Abstract
Recently we demonstrated the control of a mucosal challenge with a pathogenic chimera of simian and human immunodeficiency virus (SHIV-89.6P) by priming with a Gag-Pol-Env-expressing DNA and boosting with a Gag-Pol-Env-expressing recombinant modified vaccinia virus Ankara (DNA/MVA) vaccine. Here we evaluate the ability of the MVA component of this vaccine to serve as both a prime and a boost for an AIDS vaccine. The same immunization schedule, MVA dose, and challenge conditions were used as in the prior DNA/MVA vaccine trial. Compared to the DNA/MVA vaccine, the MVA-only vaccine raised less than 1/10 the number of vaccine-specific T cells but 10-fold-higher titers of binding antibody for Env. Postchallenge, the animals vaccinated with MVA alone increased their CD8 cell numbers to levels that were similar to those seen in DNA/MVA-vaccinated animals. However, they underwent a slower emergence and contraction of antiviral CD8 T cells and were slower to generate neutralizing antibodies than the DNA/MVA-vaccinated animals. Despite this, by 5 weeks postchallenge, the MVA-only-vaccinated animals had achieved as good control of the viral infection as the DNA/MVA group, a situation that has held up to the present time in the trial (48 weeks postchallenge). Thus, MVA vaccines, as well as DNA/MVA vaccines, merit further evaluation for their ability to control the current AIDS pandemic.
Recently, vaccines capable of eliciting high levels of antiviral T cells have successfully controlled pathogenic challenges with the 89.6P chimera of simian and human immunodeficiency virus (SHIV-89.6P) (2, 4, 22). Our successful trial used DNA priming followed by boosting with recombinant modified vaccinia virus Ankara (rMVA) (DNA/MVA) vaccine to raise high levels of antiviral T cells (2). In murine models, this heterologous prime-boost protocol has been shown to raise much higher levels of T cells than DNA priming and boosting or rMVA priming and boosting (21, 23), a phenomenon that is thought to be a reflection of the DNA focusing the immune response on the desired antigens and the poxvirus expanding this focused response, both by the expression of more antigen and by the mobilization of a proinflammatory immune response.
MVA is a highly attenuated strain of vaccinia virus that was developed toward the end of the campaign for the eradication of smallpox and safety tested with more than 100,000 people (13, 14). During over 500 passages in chicken cells, MVA lost about 10% of its genome and the ability to replicate efficiently in primate cells. Despite its limited replication, MVA has proved to be a highly effective expression vector, (25) raising protective immune responses in primates to parainfluenza virus (8), measles virus, (24), and immunodeficiency viruses (3, 18). The relatively high immunogenicity of MVA has been attributed in part to the loss of several viral anti-immune defense genes (6).
To better understand the importance of the DNA prime for the rMVA boost, we have tested rMVA priming and boosting (MVA-only vaccine) for the control of a SHIV-89.6P mucosal challenge. This allowed us to compare the immune responses raised by DNA priming and rMVA boosting to those raised by rMVA priming and boosting and to test whether the more complex heterologous prime-boost regimen provided a protective advantage in our challenge model.
MATERIALS AND METHODS
Immunogens.
The construction and production of immunogens have been previously described (2).
Immunizations and challenge.
Young adult rhesus macaques from the Yerkes breeding colony were cared for under guidelines established by the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the Emory University Institutional Animal Care and Use Committee. Macaques were typed for the Mamu-A*01 allele by PCR analyses (12). The DNA/MVA group, which was used as an example of the effects of DNA/MVA immunizations, received 2.5 mg of DNA intradermally (i.d.) at 0 and 8 weeks and of MVA at 24 weeks (group 1) (2). The MVA-only group received three sequential immunizations at 0, 8, and 24 weeks. Control animals received vector DNA, as well as MVA without inserts, at 0, 8, and 24 weeks (2). DNA immunizations were delivered in phosphate-buffered saline with a needleless jet injector (Bioject Inc., Portland, Oreg.). A total of 10 injections, 5 on each outer thigh, were delivered in a volume of 100 μl/injection. MVA or rMVA for all groups was administered both i.d. and intramuscularly with a needle for a total dose of 2 × 108 PFU, as previously described. At 7 months after the rMVA booster was administered, animals received an intrarectal challenge with SHIV-89.6P, during which 20 intrarectal infectious units (1.2 × 1010 copies of SHIV-89.6P viral RNA) was introduced 15 to 20 cm into the rectum by means of a pediatric feeding tube. All animals received the same challenge stock, which was delivered in the same manner by the same investigator. The MVA-only group was challenged approximately four months after the DNA/MVA group. Among the six control animals, four were challenged along with the DNA/MVA group and two were challenged along with the MVA-only group. Animals were identified by number as follows: 1, RBr-5*; 2, RIm-5*; 3, RQf-5*; 4, RZe-5; 5, ROm-5; 6, RDm-5; 25, RMb-5*; 26, RGy-5*; 27, RUs-4; 28, RPm-5; 29, RPs-4; 30, RKj-5; 43, RMr-4*; 44, RZt-4*; 45, RPk-5*, 46, RRk-5; 47, RKl-5; and 48, RGh-5. Rhesus monkeys with the A*01 allele are indicated with asterisks. For more detail, see reference 2.
T-cell responses.
For tetramer analyses, approximately 106 peripheral blood mononuclear cells (PBMC) were surface stained with antibodies to CD3 (FN-18; Biosource International, Camarillo, Calif.), CD8 (SK1; Becton Dickinson, San Jose, Calif.), and Gag-CM9 (CTPYDINQM)-Mamu-A*01 tetramer conjugated to different fluorochromes (for details, see reference 2). For gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays, anti-human IFN-γ antibody (clone B27; Pharmingen, San Diego, Calif.) was used for capture, and biotinylated anti-human IFN-γ antibody (clone 7-B6-1; Diapharma Group Inc., West Chester, Ohio) followed by avidin-horseradish peroxidase (Vector Laboratories Inc., Burlingame, Calif.) was used for detection (for details, see reference 2). The results of the ELISPOT assays are approximate, because different dilutions of cells have different efficiencies of spot formation in the absence of feeder layers (20).
Quantitation of SHIV copy numbers.
SHIV copy numbers were determined using a quantitative real time PCR as previously described (2, 10). All specimens were extracted and amplified in duplicate, with the mean results reported.
Intracellular p27 staining.
Approximately 106 PBMC were fixed and permeabilized with Cytofix/Cytoperm solution (Pharmingen, Inc.) and stained sequentially with anti-simian immunodeficiency virus (SIV) Gag antibody (clone FA-2, obtained from the National Institutes of Health AIDS Reagent Program) and phycoerythrin-conjugated anti-mouse immunoglobulin (Ig) (Pharmingen, Inc.) in Perm/Wash buffer (Pharmingen, Inc.) for 30 min at 4°C. Cells were washed twice with Perm/Wash buffer and incubated with antibodies to human CD3 (clone FN-18; Biosource International) and CD8 (clone SK1; Becton Dickinson) conjugated to fluorescein isothiocyanate and PerCP, respectively, in Perm/Wash buffer. Approximately 150,000 lymphocytes were acquired on a FACScalibur apparatus and analyzed using FlowJo software.
Gag and Env ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) for total anti-Gag antibody and anti-Env antibody were carried out as previously described (2). Standard curves for Gag and Env ELISAs were produced using serum from a SHIV-89.6-infected macaque with known amounts of anti-Gag or anti-Env IgG. Sera were assayed at threefold dilutions in duplicate wells. Standard curves were fitted, and sample concentrations were interpolated as micrograms of antibody per milliliter of serum by using SOFTmax 2.3 software (Molecular Devices, Sunnyvale, Calif.) (for more details, see reference 2). Avidity of the Env-specific antibodies was measured using NaSCN displacement ELISAs as previously described (2). Briefly, plates were coated overnight with 0.5 μg of recombinant gp120 89.6 per ml. The remaining steps were similar to those of anti-Env ELISAs, except for the addition of a 15-min incubation with different concentrations of NaSCN prior to the addition of anti-monkey IgG-horseradish peroxidase conjugate. All samples were assayed in duplicate over a range of dilutions, and results were expressed as the percentage of antibody bound in the absence of NaSCN.
Statistical analysis.
To examine the effect over time of doses and immunogens on parameters such as viral load, CD4 and antibody levels, and T-cell responses, linear mixed-effect models were applied to log-transformed values (19). In these analyses, differences in significant main effects for different groups are indicated by differences in the levels of parameters. Differences in the rate of change over time (slope) of a parameter for different groups are indicated by a significant group × week interaction. For determining differences in a parameter at a specific time, the t test was performed on log-transformed values.
RESULTS
Immunizations.
Our MVA vaccine expressed SIVmac239 Gag-Pol and SHIV-89.6 Env within a single recombinant MVA (termed MVA/89.6) (2). Inoculations of 2 × 108 PFU of MVA/89.6, half administered intramuscularly and half administered i.d., were given at 0, 8, and 24 weeks. For the DNA/MVA vaccine, various doses of a Gag-Pol-Env-expressing DNA (DNA/89.6) were administered at 0 and 8 weeks and a 2 × 108 PFU dose of MVA/89.6 was administered at 24 weeks (2). For comparisons with the MVA-only group, we present data from the DNA/MVA group with the highest T-cell response levels. This group was primed with 2.5 mg of DNA/89.6 i.d. (2). An intrarectal challenge with SHIV-89.6P was administered at 7 months after the final immunization. The 89.6 immunogen and the 89.6P challenge virus do not raise cross-neutralizing activity early after infection (15). Thus, the choice of immunogen and challenge approached the real-world situation, in which a human immunodeficiency virus type 1 (HIV-1) immunogen is unlikely to raise neutralizing antibody for the challenge virus.
Different patterns of vaccine-raised responses.
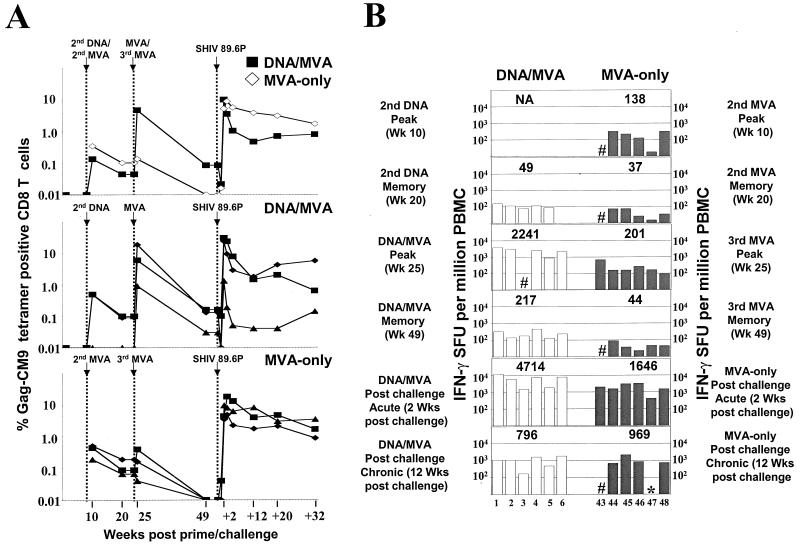
Much lower levels of Gag-specific T cells were raised in the MVA-only macaques than in the DNA/MVA-vaccinated macaques (Fig. 1). The levels of responding T cells were measured using Gag-CM9 tetramer analyses (1) and pools of overlapping Gag peptides and an ELISPOT assay (11, 20). The tetramer analyses were restricted to macaques that expressed the Mamu-A*01 histocompatibility type, whereas ELISPOT assay responses did not depend on a specific histocompatibility type. Two weeks after the second MVA inoculation, the levels of CD8 cells for the Gag-CM9 epitope in A*01 macaques had a geometric mean of 0.35%, which was slightly higher than had been achieved after DNA priming in the DNA/MVA group (Fig. 1A). The third MVA inoculation did not further boost the CD8 response in the MVA-only group. This was in sharp contrast to the results for the DNA/MVA-vaccinated animals, in which the MVA booster increased the level of tetramer-specific cells by 60- to 200-fold, with levels as high as 22% of total CD8 T cells achieved. These levels were at least 20-fold higher than those observed in MVA-only-vaccinated animals at any time prior to SHIV challenge. A similar temporal pattern of T-cell responses was observed using IFN-γ ELISPOT analyses (Fig. 1B). In these analyses, DNA/MVA-vaccinated animals had 10-fold higher levels of IFN-γ-producing cells following the MVA booster than the MVA-only group (t test; P = 0.001). At the time of challenge, IFN-γ production detected by ELISPOT assay had contracted into memory and was barely detectable in MVA-vaccinated animals compared with a geometric mean level of 217 in the DNA/MVA-vaccinated group (t test; P = 0.009).
FIG. 1.
Temporal levels of Gag-specific T cells. (A) Gag-specific CD8 T-cell responses in MVA-only and DNA/MVA-vaccinated animals. Symbols for individual animals are given in Fig. 3. (B) Gag-specific IFN-γ ELISPOTs for DNA/MVA-vaccinated (open bars) and MVA-only (hatched bars) macaques at various times before and after challenge. Three pools of 10 to 13 Gag peptides (22-mers overlapping by 12) were used for the analyses. The numbers above data bars represent the geometric means for the ELISPOTs within each group. The numbers at the bottom of the graph designate individual animals. #, data not available; ∗, less than 20 spot-forming units (SFU); NA, data not available for group. Data for the Gag-Pol-Env groups are for the group that received 2.5 mg of DNA as an intradermal prime (see reference 2).
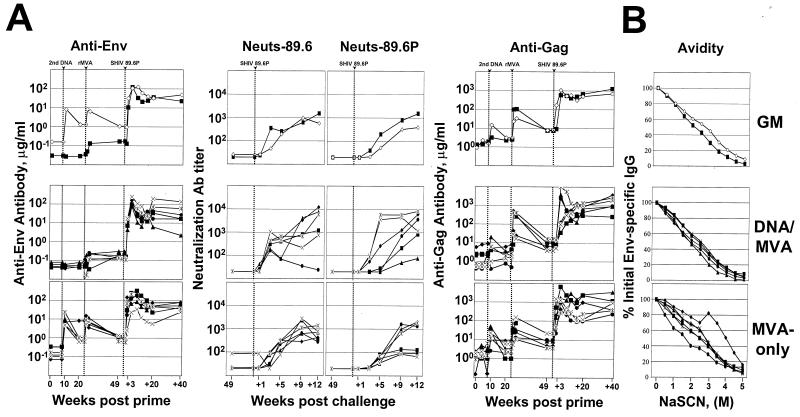
In contrast to the T-cell responses, vaccine-raised antibody responses to Env were much higher in the MVA-only group than in the DNA/MVA group (Fig. 2). The second MVA immunization raised good titers of binding antibodies for both Env and Gag (∼10 and ∼30 μg of specific antibody per ml of serum, respectively). These titers were only marginally increased by the third MVA immunization (Fig. 2). In contrast, the DNA/MVA immunizations raised very low levels of anti-Env binding antibody that could be detected only after the MVA booster (Fig. 2). Following the MVA booster, the DNA/MVA animals had good titers of anti-Gag antibody, slightly higher than in the MVA-only animals (Fig. 2). These differences were not significant (t test). Prior to challenge, none of the groups scored neutralizing antibodies to SHIV-89.6 or SHIV-89.6P (Fig. 2).
FIG. 2.
Temporal antibody responses. (A) Temporal assays of anti-Env binding, anti-Env neutralizing, and anti-Gag binding antibodies. Micrograms of total antibody against SIV239 Gag or 89.6 Env were determined using ELISAs. The titers of neutralizing antibody for SHIV-89.6 and SHIV-89.6P were determined using MT-2 cell killing and neutral red staining (16). Neutralization titers are the reciprocal of the serum dilution giving 50% neutralization of the indicated viruses grown in human PBMC. Symbols for animals are the same as those in Fig. 3. Data for the Gag-Pol-Env groups are in part reproduced from reference 2. (B) Avidity of anti-Env binding antibody at 2 weeks postchallenge. GM, geometric mean titer.
Comparable control of the SHIV 89.6P challenge.
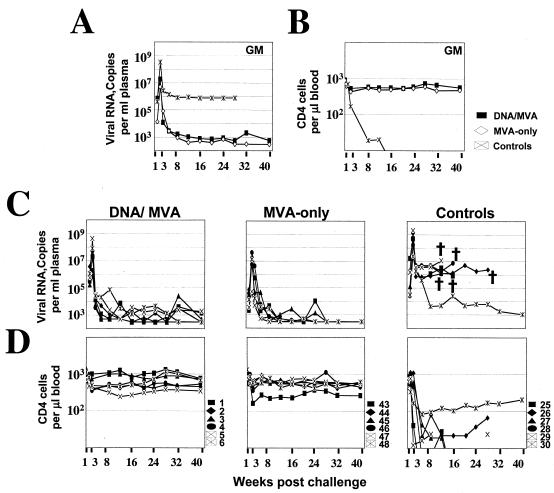
All six of the MVA-vaccinated animals controlled their postchallenge infections to the limit of detection and protected their CD4 cells (Fig. 3). At 2 weeks postchallenge, the geometric mean for the peak titers of plasma viral RNA in the MVA-only- vaccinated animals (5 × 106) was about fourfold less than that in DNA/MVA-vaccinated animals (2 × 107) and 100-fold less than that in control animals (3.3 × 108) (Fig. 3A). The rate and magnitude of virus control between weeks 2 and 3 postchallenge in the MVA-only vaccinated animals were lower than in the DNA/MVA-vaccinated animals. These differences between the two vaccine groups did not reach statistical significance. By 5 weeks postchallenge, the two groups had similar levels of viremia (Fig. 3A and C). By 40 weeks postchallenge, five out of the six control animals had succumbed to AIDS, whereas all of the MVA-only- as well as all of the DNA/MVA-vaccinated animals were healthy and were maintaining their plasma viral RNA levels at or below the level of detection (Fig. 3A and C).
FIG. 3.
Temporal viral loads and CD4 counts after challenge of vaccinated and control animals. (A) Geometric mean viral loads. (B) Geometric mean CD4 counts. (C) Viral loads. (D) CD4 counts for individual animals in the vaccine and control groups. The key to the animal numbering system is presented in panel D. Assays for the Gag-Pol-Env groups for the first 12 weeks had a background of 1,000 copies of RNA per milliliter of plasma. Animals with loads below 1,000 were scored with a load of 500. For all other assays, the background for detection was 300 copies of RNA/ml, and animals with levels of virus below 300 were scored at 300. † represents the death of an animal. Data for the DNA/MVA group are in part reproduced from reference 2. GM, geometric mean titers of each group.
Slower kinetics of T-cell expansion and contraction.
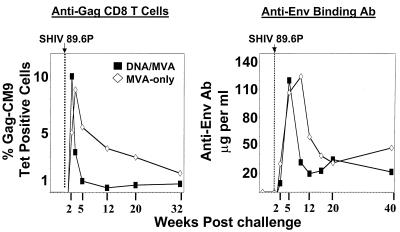
Interestingly, the control of the viral challenge in the MVA-only- vaccinated animals was associated with both a slower expansion and a lower contraction rate of antiviral T-cell response than those seen in the DNA/MVA-vaccinated animals (Fig. 1A and 4). In contrast to the DNA/MVA animals, for which the peak expansion of tetramer-positive cells in peripheral blood was observed at 2 weeks postchallenge, in the MVA-only animals the peak expansion occurred at 3 weeks postchallenge. Levels at the peak of response were very similar in the two groups (geometric means of ∼10% of total CD8 cells). The numbers of IFN-γ ELISPOTs at 2 weeks postchallenge were consistent with this slower expansion (1,646 spots per million PBMC in the MVA-only group, as opposed to 4,714 spots per million PBMC in the DNA/MVA group) (Fig. 1B). Provocatively, the decline of the tetramer-specific CD8 cells between weeks 2 and 5 in the MVA-only animals was significantly slower than in the DNA/MVA-vaccinated animals (linear mixed-effects model; P = 0.01) (Fig. 1A and 4). At 12 weeks postchallenge, both groups had controlled their levels of plasma viral RNA to similar levels. However, levels of tetramer-positive cells had fallen only twofold in the MVA-only group, as opposed to 10-fold in the DNA/MVA group. This slow contraction in levels of tetramer-positive cells for the MVA-only group has continued to the present time in the trial (48 weeks). The slow contraction was also evident in the IFN-γ ELISPOT response (Fig. 1B); by 12 weeks postchallenge, the geometric mean levels of IFN-γ ELISPOTS for MVA-vaccinated animals had fallen less than twofold (from 1,646 to 969), whereas for DNA/MVA-vaccinated animals, ELISPOTs had fallen sixfold (4,714 to 796).
FIG. 4.
Postchallenge anti-Gag CD8 T cells and anti-Env binding antibody. Data are the same as those in Fig. 1 and 2 but are plotted on a linear scale to better show the differences in postchallenge patterns of anamnestic immune responses in the DNA/MVA and MVA-only groups.
Slower emergence of anti-Env antibody.
Despite the priming of much higher titers of binding antibody for Env in the MVA-only group, binding antibodies, as well as measurable neutralizing antibodies for both 89.6 and 89.6P, emerged more slowly in this group than in the DNA/MVA group (Fig. 2A and 4). Binding antibodies for Env peaked at 5 to 9 weeks postchallenge in MVA-only animals, whereas they had peaked by 5 weeks postchallenge in DNA/MVA-vaccinated animals. The appearance of neutralizing antibodies for both 89.6 and 89.6P was also about 4 weeks later in the MVA-only animals than in the DNA/MVA-vaccinated animals. The titers of binding and neutralizing antibody for 89.6 reached similar high levels in both groups. However, the titers of neutralizing antibody for 89.6P remained about fivefold lower in the MVA-only group than in the DNA/MVA group for as long as 12 weeks postchallenge. The slower appearance of neutralizing antibodies in the MVA-only animals was not due to differences in the avidity of the binding antibody; indeed, MVA-only-vaccinated animals had slightly higher antibody avidity than DNA/MVA-vaccinated animals (Fig. 2B). As with the T-cell responses, the contraction of the binding antibody response between 5 and 12 weeks postchallenge was significantly slower in the MVA-only group than in the DNA/MVA-vaccinated group (linear mixed-effects model; P = 0.01) (Fig. 2A and 4). Postchallenge, both groups had similar anamnestic responses to Gag that peaked at close to 1 mg of anti-Gag antibody per milliliter of serum (Fig. 2A).
Higher levels of infected cells in the peripheral blood.
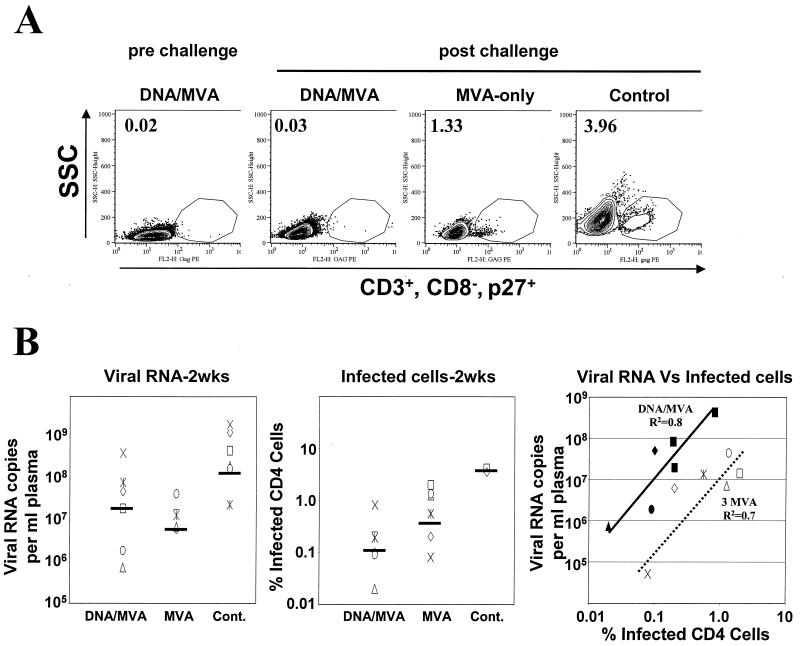
Despite lower levels of plasma viral RNA, the levels of infected CD4 cells were higher in the MVA-only group than in the DNA/MVA-vaccinated group (Fig. 5). At 2 weeks postchallenge, the levels of infected cells had geometric means of 4% in the control group, 0.5% in the MVA-only group, and 0.1% in the DNA/MVA group. Similar levels were observed in lymph nodes (data not shown). These levels and differences in levels were also seen in cocultivation assays (data not shown). This rank order did not agree with the rank order for the geometric mean titers for plasma viral RNA, in which the MVA-vaccinated group had the lowest value (Fig. 5B). When levels of plasma viral RNA were compared to levels of infected cells in the DNA/MVA and MVA-only groups, both showed direct but distinct correlations (Fig. 5B, right panel). Presumably this reflected the differences in the antiviral T-cell and antibody responses in these two sets of animals.
FIG. 5.
Viral loads and infected cells in the peripheral blood at 2 weeks postchallenge. (A) Intracellular p27 staining. PBMC were fixed and stained for intracellular Gag, CD3, and CD8. Cells were gated on lymphocytes, followed by gating for CD3+ and CD8−, and were then analyzed for Gag. The frequencies in the graph represent Gag-positive cells as the percentage of total CD4 cell levels. Representative data are shown for each group: animal 3 (prechallenge) and animals 3, 45, and 26 (postchallenge). (B) Comparison of viral loads and numbers of infected cells at 2 weeks postchallenge. Geometric means for viral RNA copies and percent infected CD4 cells are represented as horizontal bars on the respective graphs. Filled symbols represent the DNA/MVA-vaccinated animals, and open symbols represent the MVA-only-vaccinated animals. The diagonal lines represent the trend lines for the DNA/MVA-vaccinated animals (solid) and the MVA-only-vaccinated animals (dashed).
DISCUSSION
Provocatively, our MVA-only vaccine showed levels of control of plasma viremia and protection of CD4 cells as good as those of our DNA/MVA vaccine (Fig. 3). Despite similar viral control and CD4 protection levels, patterns of immune responses in the two vaccine groups were strikingly different before challenge (Fig. 1 and 2). During the immunization phase of the trial, priming of anti-Env antibody was much higher for the MVA-only group, whereas priming of T cells was much higher for the DNA/MVA group. Seven months after the final immunization, at the time of challenge, the MVA-only group had undetectable levels of specific T cells whereas the DNA/MVA group had easily detected levels in the peripheral blood. A similar pattern was also seen for Env-specific T-cell responses (data not shown). At the same time, the MVA-only group had 10-fold higher levels of binding antibody for Env than the DNA/MVA group. These differences in antibody levels were likely due to the expression of much higher levels of Env by MVA than DNA immunizations.
Surprisingly, the differences in vaccine-raised responses did not have major effects on postchallenge anamnestic responses and viral control, which were similar except for slower kinetics in the MVA-only group (Fig. 1, 2, and 4). One possibility is that immune cell trafficking differed between the MVA-only and DNA/MVA groups. This could account for the slower kinetics of postchallenge T-cell as well as neutralizing antibody responses in the peripheral blood for the MVA-only group. One of the striking differences between the MVA-only and DNA/MVA groups was in the levels of infected CD4 cells in peripheral blood. The MVA-only-vaccinated animals had much higher levels of infected CD4 cells than DNA/MVA-vaccinated animals, despite having lower levels of plasma viral RNA (Fig. 5). Both CD8 and CD4 T cells secrete chemokines, such as RANTES, macrophage inflammatory protein 1α (MIP-1α) and MIP-1β, that can block infection (7, 9). It is possible that the faster emergence of postchallenge T cells as well as neutralizing antibody in the DNA/MVA group influenced the virus entry process (Fig. 2A and 4).
The most worrying postchallenge difference between the two groups has been that of the slower contraction of immune responses in the MVA-only animals. Even at 48 weeks postchallenge, both humoral and cellular responses remained higher in the MVA-only group than in the DNA-MVA group (Fig. 1, 2, and 4). This phenomenon occurred despite viremia having been even more tightly controlled between 12 and 24 weeks postchallenge in the MVA-only group than in the DNA/MVA group (linear mixed-effects model; P = 0.02). The higher levels of persisting immune responses in the MVA-only group could be a marker for higher levels of sequestered and persisting virus in this group.
This trial achieved better and more consistent protection than has been achieved in prior MVA-only trials (3, 18). We think that an important factor contributing to this difference was the use of an intrarectal challenge. The intrarectal challenge (as opposed to an intravenous challenge) allows the immune system added time to respond to an infection that is at least transiently sequestered in the gut (5). An intrarectal challenge is also relevant to the current AIDS pandemic, in which the vast majority of infections are spread by mucosal routes during sexual intercourse. Another potentially important difference between our trial and the less-protective trial conducted using SIVSmE660 was that of the much slower appearance of neutralizing antibodies following challenge with E660 virus (17). Differences in the virulence of SIVsmE660 and SHIV-89.6P also could have contributed to our success.
The success of the MVA-only vaccine, despite its not having raised the highest T-cell response, highlights the importance of testing for protective efficacy as well as for immunogenicity during vaccine development. Our results demonstrate that different vaccine modalities can have similar postchallenge control of infection despite very different patterns of prechallenge immune responses. And finally, our results provide support for the testing of MVA-only as well as DNA/MVA vaccines in humans.
Acknowledgments
This work was supported by the Integrated Preclinical/Clinical AIDS Vaccine Development Program Project (P01 AI 43045), Emory/Atlanta Center for AIDS Research (P30 DA 12121), Yerkes Regional Primate Research Center (base grant P51 RR00165), and NIAID contract AI 85343 to D. C. Montefiori.
We thank J. Sodroski for molecularly cloned SHIV-89.6; K. Reimann for the SHIV-89.6P seed stock; D. Pauza for the plasmid pGEX 27; L. Frampton for help in preparation of the rMVA; the NIH AIDS Reagent Program for anti-SIV p27 monoclonal antibody clone FA-2; J. Pohl and the Emory Microchemical Facility for synthesis of peptides; R. Polavarapu and the Emory DNA Sequence Facility for DNA sequencing; B. Grimm, S. Sharma, M. Patel, S. Patel, and D. Campbell for excellent technical help; and H. Drake-Perrow for outstanding administrative support. We are grateful to the Yerkes Division of Research Resources for the consistent excellence of veterinary care and pathology support.
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 5.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. [DOI] [PubMed] [Google Scholar]
- 7.Chun, T. W., J. S. Justement, S. Moir, C. W. Hallahan, L. A. Ehler, S. Liu, M. McLaughlin, M. Dybul, J. M. Mican, and A. S. Fauci. 2001. Suppression of HIV replication in the resting CD4+ T cell reservoir by autologous CD8+ T cells: implications for the development of therapeutic strategies. Proc. Natl. Acad. Sci. USA 98:253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durbin, A. P., C. J. Cho, W. R. Elkins, L. S. Wyatt, B. Moss, and B. R. Murphy. 1999. Comparison of the immunogenicity and efficacy of a replication-defective vaccinia virus expressing antigens of human parainfluenza virus type 3 (HPIV3) with those of a live attenuated HPIV3 vaccine candidate in rhesus monkeys passively immunized with PIV3 antibodies. J. Infect. Dis. 179:1345-1351. [DOI] [PubMed] [Google Scholar]
- 9.Furci, L., G. Scarlatti, S. Burastero, G. Tambussi, C. Colognesi, C. Quillent, R. Longhi, P. Loverro, B. Borgonovo, D. Gaffi, E. Carrow, M. Malnati, P. Lusso, A. G. Siccardi, A. Lazzarin, and A. Beretta. 1997. Antigen-driven C-C chemokine-mediated HIV-1 suppression by CD4+ T cells from exposed uninfected individuals expressing the wild-type CCR-5 allele. J. Exp. Med. 186:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 11.Kern, F., I. P. Surel, N. Faulhaber, C. Frommel, J. Schneider-Mergener, C. Schönemann, P. Reinke, and H. D. Volk. 1999. Target structures of the CD8+-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 13.Mahnel, H., and A. Mayr. 1994. Experiences with immunization against orthopox viruses of humans and animals using vaccine strain MVA. Berl. Muench. Tieraerztl. Wochenschr. 107:253-256. (In German.) [PubMed] [Google Scholar]
- 14.Mayr, A., H. Stickl, H. K. Muller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defense mechanism. Zentbl. Bakteriol. B 167:375-390. (In German.) [PubMed] [Google Scholar]
- 15.Montefiori, D. C., K. A. Reimann, M. S. Wyand, K. Manson, M. G. Lewis, R. G. Collman, J. G. Sodroski, D. P. Bolognesi, and N. L. Letvin. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J. Virol. 72:3427-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montefiori, D. C., W. E. Robinson, Jr., S. S. Schuffman, and W. M. Mitchell. 1988. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J. Clin. Microbiol. 26:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ourmanov, I., M. Bilska, V. M. Hirsch, and D. C. Montefiori. 2000. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J. Virol. 74:2960-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinheiro, J. C., and D. M. Bates. 2001. Mixed effects models in S and S-PLUS. Springer, New York, N.Y.
- 20.Power, C. A., C. L. Grand, N. Ismail, N. C. Peters, D. P. Yurkowski, and P. A. Bretscher. 1999. A valid ELISPOT assay for enumeration of ex vivo, antigen-specific, IFNγ-producing T cells. J. Immunol. Methods 227:99-107. [DOI] [PubMed] [Google Scholar]
- 21.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S.-L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunization. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 22.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 23.Schneider, J., S. C. Gilbert, C. M. Hannan, P. Degano, E. G. Sheu, M. Plebanski, and A. V. S. Hill. 1999. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol. Rev. 170:29-38. [DOI] [PubMed] [Google Scholar]
- 24.Stittelaar, K. J., L. S. Wyatt, R. L. de Swart, H. W. Vos, J. Groen, G. van Amerongen, R. S. van Binnendijk, S. Rozenblatt, B. Moss, and A. D. Osterhaus. 2000. Protective immunity in macaques vaccinated with a modified vaccinia virus Ankara-based measles virus vaccine in the presence of passively acquired antibodies. J. Virol. 74:4236-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]