Abstract
Adult male rats were fed a low or high fat diet and given psychosocial stress (crowded and unstable housing with daily predator exposure) for 3 weeks. Neither stress nor high fat diet, alone, produced dendritic atrophy; only the group given the combination of stress and high fat diet developed a reduction of the length and number of branch points of apical dendrites of CA3 neurons. These findings indicate that a synergy between high fat diet and stress caused a retraction of CA3 dendrites. The findings are consistent with work on peripheral (e.g., cardiovascular) systems demonstrating a synergy between stress and high fat diet, and are relevant toward understanding how diet and stress interact to adversely a¡ectbrain and memory processing.
Keywords: Atrophy, Brain, CA3, High fat diet, Hippocampus, Psychological stress, Synergy
INTRODUCTION
The effects of stress or diet on health have been well-studied, but there is an insufficient understanding of how these two factors, acting alone or in concert, influence the brain and behavior. Long-term production of stress hormones, such as glucocorticoids (GCs), can impair learning [1,2] and produce atrophy in the hippocampus [3], a brain structure which is important for learning and memory [4]. Under some conditions, however, chronic stress or GCs may have only a subtle or no effect on hippocampus-dependent memory [5,6] and morphology [5,7,8]. A separate line of research has shown that rats fed a high fat diet exhibit impaired learning [9] and increased GC responses to stress [10]. Here, as well, the literature is mixed, with studies revealing that under some conditions a high fat diet can fail to produce significant effects on physiology or memory [11–13]. Thus, the actions of either chronic stress or a high fat diet may be absent or nullified depending on environmental or genetic influences.
The combination of chronic stress and a high fat diet, by contrast, consistently produces adverse effects on behavior and physiological responses. Studies on animals have shown that the combination of chronic stress and a high fat diet produces synergistic effects on physiological measures, including elevating plasma catecholamine levels [14] and increasing stress-induced mortality and cardiovascular disorders [12,13]. Studies on people also indicate that a high fat diet or stress, in isolation, may be relatively benign [15–17], but the combination of a stressful life and a high fat diet exerts a powerful adverse effect on health and well-being [16,18].
The current work was designed to extend our understanding of how stress and diet interact to affect the brain. We studied the influence of psychosocial stress or a high fat diet separately, and in concert, on dendritic morphology of neurons in the hippocampus. Whereas most previous studies have focused on the effects of chronic restraint stress on the hippocampus in rats fed standard (low fat) animal chow, here we studied the effects of an ethologically relevant stressor (predator exposure and crowded, unstable housing) on rats fed a low or high fat diet. This approach provides an animal model with which to study how psychosocial stress interacts with diet to influence brain and behavior.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at the University of South Florida and are in accordance with the applicable portions of the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals by DHHS. Thirty-two male, Sprague–Dawley rats (Harlan, Indianapolis, IN; 300 g) were housed on a 12:12 h light: dark cycle (lights on at 06:00 h) with food and water provided ad lib in their housing room. The rats were given 1 week to acclimatize to the housing facilities and then they were randomly assigned to experimental groups (Low fat diet, LFD, and high fat diet, HFD; stress/LFD, stress/HFD, control (no stress)/LFD, control (no stress)/HFD; n=8 rats/group). Each day, for 3 weeks, all rats were removed from the housing room and transported to the laboratory. There, the stress subgroups was exposed, without physical contact, to a cat for 3–6 h at random times of the day within the light phase. When rats in the stress group were not with the cat, they were housed in crowded conditions composed of 2, 3 or 4 rats/cage in a relatively small space (20 × 20 cm). In addition, rats in the stress group were housed with a different combination of rats each day (unstable housing) in a manner similar to that described previously [6,19]. Rats in the control group were housed 2 or 3/cage with the same cagemates throughout the study in a standard Plexiglas cage (40 × 20 cm).
Control (non-stress) rats were transported from the housing room to the laboratory each day along with the stress group. The control rats, unlike the stress rats, remained in their home cages within an isolated laboratory room. The animals in all four groups were restricted from food and water during the laboratory manipulations.
Body weights were measured at the beginning (day 1) and end (day 21) of the 3-week stress period. During the stress period, rats were fed either a low or high fat diet. The percentages of calories from fat were 11.2% and 37.6% for the low and high fat diets, respectively. Soybean oil contributed the primary source of fat in the low fat diet, whereas coconut oil, beef tallow and anhydrous milk fat supplied the primary source of fat in the high fat diet.
On the day after the end of the 21-day stress period, the rats were injected with an overdose of sodium pentobarbital (100 mg/kg, i.p.) and perfused transcardially with phosphate-buffered saline and buffered 4% paraformaldehyde. The brains were subsequently removed and Golgi-stained as described previously [20]. Neurons were selected for measurement provided they were located in the stratum pyramidal lower of the CA3 or CA1 regions of the hippocampus and fully stained. In addition, CA3 neurons were included in the analysis only if they were located within the CA3c region of the dorsal hippocampus, had identifiable dendrites that could be distinguished from nearby neurons, and exhibited consistent staining throughout the extent of all dendrites. Between 2 and 7 neurons were utilized per rat with 4–5 rats per group. Rats were included in the analyses for the CA3 region with the stipulation that short shaft and long shaft neurons were represented proportionately [7,20]. The neurons were traced using a camera lucida drawing tube (Olympus, U-DA drawing attachment) connected to an Olympus microscope to quantify branch points (× 400). A branch point was counted when a dendrite exhibited a bifurcation or juncture whereby two distinct branches were detected. Total dendritic length was quantified using calibrated Scion Image software (Scion Corporation, Frederick, Maryland) linked to an Olympus microscope via a video camera.
RESULTS
Representative tracings from CA3 neurons for each of the four groups are depicted in Fig. 1. For CA3 apical dendritic length, a 2 × 2 ANOVA revealed a significant main effect of stress (F(1,15)=4.27, p=0.05). The main effect was carried primarily by the stress-HFD group, as indicated by the significant interaction between stress and diet (F(1,15)=6.59, p<0.05), and that the stress-HFD group exhibited significantly shorter apical dendrites than the control-HFD and stress-LFD groups (all p≤0.05; LSD post-hoc test; Fig. 2).
Fig. 1.
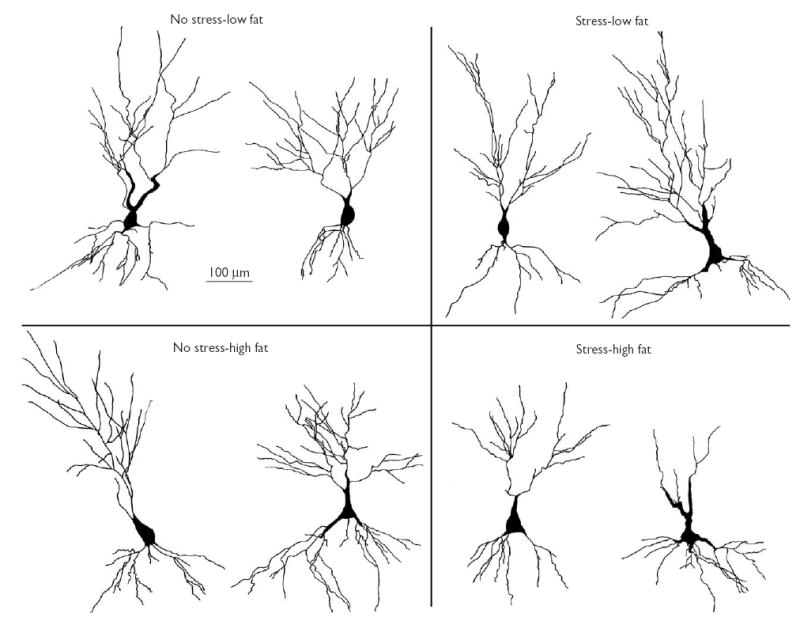
Representative neurons (2/group) from the CA3 region of the hippocampus for each experimental condition. The illustrations depict camera lucida tracings at × 400.
Fig. 2.
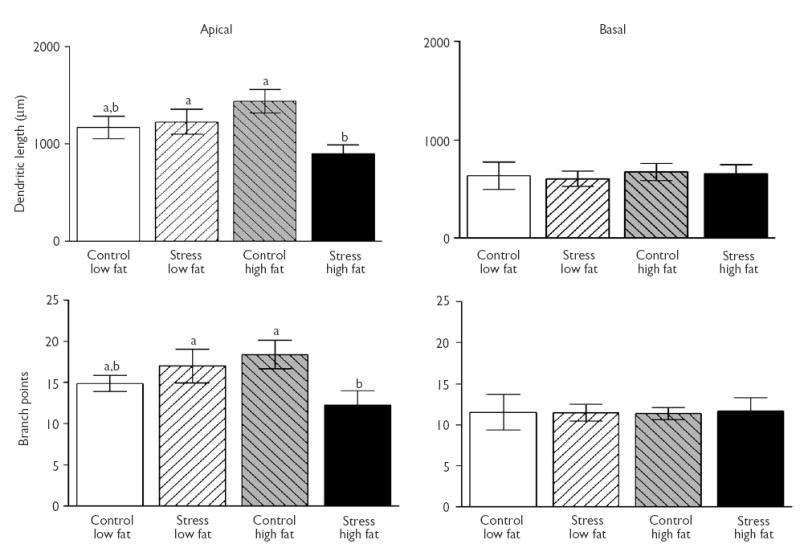
CA3 dendritic length and branch points following chronic exposure to stress and/or high fat diet. Left side: apical dendritic length (top) and branch points (bottom) are reduced in stressed rats fed a high fat diet compared to control (no stress) rats fed a high fat diet. Right side: basal dendritic length (top) and basal branch points (bottom) were not affected by a high fat diet, stress, or these two factors combined. Data in all figures represent means (±s.e.m.). Common letters represent non-significant differences across conditions and different letters represent significant differences across conditions.
The analysis for CA3 apical branch points showed a similar outcome as CA3 apical length, with a 2 × 2 ANOVA exhibiting a significant interaction between stress and diet (F(1,15)=5.56, p<0.05), without a significant main effect of stress or diet alone (p>0.1; Fig. 2). LSD post hoc tests showed that the stress-HFD group had significantly fewer apical branch points than the control-HFD group (p<0.05) and there was a marginally significant effect compared to the stress-LFD group (p=0.07). None of the groups differed statistically following a 2 × 2 ANOVA for either CA3 basal length or branch points (p>0.1; Fig. 2). There were no effects of diet, stress or the combination of both factors in dendritic length and branch points in the apical and basal arbors of CA1 neurons (data not shown; all p>0.1).
In the assessment of body weights, a 2 × 2 × 2 (stress × diet × day) mixed-factor ANOVA revealed that all groups gained weight by day 21 regardless of condition, as shown by a significant main effect of day (F(1,28)=290.33, p<0.01). Chronic stress significantly reduced body weight gain compared to control conditions, as indicated by a significant main effect of treatment (F(1,28)=8.98, p<0.01), and a significant interaction between stress and day (F(1,28)=42.58, p<0.01). Diet alone did not influence weight gain (F(1,28)=0.007, p=0.94, (Fig. 3)).
Fig. 3.
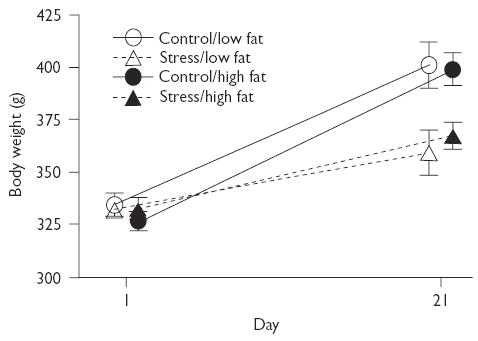
Body weights at the beginning and end of the 21-day chronic stress period. Stressed groups gained less weight than control groups regardless of diet, as indicated by the significant interaction between stress and diet (F(1,28)=42.58, p<0.01).Data points are shifted for better visibility.
DISCUSSION
The primary finding of this study is that the extent of dendritic arborization of neurons in the CA3 region of the hippocampus was influenced by the combination of high fat diet and chronic stress. Three weeks of either a high fat diet or psychosocial stress, alone, produced no effect on the dendritic arbors of neurons in the CA3 or CA1 regions. The novel finding was the synergistic interaction between high fat diet and stress, such that only the group that experienced both high fat diet and stress exhibited a significant reduction in both dendritic length and branching of apical dendrites of CA3 neurons.
The basis of the synergy between stress and high fat diet producing CA3 dendritic retraction requires more investigation, but the hypothesis that the high fat diet increased the perceived strength of the psychosocial stressor was not supported since stress attenuated weight gain to an equivalent extent in the high and low fat diet groups. A factor likely to have contributed to stress effects on CA3 morphology in the high fat diet group is an increased reactivity of the hypothalamic pituitary adrenal axis to stress, which is known to occur in animals given a high fat diet [10]. Hormones, such as GCs and epinephrine, interact with a high fat diet to exacerbate pathology in peripheral structures. For example, in rodents and non-human primates, the combination of chronic stress and a high fat diet produces the highest levels of plasma catecholamines [14] and the highest incidence of stress-induced mortality and cardiovascular disorders [12,13].
Whereas the combination of a high fat diet and stress consistently produces potent effects on physiology and behavior, the effects produced by either a high fat diet or stress, in isolation, can be nullified by salutary influences. For example, the effects of a high fat diet on the brain and peripheral organs can be blocked by a low stress environment or by exercise [15,21]. Along the same lines, the suppressive effects of intense stress on the immune system can be nullified by a low fat diet [16]. Our findings, therefore, are consistent with an extensive literature showing that the combination of chronic stress and a high fat diet exerts more deleterious effects on measures of health and well-being than either stress or high fat diet alone [18].
The absence of a significant effect of chronic stress on dendritic morphology of CA3 neurons in rats fed a low fat diet was unexpected, given that most of the literature has shown that chronic stress produced dendritic retraction in CA3 neurons in rats given standard (low fat) animal chow [3,7,20]. However, this is the first study in which predator exposure and crowding were used to induce morphological changes in CA3 dendritic arbors, whereas most studies have used restraint stress [7,20] or another form of psychosocial stress (dominance hierarchy; [22]). Chronic predator exposure has been shown to impair hippocampus-dependent learning [19], increase sensitivity to an adrenergic receptor antagonist [19], increase adrenal and thymus weights [23] and alter the endocrine response to an acute stressor [23,24]. Thus, although restraint and psychosocial stress are each effective stressors, they appear to produce dissimilar effects in the brain [24]. Despite their differences, it is notable that both types of stressors have shown that the CA3 region is more vulnerable than the CA1 region in developing stress-induced dendritic retraction [3,22].
CONCLUSION
We provide the first evidence of a synergy between high fat diet and stress on neuronal morphology. Chronic psychosocial stress or a high fat diet, separately, were ineffective at altering the dendritic morphology of CA3 neurons. Only the experimental group that was both fed a high fat diet and chronically stressed exhibited dendritic retraction in the CA3 region. These findings are relevant to naturalistic conditions in which stress increases the preference for individuals to select high, over low, fat food sources [25]. The drive toward high fat (and high sugar) foods in times of stress may be attributed to the enhanced survival value of increasing caloric intake at times of increased metabolic demands. Such a strategy may be useful as a short-term response to an acute stressor, but maintaining the pursuit of high fat foods as stress persists may become detrimental. Our work indicates that prolonged ingestion of a high fat diet in conjunction with chronic unavoidable stress produces detrimental consequences on hippocampal morphology. The repercussions of this synergy between diet and stress remain to be studied, but are likely to be manifested as a disturbance in neuroendocrine regulation and an impairment of hippocampus-dependent learning and memory.
Acknowledgments
Preliminary data for this study were presented at the 2003 annual meeting of the Society for Neuroscience. The authors acknowledge the technical assistance of Rudy Bellani, Joseph Nguyen, Scott Seganti, Lindsay Wieczorek and Kristel Zachow. Supported by the American Psychological Association Diversity Fellowship Program (S.E.B.), the Howard Hughes Medical Institute through the Undergraduate Biology Enrichment Program (J.K.K.,C.H.F.), National Institutes of Mental Health MH64727 (C.D.C.) and theVA (D.M.D.).
References
- 1.Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 2.Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 12142001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 5.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA. Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behav Brain Res. 2004;155:77–84. doi: 10.1016/j.bbr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 8.Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind ER, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav Neurosci. 1996;110:451–459. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- 11.Tannenbaum BM, Tannenbaum GS, Anisman H. Impact of life-long macronutrient choice on neuroendocrine and cognitive functioning in aged mice: differential effects in stressor-reactive and stressor-resilient mouse strains. Brain Res. 2003;985:187–197. doi: 10.1016/s0006-8993(03)03196-2. [DOI] [PubMed] [Google Scholar]
- 12.Kukreja RS, Datta BN, Chakravarti RN. Catecholamine-induced aggravation of aortic and coronary atherosclerosis in monkeys. Atherosclerosis. 1981;40:291–298. doi: 10.1016/0021-9150(81)90139-8. [DOI] [PubMed] [Google Scholar]
- 13.Sood V, Chakravarti RN. Systemic stress in the production of cardiac thrombosis in hypercholesterolaemic rats. Res Exp Med (Berl) 1976;167:31–45. doi: 10.1007/BF02180286. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi K, Goko H, Matsuoka A. Effects of electric stress on glucose metabolism, glucose-stimulated cyclic adenosine 3′,5′-monophosphate accumulation and 45 Ca+ + efflux in isolated pancreatic islets from rats fed with a high fat diet. Endocrinol Jpn. 1979;26:549–557. doi: 10.1507/endocrj1954.26.549. [DOI] [PubMed] [Google Scholar]
- 15.Stout C, Morrow J, Brandt EN, Wolf S. Unusually low incidence of death from myocardial infarction; study of an Italian American community in Pennsylvania. JAMA. 1965;188:845–847. doi: 10.1001/jama.1964.03060360005001. [DOI] [PubMed] [Google Scholar]
- 16.Garrel DR, Razi M, Lariviere F, Jobin N, Naman N, Emptoz-Bonneton A, Pugeat MM. Improved clinical status and length of care with low-fat nutrition support in burn patients. J Parenter Enteral Nutr. 1995;19:482–491. doi: 10.1177/0148607195019006482. [DOI] [PubMed] [Google Scholar]
- 17.van Meel D, de Vrij JH, Kunst AE, Mackenbach JP. Differences in risk factors for disease and health problems between monks and the general population in The Netherlands. Ned Tijdschr Geneeskd. 1992;136:1551–1555. [PubMed] [Google Scholar]
- 18.Russek HI. Role of emotional stress in the etiology of clinical coronary heart disease. Dis Chest. 1967;52:1–9. doi: 10.1378/chest.52.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- 20.Conrad CD, Magarinos AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 21.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinnilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 22.McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 24.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 25.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]


