Abstract
The relationship(s) between viral virulence and matrix metalloproteinase (MMP) expression in the central nervous system (CNS) of mice undergoing lethal and sublethal infections with neurotropic mouse hepatitis virus was investigated. Lethal infection induced increased levels of MMP-3 and MMP-12 mRNAs as well as that of tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) compared to sublethal infection. Increased induction of MMP, TIMP, and chemokine expression correlated with increased virus replication but not with inflammatory cell infiltration. Infection of immunosuppressed mice suggested that expression of most MMP, TIMP, and chemokine mRNA was induced primarily in CNS-resident cells. By contrast, MMP-9 protein activity was associated with the infiltration of neutrophils into the CNS. These data indicate an association between the magnitude of inflammatory gene expression within the CNS and viral virulence.
Inflammatory responses are a double-edged sword. They are essential for clearing invading pathogens but also have the capacity to damage both infected and bystander cells. This is of particular relevance within the central nervous system (CNS), where neuronal networks are limited in their ability to recover from injury. The CNS is also isolated from peripheral compartments by vascular endothelial cells joined by continuous tight junctions (41). These endothelial cells are in turn covered by a basal lamina and the perivascular end feet of surrounding astrocytes to form the blood-brain barrier, which limits CNS parenchymal access by blood-borne pathogens as well as leukocytes. However, during CNS infection or loss of self-tolerance to CNS antigens, rapid infiltration by inflammatory cells occurs, often resulting in immune-mediated pathology (1, 17). Thus, understanding the mechanisms which regulate CNS inflammation is crucial to comprehending the balance between effective antimicrobial immunity and immune-mediated tissue damage.
Matrix metalloproteinases (MMPs) and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), are normally involved in maintenance of the extracellular matrix. Although substrates vary between individual MMPs, as a group, MMPs are capable of breaking down all protein components of the extracellular matrix. Thus, MMPs and TIMPs are crucial in regulating leukocyte migration into infected or damaged tissues (16, 21) and have been implicated in numerous inflammatory diseases, such as rheumatoid arthritis (19) and asthma (25). In the CNS, MMP expression is associated with the inflammatory demyelinating diseases multiple sclerosis (2) and experimental autoimmune encephalitis (36). In particular, MMP-1, -2, -3, -7, and -9 and TIMP-1 have been detected in inflammatory infiltrates as well as activated astrocytes and microglia within acute multiple sclerosis lesions, suggesting that they play either a direct or an indirect role in CNS plaque formation (2, 3, 26, 28). These proteases are secreted into the extracellular space by a wide range of cell types, including endothelial cells, infiltrating inflammatory cells, and CNS-resident cells (2, 3, 26, 28).
While the mechanisms which initiate and promote leukocyte migration have been studied extensively, the relationship(s) between viral infection, subsequent inflammation, and MMP expression is less well understood (12, 13, 21, 22, 50). Infection of the CNS with mouse hepatitis virus (MHV) JHM strain (JHMV) induces an acute encephalomyelitis associated with chronic demyelination in surviving mice and has proven to be a useful model for studying both acute inflammatory responses and immune-mediated pathology within the CNS (31, 49). A variety of JHMV variants with different pathological outcomes have provided insight into the role of virulence in altering the outcome of CNS disease (30, 31, 37, 40). Two such JHMV variants with distinct pathological outcomes were used in this study to compare inflammatory processes associated with distinct clinical outcomes. DM is a plaque morphology variant which mimics the pathogenesis of parental JHMV, producing a fatal infection in adult mice associated with extensive encephalomyelitis (46). The 2.2-V-1 variant (referred to as V-1 for brevity) was derived by selection for resistance to neutralizing monoclonal antibody (MAb) J.2.2 (10). V-1 induces sublethal infections of adult mice associated with an increased incidence of demyelinating lesions in chronically infected animals (10).
In this study, the relationships between virulence, inflammation, and the induction of MMP and TIMP expression during acute lethal and sublethal infections of the CNS were compared. The lethal DM variant replicated to higher titers than the sublethal V-1 variant and was associated with a more extensive infection of CNS cells and inflammation. Expression of genes for inflammatory proteins, including MMPs, TIMPs, and most chemokines, was enhanced during lethal infection compared to sublethal infection and peaked in conjunction with virus replication, in contrast to inflammatory cell infiltration. Analysis of immunocompromised mice suggests that expression of most MMP, TIMP, and chemokines genes is associated with CNS-resident cells during early stages of acute infection. An exception was the MMP-9 protein, which was produced by inflammatory cells, and its enzymatic activity was associated with neutrophil infiltration into the CNS. These data suggest that increased expression of MMP-3, MMP-12, and TIMP-1 is a consequence of CNS viral infection as opposed to being a result of CNS inflammatory cell infiltration.
MATERIALS AND METHODS
Mice and viruses.
Male BALB/c (H-2d) mice were purchased from the National Cancer Institute (Frederick, Md.) and were maintained within an accredited animal facility at the University of Southern California. All mice were used at between 6 and 8 weeks of age. To produce a sublethal infection, mice were infected intracerebrally with 1,000 PFU of the 2.2-V-1 (V-1) variant of JHMV (10) in a volume of 30 μl of sterile endotoxin-free Dulbecco's phosphate-buffered saline (DPBS). To produce a lethal encephalomyelitis, mice were infected with 100 PFU of the DM variant of JHMV (46). Clinical scores were graded as described previously (27). Viruses were propagated and quantitated by plaque assay using the murine DBT astrocytoma cell line as previously described (46). Sham-infected mice were injected intracerebrally with 30 μl of DPBS. For immunosuppression, mice were irradiated with 850 rads from a 137Cs gamma vertical beam source 24 h prior to infection. Mice were perfused via intracardiac injection of 50 ml of PBS prior to analysis.
CNS viral titers.
Viral titers were determined from brain homogenates as described previously (46). Briefly, one half-brain from each of three or more individual mice per time point were homogenized in 4 ml of DPBS (pH 7.4) using Tenbroeck tissue homogenizers. Homogenates were clarified by centrifugation for 7 min at 800 × g at 4°C. Supernatants were stored at −70°C until assayed for infectious virus by plaque assay on DBT cell monolayers as described previously (46).
Histology.
Histopathological analysis was performed as previously described (48). Briefly, tissues were fixed for 3 h in Clark's solution (75% ethanol, 25% glacial acetic acid) and embedded in paraffin. Distribution of JHMV antigen was examined by immunoperoxidase staining (Vectastain-ABC kit; Vector Laboratory, Burlingame, Calif.) with an anti-JHMV MAb, J.3.3, specific for the viral nucleocapsid protein followed by counterstaining with hematoxylin (47, 51). The extent of inflammation and viral antigen were determined in a blinded fashion.
Isolation of CNS-infiltrating cells.
Infiltrating cells were isolated from the CNS of perfused JHMV-infected mice as described previously (5). Briefly, brains were removed, washed twice with RPMI 1640 medium supplemented with 25 mM HEPES (pH 7.2) and 1% fetal bovine serum, and homogenized in Tenbroeck tissue homogenizers. Cells were suspended in 30% Percoll (Pharmacia, Piscataway, N.J.) and concentrated onto a 1-ml cushion of 70% Percoll by centrifugation at 1,300 × g for 20 min at 4°C. Cells were collected from the interphase and washed twice prior to analysis.
Flow cytometry analysis.
Surface marker expression on cells was determined by staining with MAbs specific for CD8 (53-6.7), CD4 (GK1.5), CD11b (M1/70), Ly-6G (RB6-8C5), CD45 (30-F11), CD19 (1D3), pan-NK (DX5), and F4/80. All MAbs were purchased from BD PharMingen (San Diego, Calif.), except F4/80, which was purchased from Serotech (Raleigh, N.C.). Nonspecific binding was minimized by blocking with purified rat anti-mouse FcIII/IIR MAb (2.4G2; BD PharMingen) and naive mouse serum in PBS containing 0.5% bovine serum albumin (Sigma, Saint Louis, Mo.) for 10 min prior to staining. Cells were stained for 30 min on ice, washed twice with PBS containing 0.5% bovine serum albumin, and analyzed using a FACStar flow cytometer and Cellquest Pro software (Becton Dickinson, Mountain View, Calif.).
RNA isolation and RPA.
RNA was prepared from brains of naive and JHMV-infected mice by using TRIzol reagent (GIBCO BRL, Rockville, Md.) in accordance with the manufacturer's protocol. Plasmids containing MMP and TIMP RNase protection assay (RPA) probes (35) were gifts from Iain Campbell (Scripps Research Institute, La Jolla, Calif.). A murine chemokine RPA kit was obtained from BD PharMingen. RPA probes were synthesized using an in vitro transcription kit (BD PharMingen) to generate [α-32P]UTP-labeled probes according to the manufacturer's protocol. Probes were hybridized with 10 μg of RNA, and RPA was performed as described by Pagenstecher et al. (35). Briefly, RNA samples were hybridized with 32P-labeled probes at 56°C overnight, followed by digestion with a mixture of RNase A and RNase T1 for 45 min at 30°C. Following RNase inactivation via treatment with proteinase K for 15 min at 37°C, protected fragments were separated on 5% acrylamide denaturing gels containing 7 M urea. The intensities of protected fragments were quantitated with a PhosphorImager and Imagequant 5.0 software (Molecular Dynamics, Sunnyvale, Calif.).
Zymography.
Cells isolated from the CNS were disrupted by suspension in lysis buffer (1.0% Triton X-100, 300 mM NaCl, 50 mM Tris, pH 7.4). Lysates prepared from 2 × 105 cells were separated on a 10% acrylamide gel containing 1% gelatin (Bio-Rad, Hercules, Calif.) in the absence of reducing agents. Following electrophoresis, gels were washed at room temperature for 20 min in washing buffer (Bio-Rad). The gels were placed in developing buffer (Bio-Rad) for 20 min at room temperature prior to overnight incubation at 37°C. Gels were stained with a solution of 0.25% Coomassie brilliant blue R-250 and destained with 10% acetic acid and 10% methanol until bands became evident. Relative band intensities were quantitated using a Fluor-S imaging system (Bio-Rad).
Statistical analysis.
Results generated from three or more samples per time point are represented as the mean ± the standard deviation and were analyzed using Student's t test. Results generated from experiments involving paired samples are given as the mean ± the range of the individual data points.
RESULTS
Pathogenesis of lethal and sublethal JHMV infections.
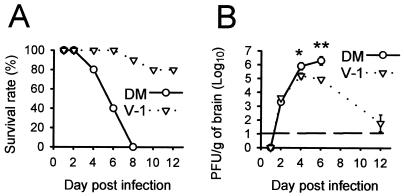
Infection of BALB/c mice with the DM isolate of JHMV resulted in a lethal infection in which almost all mice were dead or moribund by 6 days postinfection (p.i.) and none survived longer than day 8 p.i. (Fig. 1A). By contrast, more than 80% of mice survived infection with the neutralizing MAb-resistant JHMV variant V-1. Levels of virus replication were below the limit of detection at day 1 p.i. and were nearly identical at day 2 p.i. (∼5 × 103 PFU/g) (Fig. 1B). However, in accordance with the divergent clinical outcomes, by day 4 p.i. virus replication within the CNS of mice infected with the lethal variant was approximately 10-fold higher than that in mice infected with the sublethal V-1 variant (P < 0.05). By day 6 p.i. the lethal variant peaked at 107 PFU/g, approximately 15-fold higher than in mice infected with the sublethal variant (P < 0.01). Infectious V-1 virus was reduced to near the limit of detection by day 12 p.i., as previously reported (30). These data confirm previous observations with C57BL/6 mice indicating that the lethal DM variant replicates to higher titers within the CNS than the sublethal V-1 variant (37) and that infection with V-1 produces a sublethal infection in which infectious virus is cleared from the CNS by immune effector mechanisms (27, 31, 38, 40).
FIG. 1.
Mortality (A) and CNS virus replication (B) following lethal (DM) and sublethal (V-1) JHMV infections. Three or more mice from each group were sacrificed at the indicated time points. Error bars represent standard deviations from the means ∗, P < 0.05; ∗∗, P < 0.01.
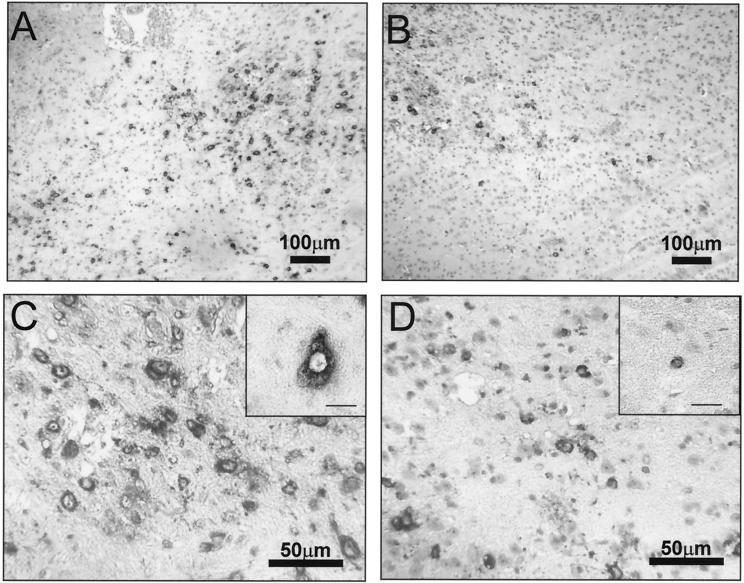
Brains from mice infected with the lethal and sublethal JHMV variants were compared to correlate virus replication with pathological changes. Inflammatory cells were detected rarely at day 2 p.i. However, both inflammatory foci and perivascular mononuclear cells were clearly evident by day 4 p.i. and increased at day 6 p.i. following either viral infection. At all time points examined, increased numbers of inflammatory cells were found in the CNS of lethally infected mice compared to mice with a sublethal infection (data not shown), which was confirmed by flow cytometry (see below). JHMV antigen was detected at day 2 p.i. and also increased with time p.i. Both viruses caused rare demyelinating lesions at day 6 p.i. and infected a variety of CNS cell types, including oligodendrocytes, microglia, and astrocytes (Fig. 2). Viral antigen was also detected in neurons of mice during lethal infection at day 6 p.i., although less frequently than in glial cells. By contrast, viral antigen was more prevalent in oligodendrocytes following sublethal V-1 infection. Consistent with the increased virus replication in the CNS of lethally infected mice, virus antigen was more prevalent in the CNS of lethally infected mice than in those of mice undergoing sublethal infection at all time points examined (Fig. 2), suggesting a greater rate of replication and/or dissemination.
FIG. 2.
CNS distribution of viral antigen. Viral antigen in brains of DM (A and C)- and V-1 (B and D)-infected mice was detected with a MAb specific for the JHMV nucleocapsid protein and counterstained with hematoxylin. The insets in panels C and D show high-power magnifications of an infected neuron (C) and an oligodendrocyte (D) identified morphologically. The bars in the insets are scaled to represent 20 μm.
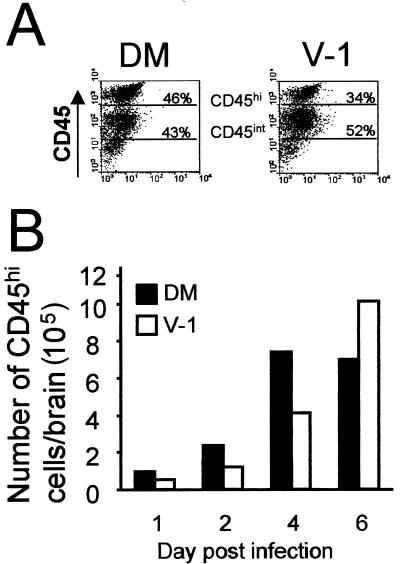
To confirm that higher levels of virus replication and viral antigen within the CNS correlated with increases of inflammation, cells were recovered from the brains of mice undergoing lethal and sublethal infections at various time points p.i. Analysis of CD45 expression allowed infiltrating inflammatory cells (CD45hi), to be distinguished from resident CNS microglia (CD45int) (Fig. 3A) (11, 44). Inflammatory cells were detected as early as 1 day p.i. with both viruses (Fig. 3B). CD45hi cells were rare in the CNS of uninfected mice, accounting for <5 × 104 cells/brain at day 1 following sham infection (data not shown). Consistent with histological examination, lethal infection induced increased infiltration of CD45hi inflammatory cells into the CNS compared to sublethal infection at all times through day 4 p.i. (Fig. 3B). Inflammatory cells declined slightly in lethally infected mice as they began to develop severe clinical symptoms and succumb to infection at day 6 p.i. By contrast, infiltrating CD45hi cells continued to increase through day 6 p.i. in the CNS of sublethally infected mice (Fig. 3B). The inflammatory cells recovered from sublethally infected mice declined by approximately 80% between days 6 and 12 p.i., coinciding with the decline in infectious virus (data not shown).
FIG. 3.
CNS inflammatory cell infiltration following infection. (A) Flow cytometric analysis of CD45-expressing cells isolated from the CNS of perfused mice at day 4 p.i. (B) Quantitation of CD45hi cell accumulation within the infected CNS. Data are representative of those from three separate experiments.
Induction of MMP expression following JHMV infection.
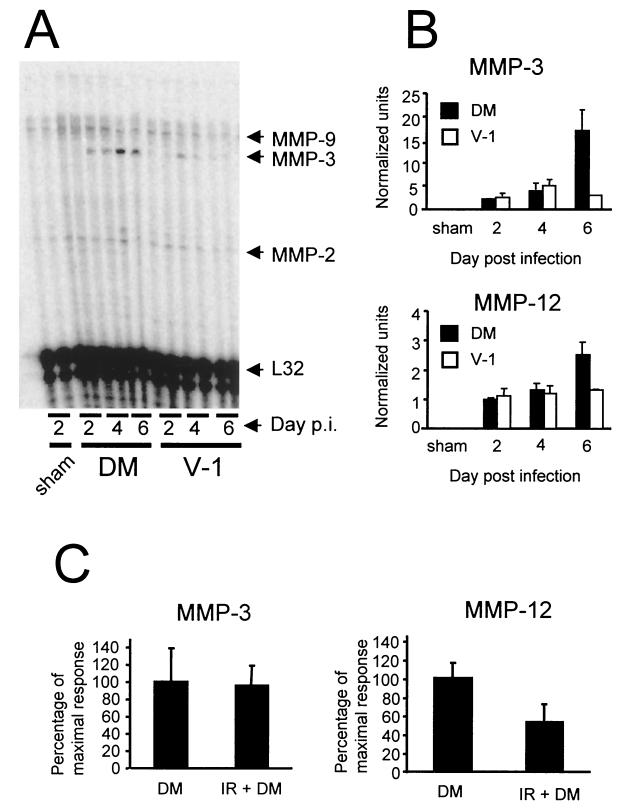
To determine correlations of MMP and TIMP mRNA expression with viral virulence and inflammation, RNA was isolated from brains of mice infected with both the lethal and sublethal JHMV variants and analyzed by RPA. The mRNAs encoding MMP-2, -9, -11, and -14 were expressed constitutively in the CNSs of BALB/c mice, with no apparent differences among naive, sham-infected, and infected mice (Fig. 4 and data not shown). Although constitutively expressed, MMP-14 (also referred to as membrane type 1 MMP) mRNA was expressed at a higher level than MMP-2, -9, or -11 mRNA, possibly due to its role as an activator of MMP proenzymes (8, 43). By contrast, expression of MMP-3 mRNA, which was not detectable within the CNS of sham-infected mice, was rapidly induced following both infections (Fig. 4A and B). MMP-3 mRNA expression was detected as early as day 2 p.i. and increased throughout the course of the lethal infection, peaking at day 6 p.i., the last time point examined. In sublethally infected mice MMP-3 mRNA was transiently expressed, peaking at day 4 p.i. at approximately 30% of the level detected during lethal infection. MMP-3 mRNA subsequently declined to undetectable levels by day 12 p.i. following sublethal infection (data not shown). The decline in MMP-3 mRNA correlated with clearance of infectious virus from the CNS. Similar to that of MMP-3 mRNA, MMP-12 mRNA expression was not detected within the CNS of sham-infected mice but rapidly increased following both infections (Fig. 4B). During lethal infection, MMP-12 mRNA expression was initially detected at day 2 p.i. and increased throughout infection, peaking at almost twice the level of expression observed in mice undergoing a sublethal infection at day 6 p.i. (Fig. 4B). In contrast to MMP-3 expression, MMP-12 mRNA expression in the CNS of sublethally infected mice remained relatively unchanged to day 30 p.i. (data not shown), well after clearance of infectious virus. Therefore, these data suggest that MMP-3 and MMP-12 mRNAs are induced following JHMV infection and that the level of expression correlates with the severity of infection.
FIG. 4.
MMP mRNA expression following JHMV infection. RNA prepared from brains of sham-, sublethally (V-1), and lethally (DM) infected mice was hybridized with probes specific for MMP-2, -3, -7, and -9 and for MMP-10, -11, -12, -13, and -14. (A) RNA hybridized with probes for MMP-2, -3, -7, and -9. Each lane represents an individual mouse. MMP-3 and MMP-12 mRNA expression was measured in brains of lethally and sublethally infected mice (B) and lethally infected immunocompetent and immunosuppressed (irradiated [IR]) mice (C). MMP mRNA expression was normalized to L32 mRNA (B), and the mean expression of the highest relative values was set to 100% (C). (B) Data were collected from two individual mice for each data point and are representative of those from three individual experiments. (C) Data were collected at 4 days p.i. from three mice per group and are representative of those from three separate experiments. Error bars represent the range of values for duplicate samples (B) or standard deviations from the mean (C).
To determine the contribution of CNS-resident cells to MMP expression following viral infection, mice were immunosuppressed by irradiation prior to infection. Irradiation resulted in a ≥95% reduction in CNS CD45hi inflammatory cells at day 4 p.i. (data not shown). Preliminary experiments demonstrated a ≤1-log-unit difference in virus replication in infected irradiated mice compared to controls at day 4 p.i. (6.2 ± 0.2 versus 5.7 ± 0.2 PFU/g of brain [log10], respectively). The mRNA levels of MMP-2, -9, -11, and -14, all expressed in naive and sham-infected mice, remained unchanged in immunosuppressed mice at day 4 p.i. (data not shown), suggesting that these genes are constitutively expressed by CNS-resident and/or vascular endothelial cells. Furthermore, neither MMP-3 nor MMP-12 mRNA was detected in naive or sham-infected immunosuppressed mice, suggesting that these genes were not induced by irradiation (data not shown). MMP-3 mRNA increased to nearly identical levels in both immunocompetent and immunosuppressed mice following infection (Fig. 4C), suggesting that MMP-3 mRNA was derived primarily from CNS-resident cells. By contrast, immunosuppression inhibited MMP-12 mRNA expression by approximately 50% (P < 0.05) (Fig. 4C). The decline in MMP-12 mRNA suggests either that MMP-12 was produced by both infiltrating inflammatory and CNS-resident cells or that uncontrolled virus replication in immunocompromised mice down-regulated MMP-12 mRNA expression by CNS-resident cells. Nevertheless, these data indicate that CNS-resident cells are responsible for the majority of inducible MMP mRNA expression following virus infection.
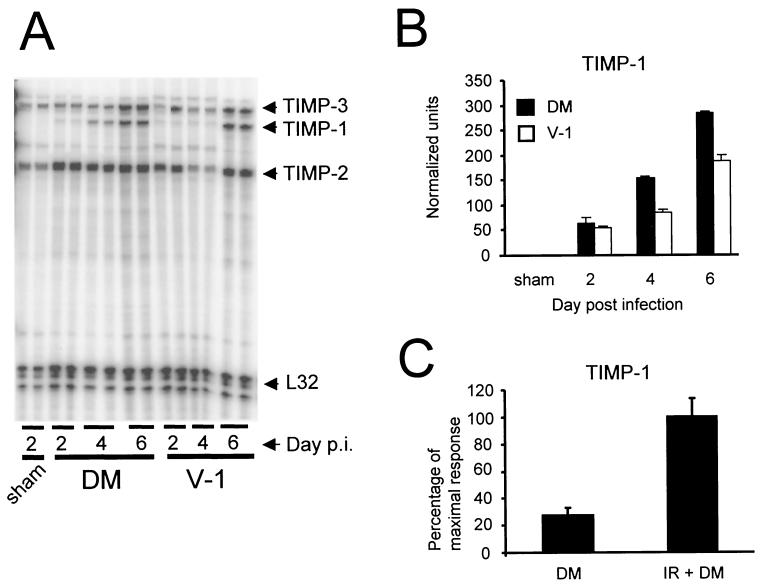
TIMPs are specific inhibitors of MMP activity, and induction of MMP expression is often associated with a coordinate induction of TIMP-1 mRNA expression. By contrast, expression of TIMP-2 and TIMP-3 mRNAs is less responsive to increases in MMP expression (14, 45). To determine whether TIMP gene expression correlated with viral replication and/or inflammation, RNAs from the brains of naive, sham-infected, and infected mice were examined by RPA. TIMP-2 and -3 mRNAs were expressed constitutively in the CNSs of naive or sham-infected mice, and expression was not altered by infection with either variant (Fig. 5A). By contrast, TIMP-1 mRNA, which was not detectable in the CNS of naive and sham-infected mice, was rapidly up-regulated following JHMV infection (Fig. 5A and B and data not shown). TIMP-1 mRNA was detected at days 2, 4, and 6 p.i. following both infections. Similar to induction of MMP-3 and -12 mRNA expression, TIMP-1 mRNA induction was higher during lethal infection than during sublethal infection (Fig. 5B). TIMP-1 mRNA was expressed transiently following sublethal infection, peaking at day 6 p.i. and returning to undetectable levels by day 12 p.i., similar to MMP-3 mRNA (Fig. 5B and data not shown).
FIG. 5.
TIMP mRNA expression following JHMV infection. (A) RNA from brains of sham-, sublethally (V-1), and lethally (DM) infected mice was hybridized with probes specific for TIMP-1, -2, and -3. Each lane represents an individual mouse. TIMP-1 mRNA expression was measured in brains of lethally and sublethally infected mice (B) and lethally infected immunocompetent and immunosuppressed (irradiated [IR]) mice (C). TIMP-1 mRNA expression was normalized to L32 mRNA (B), and the mean expression of the highest relative values was set to 100% (C). (B) Data were collected from two individual mice for each data point and are representative of those from three individual experiments. (C) Data were collected at 4 days p.i. from three mice per group and are representative of those from three separate experiments. Error bars represent the range of values for duplicate samples (B) or standard deviations from the mean (C).
To determine whether TIMP-1 mRNA was expressed primarily by CNS-resident or infiltrating cells, TIMP mRNA expression in immunosuppressed and immunocompetent mice was compared at day 4 p.i. following lethal infection. No differences in TIMP-2 or -3 expression were detected in immunosuppressed and immunocompetent mice, nor was TIMP-1 mRNA detected following sham infection of immunosuppressed mice (data not shown). TIMP-1 mRNA expression increased over threefold in immunosuppressed mice compared to immunocompetent mice following infection (Fig. 5C) (P < 0.05), which may reflect uncontrolled virus replication in the immunosuppressed host (data not shown). These data suggest that MMP-3, MMP-12, and TIMP-1 transcripts detected within the CNS following JHMV infection are produced primarily by CNS-resident cells and that expression reflects the severity of infection in contrast to the number of CNS inflammatory cells.
Induction of chemokine expression following JHMV infection.
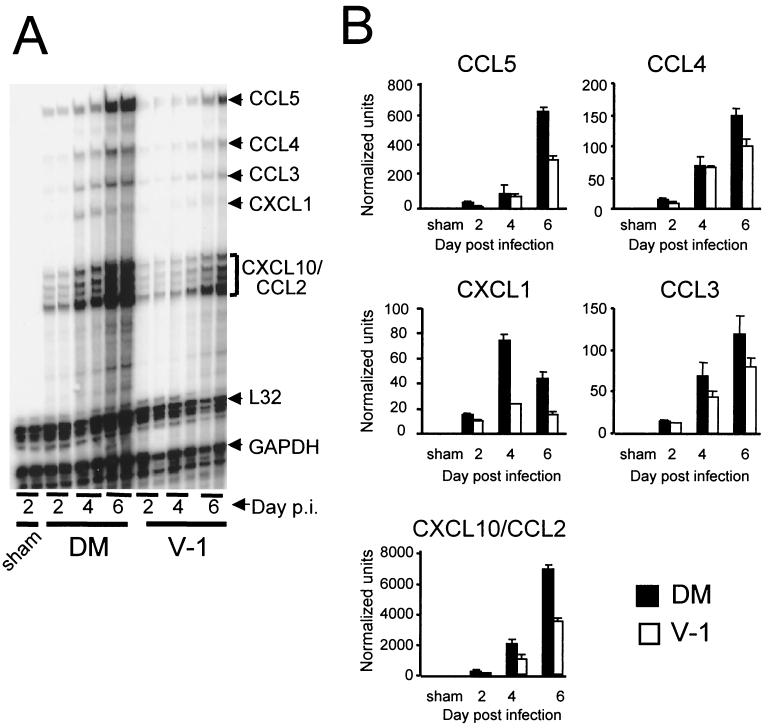
To determine whether induction of other inflammatory proteins also corresponds to viral virulence, chemokine mRNA expression in response to infection with the two JHMV variants was compared, as these genes are also rapidly induced in the CNS following MHV infection (24). No chemokine mRNA were detected in sham-infected mice, whereas chemokine mRNA levels in the CNS of mice infected with both JHMV variants showed relatively similar patterns of expression and kinetics (Fig. 6A). CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CXCL1 (MIP-2), and CXCL10 (IP-10) mRNAs were all detected as early as day 2 p.i. (Fig. 6B). The CCL2 and CXCL10 results were combined because these chemokines are not differentiated from one another by the RPA probe set in BALB/c mice (15). The mRNA levels of most chemokines continued to increase throughout acute infection and peaked at day 6 p.i. The exception was CXCL1 mRNA, which peaked at day 4 p.i. and then declined. Although chemokine mRNA declined between days 6 and 12 p.i. in sublethally infected mice, most chemokine transcripts remained detectable at day 12 p.i. (data not shown). Similar to MMP-3, MMP-12, and TIMP-1 mRNA expression induced following JHMV infection, peak chemokine mRNA expression was from approximately 20% (for CCL3) to 200% (for CXCL1) higher in the CNS of mice infected with the lethal variant than in those of mice infected with the sublethal variant. Chemokine mRNA expression, with the exception of CXCL1, correlated with that of MMP-3, MMP-12, and TIMP-1 mRNAs.
FIG. 6.
Chemokine mRNA expression following JHMV infection. (A) RNA prepared from brains of sham-, sublethally (V-1), and lethally (DM) infected mice were hybridized with chemokine-specific probes. Each lane represents an individual mouse. (B) Chemokine expression in brains of lethally and sublethally infected mice. Chemokine mRNA expression was normalized to L32 mRNA levels. Data were collected from two individual mice for each data point and are representative of those from three individual experiments. Error bars represent the range of values for duplicate samples.
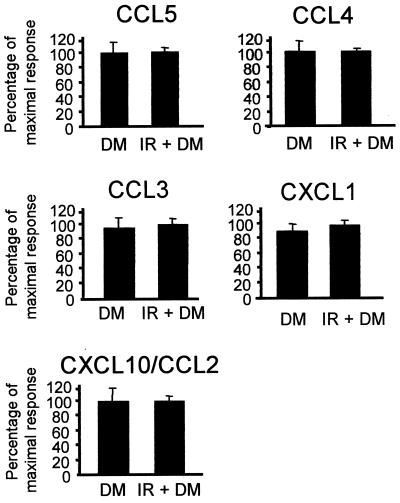
To determine whether CNS-resident or infiltrating cells were a primary source of chemokine mRNA during lethal JHMV infection, chemokine mRNA expression in immunosuppressed and immunocompetent mice was compared at day 4 p.i. No differences were detected when immunosuppressed and immunocompetent mice were compared for expression of any of the chemokines induced by JHMV infection, which included CCL2, CCL3, CCL4, CCL5, CXCL1, and CXCL10 (Fig. 7) (all P values are >0.4). Similar to the case for MMP and TIMP expression, these data suggest that infiltration by peripheral inflammatory cells is not required for chemokine expression during early stages of infection; rather, expression is dependent on the severity of infection.
FIG. 7.
Chemokine expression in infected immunosuppressed mice. RNA was prepared from brains of individual immunosuppressed (irradiated [IR]) and immunocompetent mice infected with DM virus at day 4 p.i. and hybridized with chemokine-specific RPA probes. Chemokine mRNA expression was normalized to L32 mRNA levels, and the mean expression of the highest relative values was set to 100%. Error bars represent standard deviations from the means for at least three mice per group. Data are representative of those from two separate experiments.
MMP-9 expression by infiltrating cells.
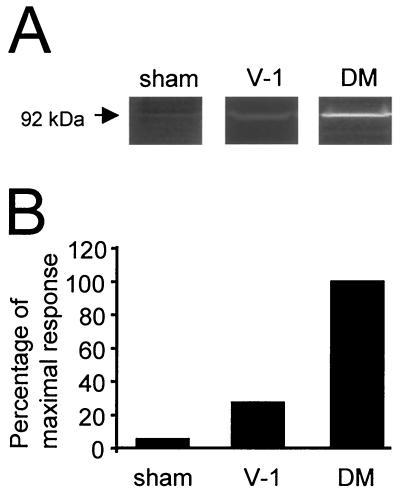
The mRNA levels of MMP-2 and -9, which are associated with activated inflammatory cells, were not altered during JHMV infection. To determine whether CNS infection altered expression at the posttranscriptional level, MMP-2 and -9 enzymatic activities were assessed. No activity was detected in homogenates prepared directly from the brains of naive, sham-infected, or JHMV-infected mice (data not shown). However, lysates prepared from CNS-derived cells isolated at day 4 p.i. exhibited MMP-9 (Fig. 8A) but not MMP-2 (data not shown) enzymatic activity. Consistent with increased recruitment of inflammatory cells, MMP-9 activity was threefold higher in lysates of cells derived from lethally infected mice than in the identical population derived following sublethal infection (Fig. 8B). MMP-9 activity was near the limit of detection in CNS cells derived from sham-infected mice (Fig. 8B). These data indicate that in contrast MMP-3, MMP-12, and TIMP-1 mRNA expression, which is associated primarily with CNS-resident cells, MMP-9 protein activity is associated with inflammatory cells.
FIG. 8.
Increased MMP-9 enzymatic activity following infection. Lysates were prepared from 2 × 105 cells isolated from the CNS of sham-, sublethally (V-1), and lethally (DM) infected mice at day 4 p.i. (A) Gelatinase activity following electrophoretic separation of cell lysates. The 92-kDa band representing pro-MMP-9 is shown. (B) Relative enzymatic activity. The gelatinase activity of lysates prepared from cells isolated from DM-infected mice was adjusted to 100% for comparison. Data are representative of those from two separate experiments.
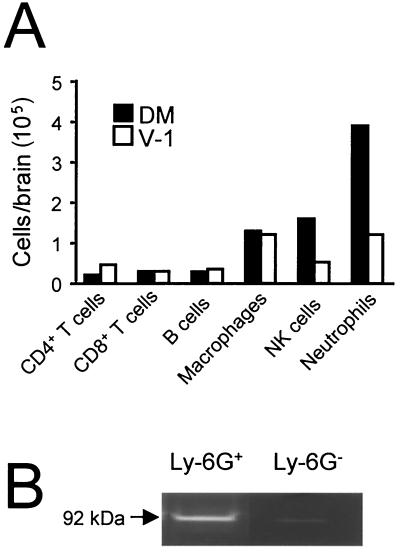
To determine whether the increase in MMP-9 activity detected during lethal infection compared to sublethal infection was attributable to a specific cell type, the composition of the CD45hi CNS-infiltrating cells were characterized at day 4 p.i. No distinct differences were observed in CD19+ B cells, CD4+ T cells, or CD8+ T cells, each of which accounted for ≤10% of the infiltrating population in comparisons of lethally and sublethally infected mice (Fig. 9A). Total cell numbers of CD45hi F4/80+ macrophages were nearly equivalent during both infections (Fig. 9A). By contrast, lethal infection induced approximately a threefold increase in both neutrophil and NK cell infiltration into the CNS compared to sublethal infection. These differences in recruitment suggested that these innate inflammatory cells could be potential sources of MMP-9. However, neutrophils store MMP-9 within secretory granules (23). To determine whether the MMP-9 protein was restricted to neutrophils, cells were isolated from the inflamed CNS at day 4 p.i. A purified neutrophil population was prepared by sorting Ly-6G+ cells. Purity of the Ly-6G+ population was verified by the distinctive nuclear morphology associated with polymorphonuclear granulocytes. Gelatinase activity consistent with the 92-kDa monomeric form of pro-MMP-9 was detected in lysates prepared from purified CNS-derived neutrophils (Fig. 9B). Little or no enzymatic activity was detected in lysates prepared from the neutrophil-depleted (Ly-6G−) population (Fig. 9B). No MMP-2 activity was detected from either population (data not shown). These data indicate that mature neutrophils, as defined by surface expression of the Ly-6G marker and distinctive multilobed nuclear morphology, are a primary source of MMP-9 within the CNS during acute virus-induced encephalitis.
FIG. 9.
MMP-9 activity is associated with neutrophils. (A) Frequencies of CD4+ T cells, CD8+ T cells, CD19+ B cells, macrophages (F4/80+), NK cells (pan-NK+), and neutrophils (Ly-6Ghi, CD11b+) within the CD45hi inflammatory cell population isolated from brains of infected mice at day 4 p.i. Data were collected from cells pooled from three or four mice per group and are representative of those from two separate experiments. (B) MMP-9 activity in purified neutrophils isolated from the infected CNS at day 4 p.i. Neutrophils were obtained by cell sorting after staining with MAb Ly-6G. Lysates prepared from both the Ly-6G + and Ly-6G − populations were analyzed for MMP-9 enzymatic activity by zymography.
DISCUSSION
In contrast to the case for chemokines and cytokines, the roles of MMPs and their inhibitors during the inflammatory processes have not been well defined. To investigate the regulation of these molecules during CNS infection, two JHMV variants associated with different clinical and pathological outcomes were analyzed for their ability to influence MMP and TIMP mRNA expression. DM virus, which induces a lethal infection in C57BL/6 mice (37, 46), replicated to higher levels in the CNS than the V-1 virus, which induces a sublethal infection (10, 37). Lethal infection of BALB/c mice was associated with a more vigorous immune response, as demonstrated by the number of CNS inflammatory cells during early stages of infection, than the sublethal infection. Kinetic analysis of MMP and TIMP mRNA expression within the CNS revealed that both viruses induced similar patterns of gene expression, including induction of MMP-3, MMP-12, and TIMP-1 mRNA expression. However, increased levels of all three mRNAs were detected during lethal infection, and peak expression of MMP-3, MMP-12, and TIMP-1 correlated with viral load. Analysis of infected immunocompromised mice indicated that MMP-3, MMP-12, and TIMP-1 induction was only minimally dependent upon infiltrating inflammatory cells, confirming the relationship between virus load and gene expression. The proinflammatory cytokines interleukin-1α, interleukin-1β, and tumor necrosis factor alpha, which are up-regulated during JHMV infection of both immunocompetent and immunosuppressed mice (37, 39), are likely mediators of MMP induction (7, 18). These cytokines are produced by CNS-resident cells (29) and are potent inducers of MMP-3 expression (7). By contrast, beta interferon, which is also up-regulated at the mRNA level following neurotropic MHV infection (52), has been associated with up-regulation of TIMP-1 expression in CNS lesions of multiple sclerosis patients (34). Thus, it is likely that uncontrolled virus replication in immunocompromised mice induced increased beta interferon, which in turn induced the additional up-regulation of TIMP-1 observed in these mice compared to controls. These data suggest that resident CNS cells may play a significant role in mediating CNS permeability via increased MMP expression in response to virus-induced cytokines.
The gelatinases MMP-2 and -9 are produced by most inflammatory cells and catalyze the breakdown of proteins which make up the basal laminae surrounding epithelial linings (reviewed in reference 33). Thus, these gelatinases are involved in mediating blood vessel permeability during inflammatory cell extravasation (42). Interestingly, mRNA levels for these gelatinases remained unchanged following JHMV infection. However, analysis of enzymatic activity within lysates prepared from CNS-derived cells demonstrated that MMP-9 protein, but not MMP-2 protein, was increased following JHMV infection, and the level of protein expression correlated with lethality. Analysis of the infiltrating populations revealed that during lethal infection a higher frequency of infiltrating neutrophils and NK cells were recruited into the CNS during sublethal infection. Neutrophils produce MMP-9 in the absence of MMP-2 whereas mononuclear cells produce both MMP-2 and MMP-9 (32). Analysis of neutrophils purified from the infected CNS confirmed that neutrophils are the primary source of MMP-9 activity detected during JHMV infection. MMP-9 protein induction was not associated with an increase in MMP-9 mRNA levels (Fig. 8 and 4, respectively). These discordant results may be attributed to the observation that neutrophils prepackage MMP-9 in granules prior to activation (32). By contrast, MMPs synthesized by mononuclear inflammatory cells and tissue-resident cells are secreted immediately following translation (32). Thus, MMP-9 protein is unique among the MMPs examined in that it may be present without detectable de novo synthesis during inflammatory responses involving neutrophils.
The relationship between enhanced mRNA expression from inflammatory genes and increased virulence was confirmed by examining the relative induction of a second family of inflammatory proteins, the chemokines. Similar to MMP and TIMP mRNA expression, chemokine mRNA expression was also enhanced during lethal infection compared to sublethal infection. Similar correlations between chemokine induction and virulence have been reported following CNS infection with Sindbis virus (20). A difference in the kinetics of chemokine induction during lethal and sublethal JHMV infection was detected for expression of CXCL1, a potent neutrophil chemoattractant (4). CXCL1 expression peaked at a higher level (>3-fold) during lethal infection than during sublethal infection, suggesting that CXCL1 may play a significant role in recruiting neutrophils into the CNS during lethal infection.
Analysis of MMP and TIMP mRNA expression during JHMV infection indicated that three genes, those for MMP-3, MMP-12, and TIMP-1 were specifically up-regulated within CNS-resident cells, whereas MMP-9 activity was associated with the inflammatory infiltrate. These results are similar to two CD4+ T-cell models of CNS inflammatory diseases. During experimental autoimmune encephalitis, expression of mRNAs for MMP-2, -3, -9, -12, and -14 is up-regulated (36), whereas MMP-1, -2, -3, -7, and -9 have been associated with CNS lesions of multiple sclerosis (2, 9, 28). Although induction of fewer types of MMPs was detected during JHMV infection, these data suggest that specific MMPs, particularly MMP-3 and MMP-9, may play prominent roles in the pathogenesis of these three distinct CNS inflammatory diseases. In contrast to JHMV, which infects primarily glial cells (10), canine distemper virus infects primarily neurons (6) and is associated with expression of MMP-14 and TIMP-1 mRNAs as well as decreased levels of MMP-9 mRNA, whereas MMP-3 and MMP-7 expression is unaffected by canine distemper virus (22). These data suggest that distinct patterns of MMP expression may be linked to cellular tropisms and/or the composition of the inflammatory processes occurring within the CNS.
In conclusion, increased virus replication during lethal infection was associated with increased levels of MMP, TIMP, and chemokine gene expression compared to sublethal infection. Whether specific genes are up-regulated as a direct result of viral infection or as result of chemokine- or cytokine-mediated signaling remains to be determined. However, these data indicate that increased inflammatory gene expression by CNS cells is predominantly due to uncontrolled viral replication, as opposed to elevated numbers of inflammatory infiltrates responding to infection.
Acknowledgments
We thank Wen-Qiang and Ernesto Barron for excellent technical assistance, Laurie LaBree for assistance with statistical analysis, and Sonia Q. Garcia for manuscript preparation. We also thank Wendy Gilmore for critical reading of the manuscript.
This work was supported by NIH grants NS 18146 and EY03040.
REFERENCES
- 1.Abbott, N. J. 2000. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 20:131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, D. C., B. Ferguson, M. K. Matyzak, K. M. Miller, M. M. Esiri, and V. H. Perry. 1997. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol. Appl. Neurobiol. 23:406-415. [PubMed] [Google Scholar]
- 3.Anthony, D. C., K. M. Miller, S. Fearn, M. J. Townsend, G. Opdenakker, G. M. Wells, J. M. Clements, S. Chandler, A. J. Gearing, and V. H. Perry. 1998. Matrix metalloproteinase expression in an experimentally-induced DTH model of multiple sclerosis in the rat CNS. J. Neuroimmunol. 87:62-72. [DOI] [PubMed] [Google Scholar]
- 4.Bell, M. D., D. D. Taub, S. J. Kunkel, R. M. Strieter, R. Foley, J. Gauldie, and V. H. Perry. 1996. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur. J. Neurosci. 8:1803-1811. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. [PubMed] [Google Scholar]
- 6.Bernard, A., M. Fevre-Montange, A. Bencsik, P. Giraudon, T. F. Wild, C. Confavreux, and M. F. Belin. 1993. Brain structures selectively targeted by canine distemper virus in a mouse model infection. J. Neuropathol. Exp. Neurol. 52:471-480. [DOI] [PubMed] [Google Scholar]
- 7.Bond, M., A. H. Baker, and A. C. Newby. 1999. Nuclear factor κB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem. Biophys. Res. Commun. 264:561-567. [DOI] [PubMed] [Google Scholar]
- 8.Cao, J., H. Sato, T. Takino, and M. Seiki. 1995. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J. Biol. Chem. 270:801-805. [DOI] [PubMed] [Google Scholar]
- 9.Cossins, J. A., J. M. Clements, J. Ford, K. M. Miller, R. Pigott, W. Vos, P. Van der Valk, and C. J. De Groot. 1997. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 94:590-598. [DOI] [PubMed] [Google Scholar]
- 10.Fleming, J. O., M. D. Trousdale, F. A. el-Zaatari, S. A. Stohlman, and L. Weiner. 1986. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 58:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford, A. L., A. L. Goodsall, W. F. Hickey, and J. D. Sedgwick. 1995. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 154:4309-4321. [PubMed] [Google Scholar]
- 12.Ghorpade, A., R. Persidskaia, R. Suryadevara, M. Che, X. J. Liu, Y. Persidsky, and H. E. Gendelman. 2001. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 75:6572-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giraudon, P., R. Szymocha, S. Buart, A. Bernard, L. Cartier, M. F. Belin, and H. Akaoka. 2000. T lymphocytes activated by persistent viral infection differentially modify the expression of metalloproteinases and their endogenous inhibitors, TIMPs, in human astrocytes: relevance to HTLV-I-induced neurological disease. J. Immunol. 164:2718-2727. [DOI] [PubMed] [Google Scholar]
- 14.Gomez, D. E., D. F. Alonso, H. Yoshiji, and U. P. Thorgeirsson. 1997. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 74:111-122. [PubMed] [Google Scholar]
- 15.Hallensleben, W., L. Biro, C. Sauder, J. Hausmann, V. C. Asensio, I. L. Campbell, and P. Staeheli. 2000. A polymorphism in the mouse crg-2/IP-10 gene complicates chemokine gene expression analysis using a commercial ribonuclease protection assay. J. Immunol. Methods 234:149-151. [DOI] [PubMed] [Google Scholar]
- 16.Hartung, H. P., and B. C. Kieseier. 2000. The role of matrix metalloproteinases in autoimmune damage to the central and peripheral nervous system. J. Neuroimmunol. 107:140-147. [DOI] [PubMed] [Google Scholar]
- 17.Hickey, W. F. 1999. Leukocyte traffic in the central nervous system: the participants and their roles. Semin. Immunol. 11:125-137. [DOI] [PubMed] [Google Scholar]
- 18.Horton, W. E., Jr., I. Udo, P. Precht, R. Balakir, and K. Hasty. 1998. Cytokine inducible matrix metalloproteinase expression in immortalized rat chondrocytes is independent of nitric oxide stimulation. In Vitro Cell Dev. Biol. Anim. 34:378-384. [DOI] [PubMed] [Google Scholar]
- 19.Ishiguro, N., T. Ito, T. Oguchi, T. Kojima, H. Iwata, M. Ionescu, and A. R. Poole. 2001. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 44:2503-2511. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, C., W. Jiang, T. Chu, and B. Levine. 2001. Identification of genes involved in the host response to neurovirulent alphavirus infection. J. Virol. 75:10431-10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston, J. B., Y. Jiang, G. van Marle, M. B. Mayne, W. Ni, J. Holden, J. C. McArthur, and C. Power. 2000. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. J. Virol. 74:7211-7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khuth, S. T., H. Akaoka, A. Pagenstecher, O. Verlaeten, M. F. Belin, P. Giraudon, and A. Bernard. 2001. Morbillivirus infection of the mouse central nervous system induces region-specific upregulation of MMPs and TIMPs correlated to inflammatory cytokine expression. J. Virol. 75:8268-8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjeldsen, L., H. Sengelov, K. Lollike, M. H. Nielsen, and N. Borregaard. 1994. Isolation and characterization of gelatinase granules from human neutrophils. Blood 83:1640-1649. [PubMed] [Google Scholar]
- 24.Lane, T. E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160:970-978. [PubMed] [Google Scholar]
- 25.Lemjabbar, H., P. Gosset, C. Lamblin, I. Tillie, D. Hartmann, B. Wallaert, A. B. Tonnel, and C. Lafuma. 1999. Contribution of 92 kDa gelatinase/type IV collagenase in bronchial inflammation during status asthmaticus. Am. J. Respir. Crit. Care Med. 159:1298-1307. [DOI] [PubMed] [Google Scholar]
- 26.Lichtinghagen, R., T. Seifert, A. Kracke, S. Marckmann, U. Wurster, and F. Heidenreich. 1999. Expression of matrix metalloproteinase-9 and its inhibitors in mononuclear blood cells of patients with multiple sclerosis. J. Neuroimmunol. 99:19-26. [DOI] [PubMed] [Google Scholar]
- 27.Lin, M. T., S. A. Stohlman, and D. R. Hinton. 1997. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda, A., and R. A. Sobel. 1996. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 55:300-309. [DOI] [PubMed] [Google Scholar]
- 29.Malipiero, U. V., K. Frei, and A. Fontana. 1990. Production of hemopoietic colony-stimulating factors by astrocytes. J. Immunol. 144:3816-3821. [PubMed] [Google Scholar]
- 30.Marten, N. W., S. A. Stohlman, R. D. Atkinson, D. R. Hinton, J. O. Fleming, and C. C. Bergmann. 2000. Contributions of CD8+ T cells and viral spread to demyelinating disease. J. Immunol. 164:4080-4088. [DOI] [PubMed] [Google Scholar]
- 31.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2001. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 32.Opdenakker, G., P. E. Van den Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. 2001. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 69:851-859. [PubMed] [Google Scholar]
- 33.Owen, C. A., and E. J. Campbell. 1999. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 65:137-150. [DOI] [PubMed] [Google Scholar]
- 34.Ozenci, V., M. Kouwenhoven, N. Teleshova, M. Pashenkov, S. Fredrikson, and H. Link. 2000. Multiple sclerosis: pro- and anti-inflammatory cytokines and metalloproteinases are affected differentially by treatment with IFN-beta. J. Neuroimmunol. 108:236-243. [DOI] [PubMed] [Google Scholar]
- 35.Pagenstecher, A., A. K. Stalder, and I. L. Campbell. 1997. RNAse protection assays for the simultaneous and semiquantitative analysis of multiple murine matrix metalloproteinase (MMP) and MMP inhibitor mRNAs. J. Immunol. Methods 206:1-9. [DOI] [PubMed] [Google Scholar]
- 36.Pagenstecher, A., A. K. Stalder, C. L. Kincaid, S. D. Shapiro, and I. Campbell. 1998. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am. J. Pathol. 152:729-741. [PMC free article] [PubMed] [Google Scholar]
- 37.Parra, B., D. R. Hinton, M. T. Lin, D. J. Cua, and S. A. Stohlman. 1997. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology 233:260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 39.Pearce, B. D., M. V. Hobbs, T. S. McGraw, and M. J. Buchmeier. 1994. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 68:5483-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlman, S., T. E. Lane, and M. J. Buchmeier. 2000. Coronaviruses: hepatitis, peritonitis, and central nervous system disease, p. 331-348. In M. W. Cunningham and R. S. Fujinami (ed.), Effects of microbes on the immune system. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 41.Reese, T. S., N. Feder, and M. W. Brightman. 1971. Electron microscopic study of the blood-brain and blood-cerebrospinal fluid barriers with microperoxidase. J. Neuropathol. Exp. Neurol. 30:137-138. [PubMed] [Google Scholar]
- 42.Rosenberg, G. A., M. Kornfeld, E. Estrada, R. O. Kelley, L. A. Liotta, and W. G. Stetler-Stevenson. 1992. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res. 576:203-207. [DOI] [PubMed] [Google Scholar]
- 43.Sato, H., T. Takino, Y. Okada, J. Cao, A. Shinagawa, E. Yamamoto, and M. Seiki. 1994. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370:61-65. [DOI] [PubMed] [Google Scholar]
- 44.Sedgwick, J. D., A. L. Ford, E. Foulcher, and R. Airriess. 1998. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J. Immunol. 160:5320-5330. [PubMed] [Google Scholar]
- 45.Shapiro, S. D., D. K. Kobayashi, and H. G. Welgus. 1992. Identification of TIMP-2 in human alveolar macrophages. Regulation of biosynthesis is opposite to that of metalloproteinases and TIMP-1. J. Biol. Chem. 267:13890-13894. [PubMed] [Google Scholar]
- 46.Stohlman, S. A., P. R. Brayton, J. O. Fleming, L. P. Weiner, and M. M. C. Lai. 1982. Murine coronaviruses: isolation and characterization of two plaque morphology variants of the JHM neurotropic strain. J. Gen. Virol. 63:265-275. [DOI] [PubMed] [Google Scholar]
- 47.Stohlman, S. A., C. Bergmann, D. Cua, H. Wege, and R. van der Veen. 1994. Location of antibody epitopes within the mouse hepatitis virus nucleocapsid protein. Virology 202:146-153. [DOI] [PubMed] [Google Scholar]
- 48.Stohlman, S. A., C. C. Bergmann, R. C. van der Veen, and D. R. Hinton. 1995. Mouse hepatitis virus-specific cytotoxic T lymphocytes protect from lethal infection without eliminating virus from the central nervous system. J. Virol. 69:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stohlman, S. A., M. Lin, B. Parra, C. C. Bergmann, and D. R. Hinton. 1997. Immune regulation of coronavirus-induced demyelinating encephalomyelitis. J. Neurovirol. 3(Suppl. 1):S56-S57. [PubMed] [Google Scholar]
- 50.Toschi, E., G. Barillari, C. Sgadari, I. Bacigalupo, A. Cereseto, D. Carlei, C. Palladino, C. Zietz, P. Leone, M. Sturzl, S. Butto, A. Cafaro, P. Monini, and B. Ensoli. 2001. Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol. Biol. Cell 12:2934-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, F. I., S. A. Stohlman, and J. O. Fleming. 1990. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J. Neuroimmunol. 30:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, Q., J. A. Haluskey, and E. Lavi. 1998. Coronavirus MHV-A59 causes upregulation of interferon-beta RNA in primary glial cell cultures. Adv. Exp. Med. Biol. 440:451-454. [DOI] [PubMed] [Google Scholar]