Abstract
Objective: To investigate the changes in CREB (cAMP response element binding protein) in hippocampus, PFC (prefrontal cortex) and NAc (nucleus accumbens) during three phases of morphine induced CPP (conditioned place preference) in rats, and to elucidate the role of CREB during the progress of conditioned place preference. Methods: Morphine induced CPP acquisition, extinction and drug primed reinstatement model was established, and CREB expression in each brain area was measured by Western Blot methods. Results: Eight alternating injections of morphine (10 mg/kg) induced CPP, and 8 d saline extinction training that extinguished CPP. CPP was reinstated following a priming injection of morphine (2.5 mg/kg). During the phases of CPP acquisition and reinstatement, the level of CREB expression was significantly changed in different brain areas. Conclusion: It was proved that CPP model can be used as an effective tool to investigate the mechanisms underlying drug-induced reinstatement of drug seeking after extinction, and that morphine induced CPP and drug primed reinstatement may involve activation of the transcription factor CREB in several brain areas, suggesting that the CREB and its target gene regulation pathway may mediate the basic mechanism underlying opioid dependence and its drug seeking behavior.
Keywords: Conditioned place preference (CPP), Morphine, Video tracking, CREB (cAMP response element binding protein)
INTRODUCTION
Drug addiction has important psychological and social causes and consequences and has been recognized to be a neurological disease involving the development of complex behaviors such as drug tolerance, dependence and craving for the drugs that are characteristic of an addictive state. It is widely thought that changes in gene expression in the central nervous system (indexed by levels of mRNA) play a critical role in drug addiction (Rhodes and Crabbe, 2005). A primary mechanism by which drugs of abuse affect gene expression is through changing the concentration of transcription factors in the nuclei of cells (Nestler et al., 2001). Several evidences suggest that changes in the expression and function of gene transcription factors in the nucleus accumbens, such as the cAMP response element binding protein (CREB), play an important role in the mechanism of drug addiction (Li et al., 2003). On the other hand, addiction results from abnormal engagement of long-term associative memory. CREB is pivotal in the switch from short-term to long-term memory, and plays a central role in the formation of long-term memory. Drug addiction and learning memory share certain intracellular signaling cascades, depend on activation of the transcription factor CREB (Nestler, 2002). CREB is a site of convergence for study of drug addiction and memory.
CREB, a ubiquitously expressed transcription product, was regulated by several intracellular signaling pathways. CREB dimers bind to specific sequences of DNA (called CREs or cAMP-response elements) in the regulatory regions of target genes. The transcriptional activity of these dimers is stimulated upon phosphorylation of CREB at Ser133 by any of several protein kinases. CREB represents a site of convergence at which diverse signaling pathways, and the external stimuli that activate those pathways, produce plasticity at the level of altered gene expression.
However, little is known about the role of CREB during the process of opioid addiction which may be an important molecular basis of craving and relapse. Whether the action of morphine on CREB in hippocampus, prefrontal cortex (PFC), and the nucleus accumbens (NAc) during different phases of addiction being associated is also unknown.
Place conditioning is commonly used to measure the rewarding or incentive properties of drugs, especially psychological dependence. At the same time, conditioned place preference (CPP) model can be used as an effective tool to investigate the mechanisms underlying drug-induced reinstatement of drug seeking after extinction (Parker and Mcdonald, 2000), and can partly evaluate the associated learning ability. In the present study, we establish the morphine induced rat CPP acquisition, extinction and reinstatement model using the computer based video-tracking CPP system, and investigated the changes of CREB in hippocampus, PFC and NAc in different CPP phases, in order to elucidate the effect of morphine induced CREB during the process of drug addiction, using CREB as the entry point to determine the function of each involved brain area, and investigate the mechanism of morphine addiction memory.
MATERIALS AND METHODS
Animals
Thirty-two male SD rats weighing 180~220 g were supplied by Zhejiang Medical Science Research Institute. All the animals were allowed to be habituated to the colony room for 2 weeks upon arrival, and housed in plastic cages with food and water and maintained on a 12 h light-dark cycle (light on at 7:00 p.m.). Room temperature was maintained at (22±2) °C.
Chemical reagents
Morphine hydrochloric (Shenyang Pharmaceutical LTD, China); anti-CREB monoclonal antibody (Sigma, USA); anti-rabbit-IgG (Rockland, USA); nuclear and cytoplasmic extraction reagents (PIERCE, USA).
Apparatus
The computer based video-tracking CPP system mainly consists of three parts, including CPP training apparatus, a video camera and a computer system with analysis software. The CPP training apparatus was made according to Shippenberg et al.(1996). Briefly, it consisted of four identical polyvinyl chloride (PVC) shuttle boxes (60 cm×30 cm×30 cm), each divided into two chambers (30 cm×30 cm×30 cm) of equal size by a separator, one white with smooth floor and the other black with wire mesh floor. A video camera was placed over the boxes and linked with a computer system. The rats’ behavior was recorded by the video camera and analyzed by RatTrack software. The whole experiment was conducted under dim illumination (15~20 lx) and stable noise (30~40 dB).
Procedures
1. Conditioned place preference
The place conditioning procedure used in this experiment was similar to that of Mueller and Stewart (2000), and included acquisition of CPP (consisting of three phases: pre-exposure, conditioning phase, and CPP test), extinction training and reinstatement. Each test animal received a pre-exposure test for 3 d before the experiment, in which they were allowed access to the entire apparatus for 15 min. The amount of time spent in each chamber was monitored. On d 1, d 3, d 5, d 7 rats were given dose of 100 mg/kg (i.p.), and then placed into the assigned chamber for 50 min. On d 2, d 4, d 6, d 8, rats received saline (10 ml/kg (i.p.)) before being confined in the other chamber for 50 min. On d 9, the guillotine door separating the two chambers was opened again, and the rats were allowed free access to the entire apparatus for 15 min without any restriction. The amount of time spent in each chamber was recorded. After conditioning and the initial CPP test, from d 10 to d 17, saline was alternate paired four times with each of the chambers, once per day, over 8 d, then on d 18, a second CPP test was given. On the next day of the second CPP test (d 19), the test animals were given a priming injection of morphine (2.5 mg/kg), 10 min later, the rats were allowed free access to the entire apparatus for 15 min, the amount of time spent in each chamber was recorded.
2. Western Blot analysis
For Western Blot analysis, the rats were sacrificed immediately after recording. Their brains were dissected out and put onto a glass plate on ice, then, the brain was cut into slices according to Paxinis and Watson (1998). The hippocampus, PFC and NAc were then separated from each slice, and put into liquid nitrogen immediately. The nuclear proteins were extracted by nuclear and cytoplasmic extraction reagents from the brain tissues. The concentration of proteins of each sample was determined using Folin assay with bovine serum albumin as the standard. Samples with equal amounts of protein were then separated by 15% polyacrylamide gel electrophoresis. Protein bands were electrically transferred to nitrocellulose membranes. The blots were blocked for 2 h in wash buffer (20 mmol/L Tris, pH 7.4; 137 mmol/L NaCl; 0.1% Tween 20) with 5% nonfat milk at room temperature with gentle rocking, and then were incubated with monoclonal rabbit anti-CREB (1:1000) antibodies at 4 °C overnight, after being washed for five times with TBST (5 min×5), the blots were incubated with horseradish peroxidase conjugated anti-rabbit secondary antibody (1:3000) for 2 h at room temperature. The washings were repeated, and the blots were developed by enhanced chemiluminescence method and visualized by exposure to GS-800 calibrated densitometer (Bio-Rad).
3. Statistical analysis
The amount of time spent in drug-paired chamber was analyzed by RatTrack software, and expressed as mean±SD, and analyzed with SPSS 11.0 statistical package. Statistical significance was determined by independent sample t-test, with statistical differences at P<0.05 considered significant. The densitometry of the bands was calculated by Image-Proplus analysis software, and data were expressed as mean±SD, and analyzed with paired sample t-test, with statistical differences at P<0.05 being considered significant.
RESULTS
Effect of morphine induced CPP
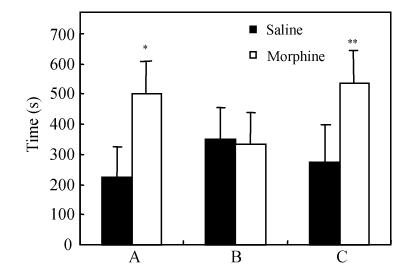
During the pre-exposure test of this experiment, the test rats showed strong preference for the black chamber, the mean time spent in the two chambers revealed significant differences (631.3±76.9, 268.7±76.9). Following morphine conditioning, there was a significant effect between the two test groups who spent time in the morphine-paired chamber (501.0±101.9, 224.4±106.9, P<0.01), shown in Fig.1. The data were analyzed and revealed significant chamber effect.
Fig. 1.
Time spent in the morphine-paired chamber in the 15 min test for CPP (mean±SD)
A: Effect of dose on the expression of a morphine-induced CPP, n=16; B: Extinction by saline pairings, n=12; C: Reinstatement by saline pairings, n=8. *Different from the saline-paired side, P<0.01; **Different from the saline-paired side, P<0.05
Effect of extinction training
After 8 d saline-paired extinction training, the mean time spent in the morphine-paired side as presented in Fig.1 revealed no differences between the two test groups (334.6±104.6, 350.5±102.9, P>0.05), rats did not present conditioned preference for the previously drug-paired side.
Reinstatement of CPP by priming dosage of morphine
After the test rats received a priming injection of morphine (2.5 mg/kg (i.p.)), the time spent in the morphine-paired chamber of two test groups revealed significant differences (535.5±123.8, 273.3±105.3, P<0.05). The time spent in morphine-paired chamber is shown in Fig.1, revealing the test rats showed significant preference for the morphine-paired chamber.
Changes in CREB protein levels in NAc, PFC and hippocampus during three phases of CPP
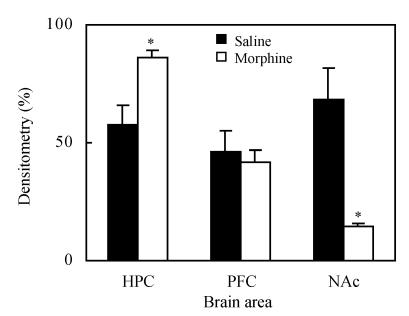
1. CREB levels on CPP acquisition day
After conditioning, the test rats showed significant preference for the white chamber, as compared to the saline group, and CREB protein expression was increased in the hippocampus (P<0.05). There were no significant changes in PFC (P>0.05), but CREB decreased in NAc (P<0.05), as shown in Fig.2 and Fig.3.
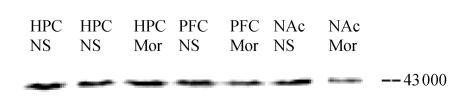
Fig. 2.
Detection of CREB expression in hippocampus (HPC), PFC, NAc of the test rats induced by saline (NS), morphine (Mor) on CPP acquisition day by Western Blot analysis (The first band is that of the hippocampus sample on CPP reinstatement day, used as the reference)
Fig. 3.
CREB levels in each brain area on CPP acquisition day. Values are expressed as mean±SD at least 3 experiments, n=3, compared with the density of the band of the hippocampus sample on CPP reinstatement day
* P<0.05, compared with saline group
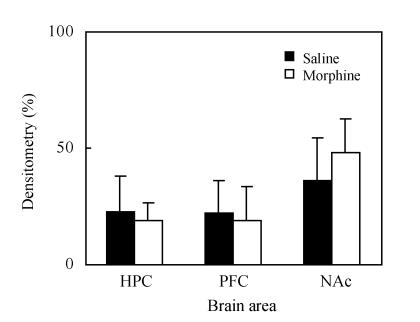
2. CREB levels on CPP extinction day
There were no significant changes of CREB levels in hippocampus. PFC and NAc are shown in Fig.4 and Fig.5.
Fig. 4.
Detection of CREB expression in hippocampus (HPV), PFC and NAc of test rats induced by saline (NS), morphine (Mor) on CPP extinction day by Western Blot analysis (The first band is that of the hippocampus sample on CPP reinstatement day, used as the reference)
Fig. 5.
CREB levels in each brain area on CPP extinction day. Values are expressed as mean±SD of at least 3 experiments, n=3, compared with the density of the hippocampus sample band on CPP reinstatement day
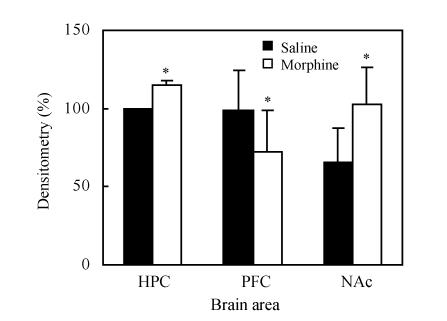
3. CREB levels on CPP reinstatement day
CREB levels in hippocampus and in NAc were increased (P<0.05), while in PFC, they were significantly decreased (P<0.05), shown in Fig.6 and Fig.7.
Fig. 6.
Detection of CREB expression in hippocampus (HPC), PFC and NAc of rats induced by saline (NS) or morphine (Mor) on CPP reinstatement day by Western Blot analysis
Fig. 7.
CREB levels in each brain area on CPP reinstatement day. Values are expressed as mean±SD of at least 3 experiments, n=3, compared with the density of the hippocampus sample band on CPP reinstatement day
* P<0.05, compared with saline group
DISCUSSION
Morphine induced CPP and drug primed reinstatement
In this study, a morphine-induced CPP was established at a dose of 10 mg/kg, and 8 d extinction with saline-conditioning injections extinguished CPP. CPP was reinstated following a priming injection of morphine (2.5 mg/kg). The results were similar to Mueller et al.(2002)’s report on cocaine research. In our research, repeated exposure to the CS (conditioned stimuli) environment without drug led to a decline in the behavioral significance of the drug-related stimuli, which demonstrated that CS plays an important role in the process of CPP. After a priming injection of low dose of morphine, the animals recovered again the drug-related stimuli, and showed significant preference for the drug-paired place. It supported Mueller and Stewart (2000)’s view that the incentive salience and attractiveness of these stimuli were renewed by the presence of the drug. Generally, the CPP model can be used as an effective tool to measure the rewarding or incentive properties of drugs, at the same time, it can be used to investigate the mechanisms underlying drug-induced reinstatement of drug seeking after extinction.
Molecular and cellular mechanisms of opiate dependence and relapse
The molecular mechanism underlying opiate dependence is not fully understood. Drugs of abuse cause long-lasting changes in the brain that underlie the behavioral abnormalities associated with drug addiction. The upregulation of the cAMP signaling pathway in the brain is the well established molecular adaptation associated with chronic opiate agonist (morphine) exposure. Modulation of transcription factors such as CREB is clearly one potential mechanism for persistent opiate-induced plasticity in the brain (García-Sevilla et al., 2004), which mediates many of the effects of the cAMP system on gene expression. Evidence for a role for CREB in drug addiction has been best established for opiate dependence in the locus coeruleus.
In recent years, the neurobiology of opiate addiction has focused on the molecular and cellular mechanisms associated with the regulation of synaptic structures (e.g. cytoskeletal proteins) and specific signaling systems (e.g. cAMP pathway) leading to neuronal plasticity in specific regions of the brain, including the neocortex.
Studies examining the effects of opiates on the cAMP signaling pathway have produced mixed results, but in general chronic morphine treatment has been associated with a compensatory upregulation of this system in the brain. Using a place preference reinstatement model, we detected the expression of transcription factor CREB in three phases of morphine CPP, in hippocampus, PFC and NAc. The major finding of the present study is the potential to use the CPP paradigm as a model for the extinction and the reinstatement of a place conditioned response induced by priming dosage of morphine, this result is consistent with the paradigm of drug-seeking behavior previously established in drug self-administration studies. We also found that during the phases of CPP acquisition and reinstatement, the level of CREB was significantly changed in different brain areas, through the way of regulating CREB levels, several brain areas play important roles in different CPP phases.
Regulation of CREB expression during morphine induced CPP acquisition, extinction and reinstatement in hippocampus, PFC and nucleus accumbens
The nucleus accumbens is an important brain region for reinforcement and motivational aspects of opioid dependence; NAc has also been shown to be an important structure for CPP learning. It suggested that levels of CREB activity in the nucleus accumbens shell can be regulated by environmental stimuli, and are a key regulator of behavioral responses to emotional stimuli (Barrot et al., 2002). Di Chiara (2002) examined a possible role for CREB in mediating the long-term effects of opiates in the NAc and found that the shell of the NAc seems to play a major role in brain reward mechanisms and motivational aspects of drug addiction. In the present experiment, on CPP acquisition day, CREB levels decreased significantly; the findings are consistent with the view of Widnell et al.(1996), who found chronic, but not acute, morphine administration decreases levels of CREB immunoreactivity in the NAc. It suggested that NAc was involved with CPP acquisition by mediating CREB levels.
The frontal cortex constitutes also a part of the reward system, but in some aspects it differs from the nucleus accumbens (Vetulani, 2001). Prelimbic cortex lesions had no effect on the acquisition of cocaine-CPP, also failed to alter rates of extinction of cocaine-CPP, but attenuated reinstatement of cocaine-CPP by cocaine priming injections (Zavala et al., 2003). Present data showed that there were no significant changes of CREB after CPP acquisition. It indicates that PFC plays a small role in CPP acquisition.
The hippocampus is a brain region known to participate in associative processes such as declarative memory. The hippocampus has direct excitatory affection to the nucleus accumbens and can activate dopaminergic neurons of the ventral tegmental area. As the NAc and the VTA are thought to be crucial for mediating the positive, rewarding aspects of ingesting drugs of abuse, so, the hippocampus is not typically considered an integral component of the “reward pathway”, but might be expected to play a significant role in the mechanism leading to the development of drug addiction (Thompson et al., 2002). In the present study, on CPP acquisition day, CREB level in hippocampus was increased significantly. It suggests that CPP acquisition is the same as the formation of associated learning process, and that the hippocampus plays an important role.
In self-administration studies, it is reported that the neuroadaptations induced by chronic drug use can be reversed or normalized by extinction training, and that extinction training can induce extensive synaptic reorganization (Self et al., 2004). In the present study, we also found that after extinction training, CREB expression in three brain areas all recovered to normal levels. It suggests that extinction training in CPP model also can profoundly reverse or ameliorate the changes of CREB after chronic drug use. In some instances their contribution may have potential benefits in the treatment of drug addiction.
Drug primed CPP reinstatement is a recall of the consolidated memory about drug reward effect. It was found that NAc plays a critical role in drug-induced CPP reactivation (Di Chiara, 2002). Present data showed that with CPP reinstatement, CREB levels in NAc increased obviously, the effect is opposite to the changes of CPP acquisition, which implied that the cAMP signaling pathway in NAc may underlie some of the long-term adaptation associated with the development of opioid dependence. But, further studies are needed in order to understand the cellular mechanisms underlying this regulation.
PFC belongs to the non-hippocampal memory systems, plays an essential role in recall of the consolidated contextual cue (Black et al., 2004). The PFC may serve as a possible common pathway for reinstatement of drug seeking by stressors, priming injections of drugs, and drug-related cues (Capriles et al., 2003; Sanchez et al., 2003). Imaging studies provided evidence for the involvement of the frontal cortex in the various aspects of drug addiction, which is likely to play an important role in the cognitive behavioral and emotional changes that perpetuate drug self-administration (Goldstein and Volkow, 2002). The present results showing that CREB levels decreased in PFC during CPP reinstatement, further refined understanding of PFC’s function in drug relapse, but the exact mechanism of how the cAMP-CREB system plays its role in this brain area is not known.
As to the role of hippocampus with drug addiction relapse, there are some different findings. Black et al.(2004) support the hypothesis that hippocampal formation may be more important for encoding and consolidating the contextual details of an episode, and less important for the long-term processing or recall of these contextual details, so they considered that drug-seeking behavior was likely regulated by nonhippocampal-dependent mechanisms. However, Vorel et al.(2001) reported that the hippocampus at theta frequency caused reinstatement of drug-taking behavior. In rats that had learned to press lever for cocaine and subsequently had this behavior extinguished by when saline was substituted with cocaine, and suggested that the hippocampal stimulation may cause a “read-out of an encoded association between the context of the cocaine experience” and the cocaine (Berke and Eichenbaum, 2001). Our studies also found that CREB levels in hippocampus increased significantly. It is well known that CREB in hippocampus is implicated in the formation of long-term memory, so the present CREB increase may be due to the aberrant memory of conditioned stimulus associated with drug administration, and plays an important role in the production of protein synthesis-dependent long-term changes in the brain.
In conclusion, we used the CPP procedure to establish the morphine induced CPP acquisition, extinction and reinstatement model, and measure CREB expression levels in hippocampus, PFC and NAc during different CPP phases. It was proved that CPP model can be used as an effective tool to investigate the mechanisms underlying drug-induced reinstatement of drug seeking after extinction. Morphine induced CPP and drug primed reinstatement may involve activation of CREB in several brain areas, leading to stable changes in the brain that are responsible for long-lasting behavioral abnormalities. The CREB and its target gene regulation pathway may mediate the basic mechanism underlying opioid dependence and its drug seeking behavior, although how these mechanisms translate into stable synaptic change and alterations in behavior remains unknown.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2003CB515402), and the Science and Technology Council of Zhejiang Province (No. 2005C23G2010166), China
References
- 1.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Neurobiology. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berke JD, Eichenbaum HB. Drug addiction and the hippocampus. Science. 2001;294(5545):1235a. doi: 10.1126/science.294.5545.1235a. [DOI] [PubMed] [Google Scholar]
- 3.Black Y, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Brain Research. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168(1-2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 5.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137(1-2):75–114. doi: 10.1016/S0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 6.García-Sevilla JA, Ferrer-Alcón M, Martín M, Kieffer BL, Maldonado R. Neurofilament proteins and cAMP pathway in brains of μ-, δ- or κ-opioid receptor gene knock-out mice: effects of chronic morphine administration. Neuropharmacology. 2004;46(4):519–530. doi: 10.1016/j.neuropharm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li YH, Yuan XR. Changes of phosphorylation of cAMP response element binding protein in rat nucleus accumbens after chronic ethanol intake: naloxone reversal. Acta Pharmacol Sin. 2003;24(9):930–936. [PubMed] [Google Scholar]
- 9.Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behavioural Brain Research. 2000;115(1):39–47. doi: 10.1016/S0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 10.Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behavioural Brain Research. 2002;136(2):389–397. doi: 10.1016/S0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiology of Learning and Memory. 2002;78(3):637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 12.Nestler EJ, Barrot M, Self DW. ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker LA, Mcdonald RV. Reinstatement of both a conditioned place preference and a conditioned place aversion with drug primes. Pharmacology Biochemistry and Behavior. 2000;66(3):559–561. doi: 10.1016/S0091-3057(00)00222-7. [DOI] [PubMed] [Google Scholar]
- 14.Paxinis G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. USA: Academic Press; 1998. pp. 1–130. [Google Scholar]
- 15.Rhodes JS, Crabbe JC. Gene expression induced by drugs of abuse. Current Opinion in Pharmacology. 2005;5(1):26–33. doi: 10.1016/j.coph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine D1-like receptor activation in the rat medial prefrontal cortex alters stress and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119(2):497–505. doi: 10.1016/S0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 17.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learning & Memory. 2004;11(5):648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shippenberg TS, Heidbreder C, Lefevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. European Journal of Pharmacology. 1996;299(1-3):33–39. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- 19.Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology. 2002;42(8):1039–1042. doi: 10.1016/S0028-3908(02)00059-X. [DOI] [PubMed] [Google Scholar]
- 20.Vetulani J. Drug addiction. Part II. Neurobiology of addiction. J VETULANI Pol J Pharmacol. 2001;53:303–317. [PubMed] [Google Scholar]
- 21.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292(5519):1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 22.Widnell KL, Self DW, Lane SB, Russell DS, Vaidya VA, Miserendino MJ, Rubin CS, Duman RS, Nestler EJ. Regulation of CREB expression: in vivo evidence for a functional role in morphine action in the nucleus accumbens. J Pharmacol Exp Ther. 1996;276(1):306–315. [PubMed] [Google Scholar]
- 23.Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Research. 2003;990(1-2):157–164. doi: 10.1016/S0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]