmacol Ther. 1999;84(2):121–131. doi: 10.1016/S0163-7258(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 6.de Smet PA. Herbal remedies. New Engl J Med. 2002;347(25):2046–2056. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 7.Delaforge M. Importance of metabolism in pharmacological studies: possible in vitro predictability. Nucl Med Biol. 1998;25(8):705–709. doi: 10.1016/S0969-8051(98)00063-8. [DOI] [PubMed] [Google Scholar]
- 8.Dreessen M, Eyssen H, Lemli J. The metabolism of sennosides A and B by the intestinal microflora: in vitro and vivo studies on the rat and the mouse. J Pharm Pharmacol. 1981;33:679–681. doi: 10.1111/j.2042-7158.1981.tb13903.x. [DOI] [PubMed] [Google Scholar]
- 9.Eddershaw PJ, Beresford AP, Bayliss MK. ADME/PK as part of a rational approach to drug discovery. Drug Discov Today. 2000;5(9):409–414. doi: 10.1016/S1359-6446(00)01540-3. [DOI] [PubMed] [Google Scholar]
- 10.Ferrini JB, Pichard L, Domergue J, Maurel P. Long-term primary cultures of adult human hepatocytes. Chemico-Biological Interactions. 1997;107(1-2):31–45. doi: 10.1016/S0009-2797(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez FP. The molecular biology of cytochrome P450s. Pharmacol Rev. 1989;40:243–288. [PubMed] [Google Scholar]
- 12.Gunaratna C. Drug metabolism and pharmacokinetics in drug discovery: a primer for bioanalytical chemists, part I. Curr Sep. 2000;19:17–23. [Google Scholar]
- 13.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95(2):153–157. doi: 10.1254/jphs.FMJ04001X4. [DOI] [PubMed] [Google Scholar]
- 14.Holtbecker N, Fromm MF, Kroemer HK, Ohnhms EF, Heidermann H. The nifedipine-rifampin interaction: evidence for induction of gut wall metabolism. Drug Metab Dispos. 1996;24:1121–1123. [PubMed] [Google Scholar]
- 15.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in’t Veld JH. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41(2):85–101. doi: 10.1016/S0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 16.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine (cites personal communication from Joshua Lederberg) Science. 2001;291(5505):881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 17.Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 2004;25(4):193–200. doi: 10.1016/j.tips.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Iyer KR, Sinz MW. Characterization of Phase I and Phase II hepatic drug metabolism activities in a panel of human liver preparations. Chemico-Biological Interactions. 1999;118(2):151–169. doi: 10.1016/S0009-2797(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 19.Kaminsky LS, Zhang QY. The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos. 2003;31(12):1520–1525. doi: 10.1124/dmd.31.12.1520. [DOI] [PubMed] [Google Scholar]
- 20.Kedderis GL. Pharmacokinetics of drug interactions. Adv Pharmacol. 1997;43:189–203. doi: 10.1016/s1054-3589(08)60206-9. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy T. Managing the drug discovery/development interface. Drug Discov Today. 1997;2(10):436–444. doi: 10.1016/S1359-6446(97)01099-4. [DOI] [Google Scholar]
- 22.Kim DH, Jung EA, Sohng IS, Han JA, Kim TH, Han MJ. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res. 1998;21:17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 23.Ko RJ. A U.S. perspective on the adverse reactions from traditional Chinese medicines. J Chin Med Assoc. 2004;67:109–116. [PubMed] [Google Scholar]
- 24.Kobashi K, Akao T. Relation of intestinal bacteria to pharmacological effects of glycosides. Bifidobacteria Microflora. 1997;16:1–7. [Google Scholar]
- 25.Kobashi K, Akao T, Hattori M, Namba T. Metabolism of drugs by intestinal bacteria. Bifidobacteria Microflora. 1992;11:9–23. [Google Scholar]
- 26.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 27.Lasker JM, Wester MR, Aramsombatdee E, Raucy JL. Characterization of CYP2C19 and CYP2C9 from human liver: respective roles in microsomal tolbutamide, S-mephenytoin, and omeprazole hydroxylations. Arch Biochem Biophys. 1998;353(1):16–28. doi: 10.1006/abbi.1998.0615. [DOI] [PubMed] [Google Scholar]
- 28.Lin JH, Lu AYH. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;5:361–390. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Li W, Li P, Deng MC, Yang SL, Yang L. The inhibitory effect of intestinal bacterial metabolite of ginsenosides on CYP3A activity. Biol Pharm Bull. 2004;27(10):1555–1560. doi: 10.1248/bpb.27.1555. [DOI] [PubMed] [Google Scholar]
- 30.Mackie R, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 31.Margolis JM, Obach RS. Impact of nonspecific binding to microsomes and phospholipid on the inhibition of cytochrome P4502D6: implications for relating in vitro inhibition data to in vivo drug interactions. Drug Metab Dispos. 2003;31(5):606–611. doi: 10.1124/dmd.31.5.606. [DOI] [PubMed] [Google Scholar]
- 32.Masimirembwa CM, Thompson R, Andersson TB. In vitro high throughput screening of compounds for favorable metabolic properties in drug discovery. Comb Chem High Throughput Screen. 2001;4:245–263. doi: 10.2174/1386207013331101. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3(5):431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 34.Obach RS, Zhang QY, Dunbar D, Kaminsky LS. Metabolic characterization of the major human small intestinal cytochrome P450s. Drug Metab Dispos. 2001;29:347–352. [PubMed] [Google Scholar]
- 35.Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, Barr DM, Gillies BS, Thummel KE. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60(1):14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 36.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St. John’s Wort. The Lancet. 2000;355(9203):547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 37.Prentis RA, Lis Y, Walker SR. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964~1985) Br J Clin Pharmacol. 1988;25:387–396. doi: 10.1111/j.1365-2125.1988.tb03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues AD, Wong SL. Application of human liver microsomes in metabolism-based drug-drug interactions. Adv Pharmacol. 1997;43:65–101. doi: 10.1016/s1054-3589(08)60202-1. [DOI] [PubMed] [Google Scholar]
- 39.Rosenblatt M, Mindel J. Spontaneous hyphema associated with ingestion of Ginkgo biloba extract. New Engl J Med. 1997;336(15):1108. doi: 10.1056/NEJM199704103361518. [DOI] [PubMed] [Google Scholar]
- 40.Schaeffeler E, Schwab M, Eichelbaum M, Zanger UM. CYP2D6 genotyping strategy based on gene copy number determination by TaqMan real-time PCR. Human Mutation. 2003;22(6):476–485. doi: 10.1002/humu.10280. [DOI] [PubMed] [Google Scholar]
- 41.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 42.Shu YZ, Hattori M, Akao T, Kobashi K, Kagei K, Fukuyama K, Tsukihara T, Namba T. Metabolism of paeoniflorin and related compounds by human intestinal bacteria. II. Structures of 7S-and 7R-paeonimetabolines I and II formed by Bacteroides fragilis and Lactobacillus brevis . Chem Pharm Bull. 1987;35:3726–3733. doi: 10.1248/cpb.35.3726. [DOI] [PubMed] [Google Scholar]
- 43.Siow YL, Gong Y, Au-Yeung KK, Woo CW, Choy PC, O K. Emerging issues in traditional Chinese medicine. Can J Physiol Pharmacol. 2005;83(4):321–334. doi: 10.1139/y05-029. [DOI] [PubMed] [Google Scholar]
- 44.Sunter WH. Warfarin and garlic. Pharm J. 1991;246:722. [Google Scholar]
- 45.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 46.Yan Z, Caldwell GW. Metabolism profiling, and cytochrome P450 inhibition and induction in drug discovery. Curr Top Med Chem. 2001;1(5):403–425. doi: 10.2174/1568026013395001. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Akao T, Kobashi K, Hattori M. Purification and characterization of a novel sennoside-hydro lyzing beta-glucosidase from Bifidobacterium sp. strain SEN, a human intestinal anaerobe. Biol Pharm Bull. 1996;19:705–709. doi: 10.1248/bpb.19.705. [DOI] [PubMed] [Google Scholar]
- 48.Yim JS, Kim YS, Moon SK, Cho KH, Bae HS, Kim JJ, Park EK, Kim DH. Metabolic activities of ginsenoside Rb1, baicalin, glycyrrhizin and geniposide to their bioactive compounds by human intestinal microflora. Biol Pharm Bull. 2004;27(10):1580–1583. doi: 10.1248/bpb.27.1580. [DOI] [PubMed] [Google Scholar]
- 49.Yue QY, Bergquist C, Gerden B. Safety of St. John’s Wort (Hypericum perforatum) The Lancet. 2000;355(9203):576–577. doi: 10.1016/S0140-6736(05)73227-X. [DOI] [PubMed] [Google Scholar]
- 50.Zhang QY, Dunbar D, Ostrowska A, Zeisloft S, Yang J, Kaminsky LS. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999;27:804–809. [PubMed] [Google Scholar]