Abstract
We constructed a chimeric human T-cell lymphotropic virus type 1 (HTLV-1) provirus in which the original envelope precursor sequence was replaced by that of ecotropic Moloney murine leukemia virus (Mo-MuLV). Chimeric particles produced by transient transfection of this chimeric provirus were infectious for murine cells, such as NIH 3T3 fibroblasts, lymphoid EL4 cells, and primary CD4+ T lymphocytes, whereas HTLV-1 particles were not. The infectivity of chimeric particles increased 10 times when the R peptide located at the carboxy terminus of the MuLV envelope glycoprotein was deleted. Primary murine CD4+ T lymphocytes, infected by the ΔR chimeric virus, released particles that could spread the infection to other naive murine lymphoid cells. This chimeric virus, with the Mo-MuLV envelope glycoprotein and the replication characteristics of HTLV-1, should be useful in studying the pathogenesis of HTLV-1 in a mouse model.
Human T-cell lymphotropic virus type 1 (HTLV-1) is the agent of adult T-cell leukemia (ATL) (37) and HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP) (10). A range of chronic inflammatory conditions are also suspected to be linked to HTLV-1 infection. However, the vast majority of infected people remain asymptomatic. The occurrence of HTLV-1-associated diseases may depend on several factors including route of infection, genetic susceptibility, immune response to the virus, proviral load, viral reactivation by environmental factors, and accumulation of lesions with time. The pathogenesis of this infection is not well understood, and several attempts have been made to obtain an animal model. Rabbits have been infected, and in some cases, they developed an acute ATL-like disease (30-32). The infection of rats has been described previously (13, 34), but long-term viral persistence has not been clearly documented (11), and the myelopathy developed by WKHA rats (13) is controversial and strictly restricted to this strain (14, 21). Several species of monkeys are susceptible to HTLV-1 infection (15, 16, 23, 36), but no pathology has been observed in these animals.
A mouse model of HTLV-1 infection would be of great value for at least two reasons. Using inbred strains would make immunological and genetic studies possible. Large numbers of animals could be infected in order to observe the occasional occurrence of disease in a small proportion of them. Several attempts at infecting mice with HTLV-1 have already been published. Severe combined immunodeficiency (SCID) mice have been grafted with human ATL cells (9, 18) which proliferated in vivo (12). Newborn C3H/HeJ mice have been injected intraperitoneally with the HTLV-1-producing MT2 cell line. Proviral DNA integrated into the mouse genome was detected by PCR (8). However, no viral expression or antibody production could be detected. The tropism of HTLV-1 for murine cells is a controversial issue. The HTLV-1 receptor appears to be ubiquitously expressed. However, mouse cells seem to be less susceptible to fusion by the HTLV-1 envelope than human cells (5, 20, 22, 33).
In order to develop a new mouse model of HTLV-1 infection, we constructed a chimeric HTLV-1 virus with the envelope glycoprotein of Moloney murine leukemia virus (Mo-MuLV). We expected the chimeric virus to infect murine cells, since Mo-MuLV infects only mouse and rat cells because of its ecotropic envelope glycoprotein. In vivo, Mo-MuLV infects CD4+ T lymphocytes as well as other cell types. Because CD4+ T lymphocytes are the main target cells of HTLV-1 in humans, we hoped that such a chimeric virus, with the ecotropic envelope of Mo-MuLV and the replicative characteristics of HTLV-1 genome, could mimic in the mouse some of the aspects of HTLV-1 infection in humans.
Construction of chimeric proviruses.
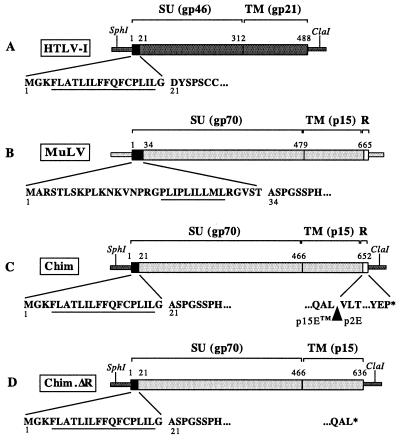
The chimeric HTLV-1 provirus was constructed by replacing the env gene of an infectious HTLV-1 cloned provirus (6) with the Mo-MuLV env gene. Figure 1 presents the structure of the parental and chimeric env genes used in this work. The pCS-HTLV-I plasmid (a gift of D. Derse, National Cancer Institute, Frederick, Md.), containing a full-length infectious HTLV-1, and the pFBMO-SALF plasmid (a gift from O. Schwartz, Institut Pasteur, Paris, France), containing a complete Mo-MuLV env sequence, were modified by site-directed mutagenesis. Using newly introduced EcoRI and BclI restriction sites, the sequence coding for the HTLV-1 Env signal peptide (positions 1 to 20) (Fig. 1A) was recombined with the Mo-MuLV sequence corresponding to gp70 and p15E (positions 35 to 675) (Fig. 1B) to give plasmid pCS-HTLV-chim (Fig. 1C). The HTLV-1 Env signal peptide was kept in the construct, because it contains the initiator codon for the tax gene and the splice donor site for the tax, rex, tof, and rof genes. As a result, the cell surface expression of Mo-MuLV Env is controlled by the HTLV-1 signal peptide. The resulting chimeric plasmid was mutagenized back in order to recover the initial sequence, and the junctions were sequenced.
FIG. 1.
Chimeric envelope constructs. (A and B) HTLV-1 and Mo-MuLV env precursor genes. The amino acid sequences of signal peptides are shown (hydrophobic regions are underlined). Numbering of amino acids begins at the first methionine. (C) Representation of the HTLV/Mo-MuLV chimeric (Chim) Env precursor. The HTLV-1 Env sequence (amino acids 21 to 488) was replaced by the corresponding MuLV Env sequence (amino acids 34 to 665). (D) Representation of the ΔR chimeric (Chim.ΔR) Env precursor in which the last 16 amino acids (R peptide) of the TM part of MuLV Env were deleted (amino acids 649 to 665). The stop codon (∗) of the Env sequence is indicated.
The fusogenic activity of MuLV virions has been linked to the cleavage of the C-terminal R peptide of Env by the viral protease (27, 28). Since it is unlikely that the HTLV-1 protease will perform this cleavage, we also constructed a chimeric plasmid in which the R peptide was deleted (pCS-HTLV-chim.ΔR [Fig. 1D]). The deletion was achieved by PCR-mediated mutagenesis. Two fragments flanking the R-peptide sequence were amplified using primers that contained new restriction sites and an overlapping sequence. They were annealed together to obtain a MuLV env fragment (1,557 nucleotides) with the 48 nucleotides of the R peptide deleted. After cloning and sequencing, this ΔR fragment was introduced into the pCS-HTLV-chim plasmid to give the pCS-HTLV-chim.ΔR plasmid.
Expression of the chimeric proviruses after transfection.
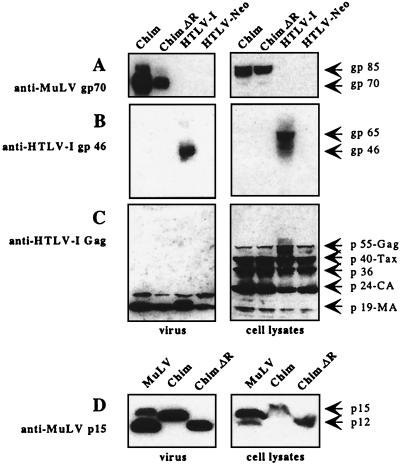
To examine the synthesis and processing of virion proteins by the chimeric proviruses, human 293T cells were transfected with chimeric and original proviral DNA together with a small amount of a plasmid expressing Tax (pCMV-envΔPvuII) (4, 6). Control transfections performed without this plasmid yielded no viral expression. The production of viral proteins was examined by Western blotting of cell lysates and of culture medium (Fig. 2). Large amounts of HTLV-1 Gag polyprotein p55, Gag-processing intermediate p36, and fully processed capsid p24 and matrix p19 proteins were detected in cell extracts (Fig. 2C). The p40 Tax protein was found in large amounts because of the presence of the pCMV-envΔPvuII plasmid. The capsid p24 and matrix p19 proteins were also detected in the culture medium. The lysates of cells transfected with the pCS-HTLV-chim and pCS-HTLV-chim.ΔR plasmids contained the gp85 Mo-MuLV Env glycoprotein precursor, but not the HTLV-1 Env glycoprotein (Fig. 2A). The culture medium of cells transfected with the parental HTLV-1 plasmid or the chimeric plasmid contained gp46 HTLV-1 Env protein or processed gp70 Mo-MuLV Env glycoprotein, respectively (Fig. 2A and B). Thus, the structural precursor proteins of the chimeric viruses were efficiently produced and correctly matured after transfection. Supernatants of 293T cells transfected with pCS-HTLV-chim contained mostly the unprocessed MuLV p15E protein, indicating that the HTLV-1 protease was unable to cleave the MuLV R peptide (Fig. 2D). In contrast, supernatants and lysates of 293T cells transfected with pCS-HTLV-chim.ΔR contained only the p12E protein. As expected, lysates of NIH 3T3 cells infected with Mo-MuLV contained more p15E than p12E, while the ratio was reversed in supernatants (28).
FIG. 2.
Western blots of viral proteins 24 h after transfection of 293T cells with chimeric (Chim, Chim.ΔR) and parental plasmids. Plasmids pCS-HTLV, pCS-HTLV-chim, pCS-HTLV-chim.ΔR, and pCS-HTLV-Neo (with a deletion in the env gene) were transfected in 293T cells (3 μg of plasmid/106 cells by the calcium phosphate procedure) together with 0.5 μg of pCMV-envΔPvuII. Cells were washed extensively 5 h later in order to eliminate excess plasmids and grown in a small volume of medium. Viral proteins were analyzed in virus pellets obtained by ultracentrifugation of medium (left panels) and in cell lysates (right panels). They were visualized on Western blots probed with different antibodies (serum and antibodies were previously adsorbed on 293T cells). Protein concentrations were determined by a Bradford assay, and the amount of protein loaded on a sodium dodecyl sulfate-polyacrylamide gel was adjusted as follows: 50 μg of protein for transfections with chimeric plasmids and 100 μg of protein for transfections with parental pCS-HTLV-I. (A) Mo-MuLV Env glycoprotein detected with an anti-MuLV gp70 polyclonal antibody (National Institutes of Health AIDS Research and Reference Reagent Program). (B) HTLV-1 Env glycoprotein detected with an anti-HTLV-1 gp46 monoclonal antibody (Cellular Products, Inc., Buffalo, N.Y.). (C) HTLV-1 Gag and Tax proteins detected with an anti-HTLV-1 serum sample from a HAM/TSP patient (a gift from A. Gessain, Institut Pasteur). (D) Analysis of the p15E/p12E cleavage of the Mo-MuLV Env glycoprotein. Proteins (50 μg) extracted from 293T cells after transfection with chimeric plasmids or from NIH 3T3 cells infected by Mo-MuLV were detected with an anti-MuLV p15E antibody (a gift from J. Cunningham, Harvard Medical School).
Transfected cells were examined by immunofluorescence microscopy for the presence of Mo-MuLV Env glycoprotein (not shown). The MuLV envelope glycoprotein was expressed at the surfaces of cells transfected with the chimeric plasmids, indicating that the HTLV-1 signal peptide is functional for the Mo-MuLV Env protein. Because the receptor for Mo-MuLV Env glycoprotein is not present on human cells, the chimeric viruses did not fuse to 293T cells, whereas the original HTLV-1 did.
Production of chimeric viral particles.
To assess the relative efficiency with which viral proteins were released after transfection with chimeric and HTLV-1 proviral DNA, clarified supernatants of 293T transfected cells were analyzed for the presence of p19 protein (detected with an HTLV-I Antigen ELISA kit [ZeptoMetrix, Buffalo, N.Y.]). Cells transfected with chimeric proviruses released reproducibly three to five times more p19 protein in the medium than cells transfected with the original HTLV-1 DNA. The same increase was observed in viral pellets after ultracentrifugation of culture medium. This result indicates that chimeric proviruses were more efficient at releasing particles in the medium.
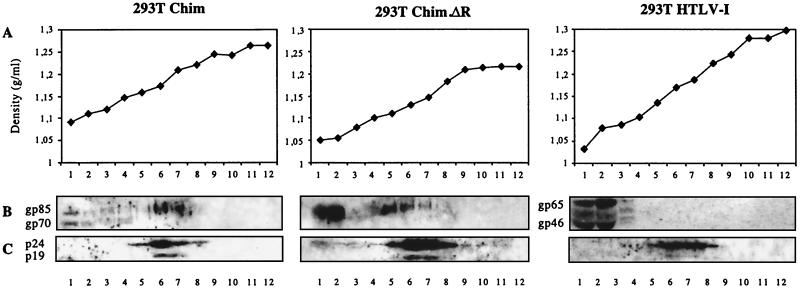
To determine whether chimeric particles were released, clarified supernatants of 293T cells transfected with the proviral plasmids were loaded on 15 to 60% sucrose gradients and the fractions of the gradients were analyzed by Western blotting for the presence of viral proteins (Fig. 3). The Gag proteins sedimented at a density of 1.16 g/ml, characteristic of retroviral particles. The incorporation of Env glycoproteins in the particles was also analyzed. With supernatants of cells transfected with chimeric plasmids, the Mo-MuLV Env protein sedimented both at the top of the gradient and together with the HTLV-1 Gag proteins, indicating that it was associated with the chimeric particles. In contrast, with supernatants of pCS-HTLV-I-transfected cells, the HTLV-1 Env protein was present only at the top of the gradient, indicating that it was loosely bound to the viral particles. This difference may be due to the fact that the surface and transmembrane subunits of the HTLV-1 envelope glycoprotein are noncovalently linked (26), while MuLV glycoproteins are linked by a disulfide bond (25).
FIG. 3.
Sucrose gradient analysis of chimeric (Chim, Chim.ΔR) and parental viral particles produced after transfection of 293T cells. (A) Density determination. (B) MuLV and HTLV-1 Env proteins detected with anti-MuLV gp70 polyclonal antibody and anti-HTLV-1 gp46 monoclonal antibody, respectively. (C) HTLV-1 Gag proteins detected with an anti-HTLV-1 serum sample from a HAM/TSP patient.
Transmission electron microscopy of 293T cells transfected with chimeric proviruses showed the presence of particles in 5% of the cells (Fig. 4). The vast majority of chimeric particles were spherical, homogeneous in size and shape, and had a diameter of approximately 100 nm (Fig. 4A, C, E, and F). Particles were often trapped inside membrane folds (Fig. 4E). Glycoprotein spikes could be observed at the surfaces of chimeric virions (Fig. 4C). On occasion, a chimeric particle was observed in a late budding stage (Fig. 4F). In contrast, HTLV-1 particles produced by MT2 cells (Fig. 4B) or by 293T cells transfected with pCS-HTLV-I (Fig. 4D) were heterogeneous in size and shape, and budding particles were never observed. This observation indicates that chimeric particles bud more efficiently than HTLV-1, which is consistent with the presence of more p19 protein in the medium of cells transfected with chimeric provirus than in the medium of cells transfected with the original HTLV-1 provirus. Taken together, these results indicate that chimeric virions are produced in larger amounts than HTLV-1 is.
FIG. 4.
Ultrathin-section electron microscopic analysis. Transfected or infected cells were fixed in 1.6% glutaraldehyde, washed with Sörensen phosphate buffer (0.1 M, pH 7.2), and postfixed in 1% osmic acid. Cells were rinsed, dehydrated, and embedded in either Epon or propylene oxide for the floating sheet method (2). The sections were examined with a JEOL 1200 EX electron microscope. (A) 293T cell transfected with the pCS-HTLV-chim plasmid. (B) HTLV-1-infected (chronically infected) MT2 cell. (C) Higher magnification of chimeric particles showing Env spikes on the particle on the right. (D) Higher magnification of HTLV-1 particles produced by 293T cells transfected with the parental pCS-HTLV-I plasmid. (E) Chimeric particles trapped in a membrane fold in 293T cells. (F) A chimeric particle in a late budding stage. Magnifications: ×35,000 (A and B), ×115,000 (C and D), and ×70,000 (E and F).
Cell tropism of chimeric viruses.
The tropism of chimeric viral particles was examined with binding assays on primate and murine cells. Chimeric viral particles produced by transfecting 293T cells with either pCS-HTLV-chim or pCS-HTLV-chim.ΔR plasmid were incubated for 15 min at 37°C with human 293T or murine NIH 3T3 cells. After the cells were washed, they were immunolabeled with an anti-Mo-MuLV Env monoclonal antibody (83A25 [7], a gift of O. Schwartz, Institut Pasteur) and then with a secondary R-phycoerythrin-conjugated antibody and analyzed by fluorescence-activated cell sorting (not shown). Both chimeric viruses (with and without the R peptide) bound to murine NIH 3T3 cells, whereas HTLV-1 did not. Chimeric viruses did not bind to human cells, whereas HTLV-1 did. For a control, human cells expressing the ecotropic Mo-MuLV receptor (1) were constructed by transfecting 293T cells with a pJET plasmid expressing mCAT-1 (a gift from J. Cunningham, Harvard Medical School, Boston, Mass.) and a plasmid expressing resistance to hygromycin. After cloning by limiting dilution, cell clones were tested for their susceptibility to MuLV using a previously described method (3). A highly susceptible clone, named 293T4, was selected. Both chimeric viruses bound efficiently to 293T4 cells expressing the murine Mo-MuLV receptor. Hence, the HTLV-1/Mo-MuLV chimeric viruses have acquired the cell binding properties of Mo-MuLV particles.
To test the fusogenic properties of cells transfected with chimeric or HTLV-1 proviral DNA, a cell-to-cell fusion assay was developed. Indicator cells were obtained by transduction of 293T, 293T4, and NIH 3T3 cells with a lentiviral vector (38) expressing the green fluorescent protein under the control of the HTLV-1 long terminal repeat (LTR). These indicator cells were cocultivated for 24 h with 293T cells transfected with either the chimeric or parental HTLV-1 proviral plasmid, and green fluorescent protein expression was monitored (not shown). Transfection with the chimeric plasmids induced fusion only with murine cells or with human cells expressing the ecotropic MuLV receptor, thus confirming that the chimeric virus acquired the cell tropism of Mo-MuLV. The ΔR Env glycoprotein was more fusogenic than the full-length Env protein was. The HTLV-1 Env glycoprotein expressed after transfection with pCS-HTLV-I fused human cells and murine cells, while HTLV-1 particles bound only human cells. The fusion of murine cells by HTLV-1 Env has already been described (24). However, the role of cell-to-cell fusion in the propagation of HTLV-1 infection in vivo is not clear.
Infection of murine cells by chimeric viruses.
The ability of chimeric viruses to infect and replicate in murine cells was examined. To perform cell-free infections, chimeric and HTLV-1 viruses were prepared as follows: 293T cells were transfected with the appropriate plasmid, washed extensively 5 h later, and allowed to grow for 36 additional hours. Supernatants were clarified, filtered through 0.45-μm-pore-size filters (Sartorius), treated with RNase-free DNase (225 U; Amersham Pharmacia Biotech) for 30 min at 37°C to eliminate any residual plasmid DNA, and supplemented with 10 μg of DEAE-dextran per ml. Cell-free infections were performed by incubating 0.5 ml of supernatant for 2 h at 37°C with 5 × 105 target cells. Because the p19 protein was three to five times more concentrated in supernatants from cells transfected with chimeric plasmids than in supernatants from cells transfected with HTLV-1, all virus preparations were diluted to a concentration of 60 to 80 ng of p19 per ml in order to infect target cells with the same amount of particles. Fresh medium was added, and the cells were grown for 4 days. The target cells used were NIH 3T3, EL4 (a murine CD4 T-cell lymphoma obtained from the American Type Culture Collection), 293T, 293T4, HOS (a human osteosarcoma-derived fibroblast cell line permissive for HTLV-1 [19, 35]), and B5 (a cell line derived from the DBS-FRhL rhesus monkey lung fibroblast line that is permissive for HTLV-1 [6]).
Infectivity was defined as the ability of cell-free virions to promote the synthesis of new provirus, detected by PCR amplification of part of the gag gene (positions 1616 to 1821 in Seiki ATK1 sequence), after 4 days of incubation. For a control, the plasmid DNA was also looked for by PCR. All controls were negative. Figure 5A shows that cell-free chimeric HTLV/Mo-MuLV viruses were infectious for murine cells, whereas cell-free HTLV-1 was not. The chimeric viruses, in particular that produced by the pCS-HTLV-chim.ΔR plasmid, were also infectious for 293T4 human cells expressing the Mo-MuLV receptor. No infectivity was detected with HTLV-1 particles on 293T cells. Only a faint band was detected with this virus on B5 cells and sometimes on HOS cells (not shown).
FIG. 5.
Cell-free viral infections of murine cells. The HTLV-1 gag gene (205 bp, positions 1616 to 1821 in Seiki ATK1 sequence [29]) was detected by PCR amplification of DNA extracted from cells infected with cell-free chimeric viruses or cell-free HTLV-1. Amplified products were detected by Southern blot hybridization with specific oligonucleotide internal probes end labeled with 32P using 3′ terminal transferase. Radioactive signals were detected and quantified using a PhosphorImager (Molecular Dynamics) and ImageQuant software. To detect plasmid contamination, PCR was also performed with primer 5′-GGCTCGTATGTTGTGTGGAA-3′ in pUC19 and primer 5′-TTAGCCATATGCGTGCCATG-3′ in the HTLV-1 LTR. (A) PCR detection of the HTLV-1 gag gene and of control plasmid in DNA extracted from different cell lines infected by chimeric (Chim, Chim.ΔR) and HTLV-1 particles. The positive control (+) corresponds to 1 copy of the HTLV-1 genome from MT2 cells. The negative control (−) is 1 μg of DNA from naïve 293T cells. (B) Standard curve for PCR detection of HTLV-1 gag gene. Dilutions of pCS-HTLV-I plasmid (top) and MT2 cell DNA (bottom) are shown. (C) Evidence for infection of mouse lymphocytes by ΔR chimeric cell particles. PCR detection of the HTLV-1 gag gene and of control plasmid in DNA. Detection by reverse transcription-PCR of the doubly spliced viral mRNA for the Tax protein (221-bp spliced fragment, primers in positions 5098 to 7438 in Seiki ATK1 sequence). The GAPDH gene was also amplified by reverse transcription-PCR for a control. The positive controls are as follows: for the Gag panel, 1 copy of the HTLV-1 genome from MT2 cells; for the pUC panel, 1 μg of pCS-HTLV-1 plasmid; and for the Tax mRNA and GAPDH panels, MT2 RNA. (D) PCR detection of HTLV-1 gag gene in mouse lymphocytes infected with ΔR chimeric particles. Lanes 1, 2, and 3 show the spread of the infection from primary infected, male CD4+ lymphocytes placed in the lower chamber of a transwell to other cells placed in the upper chamber. Lane 1, DNA from primary infected cells; lane 2, DNA from secondary infected EL4 cells; lane 3, DNA from secondary infected female 129sv CD4+ lymphocytes. Lanes 4, 5, and 6 illustrate the spread of the infection from primary infected EL4 cells placed in the lower chamber of a transwell to other cells placed in the upper chamber. Lane 4, DNA from primary infected EL4 cells; lane 5, DNA from secondary infected male 129sv CD4+ lymphocytes; lane 6, DNA from secondary infected EL4 cells. The control primers to detect any contamination in secondary infections were 5′-GACTAGACATGTCTTAACATCTGTCC-3′ and 5′-CCTATTGCATGGACAGCAGCTTATG-3′ in the Zfy gene (murine Y chromosome [Y Chr.]). The positive control for the Gag panel is 1 copy of the HTLV-1 genome from MT2 cells, while that for the Y Chr. panel is 1 μg of DNA from male mouse splenocytes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The number of copies of proviral DNA in infected cells was estimated by a semiquantitative PCR assay as follows (Fig. 5B). Decreasing amounts of HTLV-1 DNA were added to a constant amount of 293T cell genomic DNA (1 μg, which is equivalent to 105 cell genomes), prior to PCR amplification of HTLV-1 gag sequence. One copy of proviral DNA per cell corresponded to 3 pg of pCS-HTLV-I plasmid diluted in 1 μg of genomic DNA (Fig. 5B, top blot). Serial dilutions of genomic DNA from MT2 cells in a constant amount of 293T cell DNA were also amplified (Fig. 5B, bottom blot). Because MT2 cells contain six copies of HTLV-1 gag DNA (17), one proviral copy per cell corresponded to 167 ng of MT2 cell DNA in 1 μg of DNA from 293T cells. With this assay, we determined that the infection of murine NIH 3T3 and EL4 cells by chimeric particles yielded 100 copies of provirus per 105 cells. The ΔR chimeric virus was 10 times more infectious (1,000 copies of proviral DNA per 105 cells). Human 293T4 cells expressing the MuLV receptor could also be infected by the ΔR chimeric virus, but at a lower level (100 proviral DNA copies per 105 cells). Under the same conditions, HTLV-1 particles were not infectious for murine cells (NIH 3T3 and EL4) or 293T and 293T4 human cells, but they were infectious for B5 cells (10 proviral DNA copies per 105 cells) and for CEM human CD4+ T cells (100 copies of proviral DNA per 105 cells [not shown]).
Thus, the chimeric viruses were able to accomplish successfully the early steps of infection, namely, cell entry, reverse transcription, and provirus integration. As determined by a semiquantitative test, the ΔR chimeric particles were 10 to 1,000 times more infectious for murine cells than HTLV-1 particles were for human cells, depending on the cells tested.
Secondary infection in murine primary lymphoid cells.
The ability of ΔR chimeric virus to infect mouse primary lymphoid cells was analyzed. Primary splenocytes from BALB/c, C3H, or 129sv mice were activated for 2 days with concanavalin A and interleukin-2. In the case of 129sv mice, CD4+ cells were selected from the activated splenocytes with magnetic beads (MACS; Miltenyi Biotech). Total splenocytes from BALB/c and C3H mice and CD4+ lymphocytes from 129sv mice were infected with cell-free ΔR chimeric particles as described above. Infectivity was detected by PCR amplification of part of the gag gene after 4 days of incubation. Figure 5C shows that primary murine lymphoid cells were efficiently infected. The proviral copy number was higher with CD4+ cells (129sv and EL4) than with total splenocytes, suggesting more efficient infection of CD4+ cells. The presence of the doubly spliced Tax mRNA was detected by RT-PCR assay in infected cells (Fig. 5C), showing that the chimeric provirus was expressed in infected lymphoid cells. This expression persisted during several weeks of culture (not shown).
In order to determine whether cells that had been previously infected with viral particles released new infectious virions, the ability of ΔR chimeric virus to spread the infection to fresh cells was investigated. Primary splenocytes from male 129sv mice were activated by treatment with concanavalin A and interleukin-2 for 2 days. CD4+ cells were selected from the activated splenocytes using magnetic beads (MACS; Miltenyi Biotech) and infected with cell-free ΔR chimeric particles. Thirty-six hours later, the infected CD4+ T cells were washed extensively and placed in the inferior chamber of a transwell (3-μm pore size). Fresh EL4 cells (from a female C57BL/6 mouse) or primary CD4+ T lymphocytes (from a female 129sv mouse) were seeded in the upper chamber as target cells. Four days after initiation of the transwell coculture, target cells were removed from the upper chamber, and the presence of newly synthesized provirus was looked for as described above. The Zfy gene (present on mouse Y chromosome) was looked for as a control to detect any contamination by primary infected cells. Figure 5D shows that EL4 cells or primary CD4+ T lymphocytes from the upper chamber of the transwell were infected by ΔR chimeric virus. The reverse experiment was also performed: infected EL4 cells were seeded in the lower chamber of the transwell, and naive primary CD4+ lymphocytes from male 129sv mice (cells previously activated) or naive EL4 cells were added to the upper chamber. The secondary infection was also observed in this case (Fig. 5D). Thus, the ΔR chimeric virus was infectious in secondary infection of murine lymphoid cells.
In conclusion, the ΔR Mo-MuLV/HTLV-1 chimeric virus described in this work was infectious for murine cells which were able to spread the infection to primary murine lymphocytes. In vivo experiments showed that chimeric proviral DNA could be found in the splenocytes of mice weeks after inoculation with the ΔR chimeric virus (unpublished results), indicating that in vivo infection occurs in mice. Therefore, this ΔR chimeric virus should provide a new animal model of infection by HTLV-1.
Acknowledgments
We thank Sandrine Luce and Emmanuelle Perret for excellent technical assistance; Marie Christine Dokhélar, Isabelle Leblanc, and Loraine Albritton for helpful discussions and advice; David Derse for kindly providing the infectious molecular clone of HTLV-1; Jim Cunningham for providing the mCAT-1 pJET plasmid; Marie Christine Dokhélar for providing cell lines (HOS and B5) and plasmids; Pierre Charneau for providing the HIV-Triplex vector; Olivier Schwartz and Pierre Rodrigues for providing the MuLV plasmid; and Antoine Gessain for providing patient sera.
This work was supported in part by a grant from the Agence Nationale pour la Recherche contre le SIDA (ANRS grant no. 99004) and institutional grants from the CNRS and Pasteur Institute.
REFERENCES
- 1.Albritton, L. M., L. Tseng, D. Scadden, and J. M. Cunningham. 1989. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57:659-666. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, J. R., and P. J. Boor. 1986. Improved transmission electron microscopy (TEM) of cultured cells through a “floating sheet” method. J. Ultrastruct. Mol. Struct. Res. 94:30-36. [DOI] [PubMed] [Google Scholar]
- 3.Bassin, R. H., N. Tuttle, and P. J. Fischinger. 1971. Rapid cell culture assay technique for murine leukaemia viruses. Nature 229:564-566. [DOI] [PubMed] [Google Scholar]
- 4.Delamarre, L., A. R. Rosenberg, C. Pique, D. Pham, and M. C. Dokhelar. 1997. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J. Virol. 71:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denesvre, C., P. Sonigo, A. Corbin, H. Ellerbrok, and M. Sitbon. 1995. Influence of transmembrane domains on the fusogenic abilities of human and murine leukemia retrovirus envelopes. J. Virol. 69:4149-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derse, D., J. Mikovits, M. Polianova, B. K. Felber, and F. Ruscetti. 1995. Virions released from cells transfected with a molecular clone of human T-cell leukemia virus type 1 give rise to primary and secondary infections of T cells. J. Virol. 9:1907-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, L. H., R. P. Morrison, F. G. Malik, J. Portis, and W. J. Britt. 1990. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J. Virol. 64:6176-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang, J., S. Kushida, R. Feng, M. Tanaka, T. Kawamura, H. Abe, N. Maeda, M. Onobori, M. Hori, K. Uchida, and M. Miwa. 1998. Transmission of human T-cell leukemia virus type 1 to mice. J. Virol. 72:3952-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuer, G., J. A. Zack, W. J. Harrington, Jr., R. Valderama, J. D. Rosenblatt, W. Wachsman, S. M. Baird, and I. S. Chen. 1993. Establishment of human T-cell leukemia virus type I T-cell lymphomas in severe combined immunodeficient mice. Blood 82:722-731. [PubMed] [Google Scholar]
- 10.Gessain, A., E. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de Thé. 1985. Antibodies to human T-lymphotropic virus type-1 in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed]
- 11.Ibrahim, F., L. Fiette, A. Gessain, N. Buisson, G. de-The, and R. Bomford. 1994. Infection of rats with human T-cell leukemia virus type-I: susceptibility of inbred strains, antibody response and provirus location. Int. J. Cancer 58:446-451. [DOI] [PubMed] [Google Scholar]
- 12.Imada, K., A. Takaori-Kondo, T. Akagi, K. Shimotohno, K. Sugamura, T. Hattori, H. Yamabe, M. Okuma, and T. Uchiyama. 1995. Tumorigenicity of human T-cell leukemia virus type I-infected cell lines in severe combined immunodeficient mice and characterization of the cells proliferating in vivo. Blood 86:2350-2357. [PubMed] [Google Scholar]
- 13.Ishiguro, N., M. Abe, K. Seto, H. Sakurai, H. Ikeda, A. Wakisaka, T. Togashi, M. Tateno, and T. Yoshiki. 1992. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J. Exp. Med. 176:981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazanji, M., F. Ibrahim, L. Fiette, R. Bomford, and G. De The. 1997. Role of the genetic background of rats in infection by HTLV-I and HTLV-II and in the development of associated diseases. Int. J. Cancer 73:131-136. [DOI] [PubMed] [Google Scholar]
- 15.Kazanji, M., J. P. Moreau, R. Mahieux, B. Bonnemains, R. Bomford, A. Gessain, and G. De Thé. 1997. HTLV-I infection in squirrel monkeys (Saïmiri sciureus) using autologous, homologous or heterologous HTLV-I-producing cell lines. Virology 231:258-266. [DOI] [PubMed] [Google Scholar]
- 16.Kazanji, M., A. Ureta-Vidal, S. Ozden, F. Tangy, B. de Thoisy, L. Fiette, A. Talarmin, A. Gessain, and G. de The. 2000. Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): provirus expression, persistence, and humoral and cellular immune responses. J. Virol. 74:4860-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, N., H. Konishi, H. Sabe, K. Shigesada, T. Noma, T. Honjo, and M. Hatanaka. 1984. Genomic structure of HTLV (human T-cell leukemia virus): detection of defective genome and its amplification in MT-2 cells. EMBO J. 3:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo, A., K. Imada, T. Hattori, H. Yamabe, T. Tanaka, M. Miyasaka, M. Okuma, and T. Uchiyama. 1993. A model of in vivo cell proliferation of adult T-cell leukemia. Blood 82:2501-2509. [PubMed] [Google Scholar]
- 19.Landau, N. R., K. A. Page, and D. R. Littman. 1991. Pseudotyping with human T-cell leukemia virus type 1 broadens the human immunodeficiency virus host range. J. Virol. 65:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Q. X., D. Camerini, Y. Xie, M. Greenwald, D. R. Kuritzkes, and I. S. Chen. 1996. Syncytium formation by recombinant HTLV-II envelope glycoprotein. Virology 218:279-284. [DOI] [PubMed] [Google Scholar]
- 21.Mizusawa, H., S. Kushida, M. Matsumura, H. Tanaka, Y. Ami, M. Hori, M. Kobayashi, K. Uchida, K. Yagami, T. Yoshizawa, et al. 1994. A neuropathological study of paraparetic rats injected with HTLV-I-producing T cells. J. Neurol. Sci. 126:101-108. [DOI] [PubMed] [Google Scholar]
- 22.Nagy, K., P. Clapham, R. Cheingsong-Popov, and R. A. Weiss. 1983. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int. J. Cancer 32:321-328. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura, H., Y. Tanaka, A. Komuro-Tsujimoto, K. Ishikawa, K. Takadaya, H. Tozawa, H. Tsujimoto, S. Honjo, and M. Hayami. 1986. Experimental inoculation of monkeys with autologous lymphoid cell lines immortalized by and producing human T-cell leukemia virus type-I. Int. J. Cancer 38:867-875. [DOI] [PubMed] [Google Scholar]
- 24.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 25.Opstelten, D. J., M. Wallin, and H. Garoff. 1998. Moloney murine leukemia virus envelope protein subunits, gp70 and Pr15E, form a stable disulfide-linked complex. J. Virol. 72:6537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pique, C., D. Pham, T. Tursz, and M. C. Dokhelar. 1992. Human T-cell leukemia virus type 1 envelope protein maturation process: requirements for syncytium formation. J. Virol. 66:906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragheb, J. A., and W. F. Anderson. 1994. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 68:3220-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1983. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 80:3618-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seto, A., M. Kawanishi, S. Matsuda, K. Ogawa, and I. Miyoshi. 1988. Adult T cell leukemia-like disease experimentally induced in rabbits. Jpn. J. Cancer Res. 79:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson, R. M., M. Leno, B. S. Hubbard, and T. J. Kindt. 1996. Cutaneous manifestations of human T cell leukemia virus type I infection in an experimental model. J. Infect. Dis. 173:722-726. [DOI] [PubMed] [Google Scholar]
- 32.Simpson, R. M., T. M. Zhao, B. S. Hubbard, S. Sawasdikosol, and T. J. Kindt. 1996. Experimental acute adult T cell leukemia-lymphoma is associated with thymic atrophy in human T cell leukemia virus type I infection. Lab. Investig. 74:696-710. [PubMed] [Google Scholar]
- 33.Sommerfelt, M. A., B. P. Williams, P. R. Clapham, E. Solomon, P. N. Goodfellow, and R. A. Weiss. 1988. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science 242:1557-1559. [DOI] [PubMed] [Google Scholar]
- 34.Suga, T., T. Kameyama, T. Kinoshita, K. Shimotohno, M. Matsumura, H. Tanaka, S. Kushida, Y. Ami, M. Uchida, K. Uchida, et al. 1991. Infection of rats with HTLV-1: a small-animal model for HTLV-1 carriers. Int. J. Cancer 49:764-769. [DOI] [PubMed] [Google Scholar]
- 35.Sutton, R., and D. Littman. 1996. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J. Virol. 70:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamanouchi, K., K. Kinoshita, R. Moriuchi, S. Katamine, T. Amagasaki, S. Ikeda, M. Ichimaru, T. Miyamoto, and S. Hino. 1985. Oral transmission of human T-cell leukemia virus type-I into a common marmoset (Callithrix jacchus) as an experimental model for milk-borne transmission. Jpn. J. Cancer Res. 76:481-487. [PubMed] [Google Scholar]
- 37.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]