Abstract
Objective: To investigate a possible mechanism responsible for anti-apoptotic effects of melatonin and provide theoretical evidences for clinical therapy. Methods: Ischemia-reperfusion mediated neuronal cell injury model was constructed in cerebellar granule neurons (CGNs) by deprivation of glucose, serum and oxygen in media. After ischemia, melatonin was added to the test groups to reach differential concentration during reperfusion. DNA fragmentation, mitochondrial transmembrane potential, mitochondrial cytochrome c release and caspase-3 activity were observed after subjecting cerebellar granule neurons to oxygen-glucose deprivation (OGD). Results: The results showed that OGD induced typical cell apoptosis change, DNA ladder and apoptosis-related alterations in mitochondrial functions including depression of mitochondrial transmembrane potential (its maximal protection ratio was 73.26%) and release of cytochrome c (its maximal inhibition ratio was 42.52%) and the subsequent activation of caspase-3 (its maximal protection ratio was 59.32%) in cytoplasm. Melatonin reduced DNA damage and inhibited release of mitochondrial cytochrome c and activation of caspase-3. Melatonin can strongly prevent the OGD-induced loss of the mitochondria membrane potential. Conclusion: Our findings suggested that the direct inhibition of mitochondrial pathway might essentially contribute to its anti-apoptotic effects in neuronal ischemia-reperfusion.
Keywords: Cerebellar granule cell, Ischemia-reperfusion, Cytochrome c, Melatonin
INTRODUCTION
Oxidative stress plays a critical role in neurodegeneration disorders in the central nervous system (CNS), including Parkinson’s disease (PD), Alzheimer’s disease (AD) and so on (Aliev et al., 2004; Moreira et al., 2005; Gu et al., 2005). The neuronal injury induced by ischemia-reperfusion is the most familiar clinical type. Most cerebral ischemia induced by various factors can be transiently reversed, although reperfusion produces further neuron damage. During this process, the major pathogenetic mechanism of ischemia-reperfusion injury includes neuron apoptosis induced by excitotoxicity, disturbed calcium ion homeostasis, over production of nitric oxide and other free radicals.
Melatonin, the main secretory product of the pineal gland, is well known for its protective effects that are currently attributed mainly to its radical scavenging and antioxidant properties (Tan et al., 2000; Fischer et al., 2004; Jou et al., 2004). The endogenous compound that readily crosses the blood-brain barrier was accordingly found to reduce the infarct size and neuronal injury in experimental ischemia (Pei et al., 2002; 2003). Furthermore, melatonin reduces oxidative stress and rescues dopaminergic neurons in different models of Parkinson’s disease (Joo et al., 1998; Acunna-Castroviejo et al., 1997). Besides its direct and indirect antioxidant potential, several other mechanisms such as interactions with calmodulin have been found. This research aimed to establish the ischemia-reperfusion model to investigate the possible mechanism of direct inhibition mitochondrial function responsible for the anti-apoptotic effects of melatonin.
MATERIALS AND METHODS
Materials
Basal medium eagle (BME) and fetal bovine serum were purchased from GIBCO Company (USA). Melatonin, AC-DEVD-pNA and fluorescence dye Rhodamine 123 were purchased from Sigma Chemical Co. Anti-cytochrome c monoclonal antibody and horseradish peroxidase-conjugated rabbit anti-mouse polyclonal antibody were purchase from R & D Company (USA). ECL (enhanced chemiluminescence) kit was from Amersham Pharmacia Biotech (France).
Preparation of cultured CGNs
Rat CGNs (cerebellar granule neurons) were prepared from postnatal day 8 Sprague Dawlay rat pups (Tongji Medical College of Huazhong University of Science and Technology’s animal farms) as described by Lu et al.(2005).
Exposure of CGNs to oxygen-glucose deprivation
CGNs were maintained in standard medium (BME, 100 ml/L fetal bovine serum, 25 mmol/L KCl, 2 mmol/L glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin) for 7 d and then the medium was preserved and replaced with balanced salt solution (116 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4, 1 mmol/L NaH2PO4, 0.9 mmol/L CaCl2, and 10 mg/L phenol red) and incubated at 37 °C in humidified 95% N2, 5% CO2 for 90 min, followed by replacement of the preserved medium and incubation at 37 °C in humidified atmosphere with 5% CO2 for corresponding time for the experiments. The cells were divided into 5 groups: control group (without any treatment); OGD (oxygen-glucose deprivation) group; OGD+MT (melatonin) group (melatonin was added during reperfusion after OGD, with their final concentration being 10−5, 10−7and 10−9 mol/L respectively).
Detection of apoptosis
DNA fragmentation was detected by electrophoresis as described by Zhao et al.(2004).
Analysis of mitochondrial transmembrane potential
Fluorescent probe Rhodamine123 was used to analyze the mitochondrial transmembrane potential (ΔΨ m) by fluorescence spectrophotometry. After treatment, cells were washed with cold phosphate buffered saline (PBS), and then were incubated in 5 μmol/L Rhodamine123 for 30 min at 37 °C. The cultures were washed thrice and fluorescence intensity was measured at excitation wavelength 488 nm and emission wavelength 527 nm.
Release of cytochrome c assay by Western-Blot
Immunoblot analysis was performed on mitochondrial extracts from control and apoptotic cultures as described by Bobba et al.(1999). In both cases, cells were washed once with PBS and collected by centrifugation at 2000×g for 5 min at 4 °C. The cell pellet was resuspended in 500 μl of extraction buffer containing 250 mmol/L sucrose, 50 mmol/L Tris-HCl, 1 mmol/L EGTA (ethyleneglycol bis(2-aminoethyl ether) tetraacetic acid), 1 mmol/L EDTA (ethylenediamine tetraacetic acid), 1 mmol/L DTT (dithiothreitol), 1 mmol/L 1,10-phenantroline, 0.1 mmol/L PMSF (phenylmethylsulphone fluoride) pH 7.4. The cells were homogenized in a Teflon/glass homogenizer (10 strokes) and after 5 min on ice; the suspension was centrifuged at 15 000×g for 30 min. The supernatants (i.e. cytosolic fractions) were removed and stored at −80 °C until analysis by gel electrophoresis. The pellets were resuspended in 500 μl of 20 mmol/L Tris-HCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mol/L DTT, 1 mmol/L 1,10-phenantroline, 0.1 mmol/L PMSF pH 7.4 and homogenized on ice (10 strokes). After centrifugation at 750×g for 10 min at 4 °C to palletize the nuclei, 400 μl of the resulting supernatant was supplemented with 400 mmol/L NaCl and 1% Triton X-100. After 5 min incubation on ice, the samples were centrifuged at 15 000×g for 5 min at 4 °C to remove insoluble materials. Supernatants in the solubilized mitochondrial protein fraction were aliquoted and stored at −80 °C. The mitochondrial fractions were quantified by BCA (bicinchoninic acid) kit. Thirty micrograms of mitochondrial proteins was loaded onto a 12% (w/V) SDS (sodium dodecylsulphate) gel electrophoresis and eventually transferred to polyvinylidene difluoride (PVDF) membranes by conventional methods. The procedure for immuno-detection included blocking of the membrane incubation with the primary antibody (1:1000), washing membranes and incubation with peroxidase-conjugated secondary antibodies (1:5000). After washing, detection of bound antibodies was visualized by chemiluminescence using the ECL-plus reagent.
Caspase-3 activity assay
The proteolytic activity of caspases-3 was measured spectrophotometrically (λ max=405 nm) by the cleavage of Ac-DEVD-pNA, a substrate of caspase-3. Cells were scraped off in PBS, collected by centrifugation and lysed at 4 °C in hypotonic buffer (10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L DTT, 2.0 mmol/L MgCl2, 20 mmol/L HEPES (hydroxyethyl piperazine ethanesulfonic acid) pH 7.5, 0.1 mmol/L PMSF) for 30 min. Lysates were clarified by centrifugation at 13000×g for 10 min. The supernatant was quantified by BCA kit. Forty μg proteins were analyzed in 100 μl reaction mixture containing assay buffer (20 mmol/L HEPES pH 7.5, 10% (V/V) glycerol, 2 mmol/L DTT and 20 μmol/L Ac-DEVD-pNA). After 2 h incubation at 37 °C in the dark, enzymatic activity was measured by a Spectrophotometer.
Statistics
Data were expressed as xݱS and ANOVA (analysis of variance) was applied to assess statistical significance. Differences were considered significant when P values were less than 0.05.
RESULTS
OGD induces apoptosis in cultured CGNs
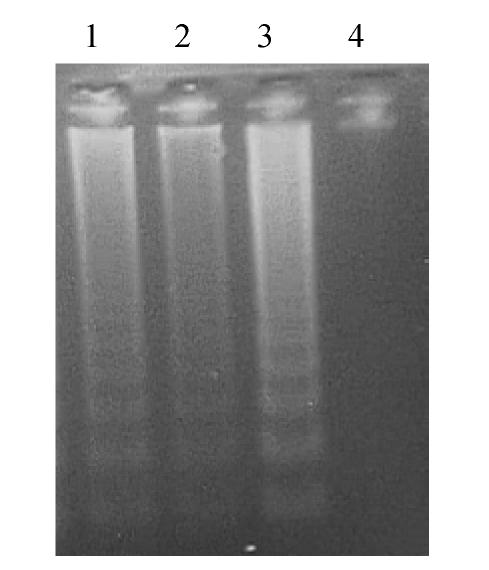
OGD and reperfusion for 24 h induced a typical apoptotic DNA ladder with 200-base pair range in OGD group; melatonin could protect cerebellar granule neurons from apoptosis (Fig.1).
Fig. 1.
DNA fragmentation of CGNs revealed by agarose gel electrophoresis
Lane 1: Precaution group; Lane 2: OGD+10−5 mol/L MT; Lane 3: OGD group; Lane 4: Control
Analysis of mitochondrial transmembrane potential by rhodamine 123
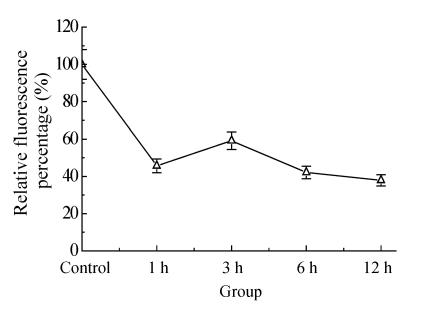
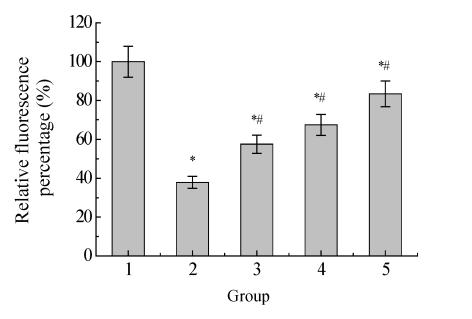
The mitochondrial transmembrane potential was first decreased and then increased transiently and decreased again at 3 h after OGD. Melatonin partly inhibited the decrease of mitochondrial transmembrane potential with the effect being dose-dependent (Fig.2 and Fig.3).
Fig. 2.
The time-change of mitochondrial transmembrane potential after ischemia-reperfusion as revealed by spectrophotofluorimetry assay
Fig. 3.
Effect of melatonin on mitochondrial transmembrane potential after 12 h ischemia-reperfusion
1: Control; 2: Ischemia-reperfusion; 3: OGD+10−9 mol/L MT; 4: OGD+10−7 mol/L MT; 5: OGD+10−5 mol/L MT; * P<0.05 vs control; # P<0.05 vs ischemia-reperfusion
Detection of cytochrome c release from mitochondria
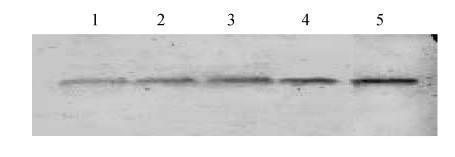
Cytochrome c released from mitochondria to cytosol after 6 h ischemia-reperfusion showing that melatonin could inhibit the release of mitochondrial cytochrome c (Fig.4).
Fig. 4.
Mitochondrial cytochrome c detected by Western-Blot after 6 h ischemia-reperfusion
Lane 1: Ischemia-reperfusion; Lane 2: OGD+10−9 mol/L MT; Lane 3: OGD+10−7 mol/L MT; Lane 4: OGD+10−5 mol/L MT; Lane 5: Control
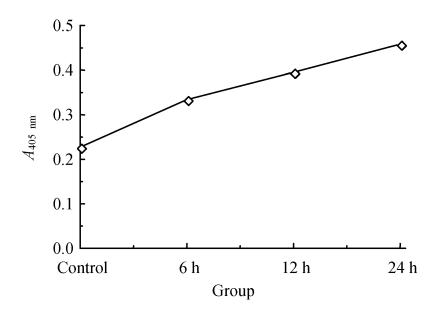
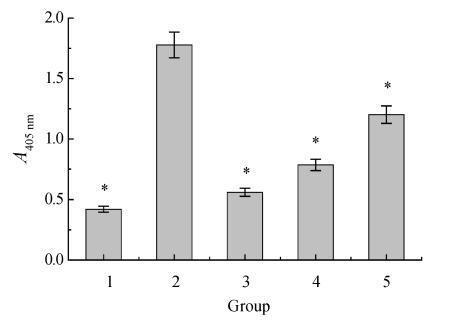
Analysis of the activity of caspase-3
The activity of caspase-3 was time-changed after ischemia-reperfusion that increased along with prolongation of reperfusion time. Melatonin could partly block the activation of caspase-3 (Fig.5 and Fig.6).
Fig. 5.
Caspase-3 was activated after CGNs suffered from OGD insults
Fig. 6.
Effects of melatonin on caspase-3 activity after 24 h ischemia-reperfusion
1: Control; 2: Ischemia-reperfusion; 3: OGD+10−5 mol/L MT; 4: OGD+10−7 mol/L MT; 5: OGD+10−9 mol/L MT; * P<0.05
DISCUSSION
Most cerebral ischemia could be promptly reversed in clinical; reperfusion produced further neuron damage even though neuron death is delayed. Cell apoptosis is commonly mediated by death receptor pathway or mitochondria pathway. Recently, evidences showed that mitochondria matrix swells, outer membrane ruptures and pro-apoptotic factor is released from the intermembrane space in the early stage of apoptosis (Polster and Fiskum, 2004; Bras et al., 2005).
During apoptosis, mitochondria suffer specific damages that result in loss of their function. ROS as a second pro-apoptotic messenger induces opening of permeability transition pore located on inner membrane, loss of mitochondrial membrane potential, release of pro-apoptotic factors including cytochrome c and AIF (Kowaltowski et al., 2001). Cytochrome c release from mitochondria was thought to occur in early events in apoptosis (Budd and Reed, 2000). It was controversial how cytochrome c was released from mitochondria to cytosol under apoptotic signal stimuli (Iijima et al., 2003).
Previous studies using various other mtPTP blocking agents showed that in pathological conditions such as ischemia an excessive loading of Ca2+ into the mitochondria induces apoptosis by stimulating the release of apoptosis-promoting factors like cytochrome c, AIF, Smac/DiaBLO, and pro-caspases from the mitochondrial intermembrane space into the cytoplasm via a permeability transition mechanisms (Wang, 2001). The release mechanism was believed to be accompanied by mitochondrial depolarization that follows the mitochondrial permeability transition. Some contradicting studies on isolated mitochondria suggested that the release of cytochrome c might occur also independently of the mtPTP, even before the opening of PTP (permeability transition pore) (Chiu and Oleinick, 2001). When mitochondrial transmembrane potential irreversibly decreased, cytochrome c was released from mitochondria to cytosol. Cytosolic cytochrome c binds to Apaf-1, a cytosolic protein containing a caspase-recruitment domain and caspase-9 to form a complex, which activates procaspase-3. Subsequently, the activated caspase-3 effect on target cell leads to cell apoptosis.
At the early stage of reperfusion, ΔΨ m decreased due to opening of PTP, however, transient opening of PTP did not cause cytochrome c release from mitochondria. Along with the prolongation of reperfusion time, persistent PTP opening leads to decrease of ΔΨ m and cytochrome c release from mitochondria. Our findings showed that ΔΨ m decreased after reperfusion but transiently increased at 3 h. The activity of caspase-3 began to rise after 6 h reperfusion and peaked at 24 h.
We followed the cascade of events extending downstream from the mtPTP-mediated cytochrome c release by examining how melatonin affects the caspase-3 activation and the subsequent DNA fragmentation. Our model showed inhibition of caspase-3 activation by melatonin which consequentially also prevented DNA fragmentation. The melatonin-induced anti-apoptotic effects presented here accorded with results of other studies showing that melatonin inhibits apoptosis in ischemic kidney (Kunduzova et al., 2003) and in amyloid β-peptide injury in hippocampal neurons (Shen et al., 2002) and NO-induced cell death in PGT-β (a cell line of pineal gland tumor) immortalized pineal cells (Yoo et al., 2002). It is interesting that melatonin is not protective in all models of apoptotic cell death (Harms et al., 2000), which may be explained by the fact that not all the investigated noxious stimuli trigger mtPTP-mediated apoptotic pathways.
The results of the present study therefore open a new field for investigating other regulatory principles in melatonin-controlled mechanisms. Taken together, our results show that melatonin has direct effect on mitochondria and that this effect may contribute to the anti-apoptotic properties of melatonin. The inhibition of mitochondria provides an evidence of an alternative mechanism used by melatonin to provide neuroprotection. As melatonin is an antioxidant and inhibitor of the mitochondria-mediate pathway, therapeutic intervention by melatonin may provide beneficial clinical applications for treating stroke neurodegenerative disorders. Since melatonin is safe and nontoxic, more experimental studies should be conducted to explore the synergetic actions of melatonin with other drugs that are presently applied clinically.
Footnotes
Project (No. WJ01510) supported by the Natural Science Foundation of Hygienic Committee of Hubei Province, China
References
- 1.Acunna-Castroviejo D, Coto-Montes A, Gaia-Monti M, Ortiz GG, Reiter RJ. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci. 1997;60:23–29. doi: 10.1016/s0024-3205(96)00606-6. [DOI] [PubMed] [Google Scholar]
- 2.Aliev G, Smith MA, de la Torre JC. Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer’s disease. Mitochondrion. 2004;4(5-6):649–663. doi: 10.1016/j.mito.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Bobba A, Atlante A, Giannattasio S, Sgaramella G, Calissano P, Marra E. Early release and subsequent caspase-mediated degradation of cytochrome c in apoptotic cerebellar granule cells. FEBS Letters. 1999;457(1):126–130. doi: 10.1016/S0014-5793(99)01018-2. [DOI] [PubMed] [Google Scholar]
- 4.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Moscow) 2005;70(2):231–239. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 5.Budd SL, Reed JC. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc Natl Acad Sci USA. 2000;97:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu SM, Oleinick NL. Dissociation of mitochondrial depolarization from cytochrome c release during apoptosis induced by photodynamic therapy. Br J Cancer. 2001;84(8):1099–1106. doi: 10.1054/bjoc.2000.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer TW, Scholz G, Knoll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J Pineal Res. 2004;37(2):107–112. doi: 10.1111/j.1600-079X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 8.Gu Z, Nakamura T, Yan D. Nitrosative and oxidative stress links dysfunctional ubiquitination to Parkinson’s disease. Cell Death and Differentiation. 2005;12(9):1202–1204. doi: 10.1038/sj.cdd.4401705. [DOI] [PubMed] [Google Scholar]
- 9.Harms C, Lauternschlager M, Bergk A. Melatonin is protective in necrotic but not in caspase-dependent, free radical-independent apoptotic neuronal cell death in primary neuronal cultures. FASEB J. 2000;14(12):1814–1824. doi: 10.1096/fj.99-0899com. [DOI] [PubMed] [Google Scholar]
- 10.Iijima T, Mishima T, Akagawa T. Mitochondrial hyperpolarization after transient oxygen-glucose deprivation and subsequent apoptosis in cultured rat hippocampal neurons. Brain Res. 2003;993(1-2):140–145. doi: 10.1016/j.brainres.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Joo WS, Jin BK, Park CW, Maeng SH, Kim YS. Melatonin increases striatal dopaminergic function in 6-OHDA-lesioned rats. Neuroreport. 1998;9:4123–4126. doi: 10.1097/00001756-199812210-00022. [DOI] [PubMed] [Google Scholar]
- 12.Jou MJ, Pang TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res. 2004;37(1):55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 13.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495(1-2):12–15. doi: 10.1016/S0014-5793(01)02316-X. [DOI] [PubMed] [Google Scholar]
- 14.Kunduzova OR, Escourrou G, Seguelas MH. Prevention of apoptotic and necrotic cell death, caspase-3 activation and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J. 2003;17:872–874. doi: 10.1096/fj.02-0504fje. [DOI] [PubMed] [Google Scholar]
- 15.Lu ZQ, Wang XM, Duan QH. Mechanism of melatonin protecting neurons from ischemia/reperfusion-induced injury. J Acta Med Univ Sci Technol Huazhong. 2005;34:149–152. (in Chinese) [Google Scholar]
- 16.Moreira PI, Smith MA, Zhu X. Oxidative stress and neurodegeneration. Ann N Y Acad Sci. 2005;1043(1):545–552. doi: 10.1196/annals.1333.062. [DOI] [PubMed] [Google Scholar]
- 17.Pei Z, Pang SF, Cheung RT. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J Pineal Res. 2002;32(3):168–172. doi: 10.1034/j.1600-079x.2002.1o847.x. [DOI] [PubMed] [Google Scholar]
- 18.Pei Z, Pang SF, Cheung RT. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34(3):770–775. doi: 10.1161/01.STR.0000057460.14810.3E. [DOI] [PubMed] [Google Scholar]
- 19.Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90(6):1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- 20.Shen YX, Xu SY, Wei W. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res. 2002;32(3):163–167. doi: 10.1034/j.1600-079x.2002.1o839.x. [DOI] [PubMed] [Google Scholar]
- 21.Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept. 2000;9(3-4):137–159. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- 22.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 23.Yoo YM, Yim SV, Kim SS. Melatonin suppresses NO-induced apoptosis via induction of Bcl-2 expression in PGT-beta immortalized pineal cell. J Pineal Res. 2002;33(3):146–150. doi: 10.1034/j.1600-079X.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao HG, Li WB, Chen XL. Limb ischemia preconditioning attenuates apoptosis of pyramidal neurons in the CA1 hippocampus induced by cerebral ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:407–412. [PubMed] [Google Scholar]