Abstract
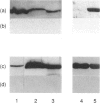
Although the pathology of discoid lupus erythematosus is well documented the causative agents are not known. Here, we report the identity of the target antigen of an autoantibody present in high titre in the serum of a patient with discoid lupus erythematosus. We have demonstrated that the antigen is enolase; first, because it has properties consistent with this glycolytic enzyme (47,000 MW, cytosolic localization and ubiquitous tissue distribution). Secondly, limited amino acid sequence determination after trypsin digestion shows identity with alpha-enolase. Finally, the autoimmune serum immunoblots rabbit and yeast enolase and predominantly one isoelectric form of enolase (PI approximately 6.1). These results indicate that the reactive autoepitopes are highly conserved from man to yeast. The results also suggest that the autoantibodies are most reactive to the alpha-isoform of enolase, although it is possible that they may also be reactive with gamma-enolase, and have least reactivity to beta-enolase. The anti-enolase autoantibodies belong to the immunoglobulin G1 (IgG1) isotype. This is the first report of IgG1 autoantibodies to evolutionarily conserved autoepitopes of enolase in the serum of a patient with discoid lupus erythematosus. Previous reports of autoantibodies to enolase have suggested associations with autoimmune polyglandular syndrome type I and cancer-associated retinopathy. This report and an earlier report of what is likely to be enolase autoantibodies in two patients without systemic disease suggest that enolase autoantibodies have a broad association and are not restricted to any particular disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamus G., Aptsiauri N., Guy J., Heckenlively J., Flannery J., Hargrave P. A. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996 Feb;78(2):120–129. doi: 10.1006/clin.1996.0021. [DOI] [PubMed] [Google Scholar]
- Callen J. P. Chronic cutaneous lupus erythematosus. Clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982 Jun;118(6):412–416. doi: 10.1001/archderm.118.6.412. [DOI] [PubMed] [Google Scholar]
- Callen J. P., Fowler J. F., Kulick K. B. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol. 1985 Nov;13(5 Pt 1):748–755. doi: 10.1016/s0190-9622(85)70217-4. [DOI] [PubMed] [Google Scholar]
- Elkon K., Bonfa E., Llovet R., Danho W., Weissbach H., Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledermann J. A. Serum neurone-specific enolase and other neuroendocrine markers in lung cancer. Eur J Cancer. 1994;30A(5):574–576. doi: 10.1016/0959-8049(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Millard L. G., Rowell N. R. Abnormal laboratory test results and their relationship to prognosis in discoid lupus erythematosus. A long-term follow-up study of 92 patients. Arch Dermatol. 1979 Sep;115(9):1055–1058. [PubMed] [Google Scholar]
- Peterson P., Perheentupa J., Krohn K. J. Detection of candidal antigens in autoimmune polyglandular syndrome type I. Clin Diagn Lab Immunol. 1996 May;3(3):290–294. doi: 10.1128/cdli.3.3.290-294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prystowsky S. D., Herndon J. H., Jr, Gilliam J. N. Chronic cutaneous lupus erythematosus (DLE)--a clinical and laboratory investigation of 80 patients. Medicine (Baltimore) 1976 Mar;55(2):183–191. doi: 10.1097/00005792-197603000-00006. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Martin L., Waisman D. M., Johnstone S. A., Fritzler M. J. Autoantibodies to the centrosome (centriole) react with determinants present in the glycolytic enzyme enolase. J Immunol. 1991 Apr 1;146(7):2341–2344. [PubMed] [Google Scholar]
- Riedel N., Wolin S., Guthrie C. A subset of yeast snRNA's contains functional binding sites for the highly conserved Sm antigen. Science. 1987 Jan 16;235(4786):328–331. doi: 10.1126/science.2948278. [DOI] [PubMed] [Google Scholar]
- Schickedanz J., Scheidtmann K. H., Walter G. Kinetics of nuclear transport and oligomerization of simian virus 40 large T antigen. Virology. 1986 Jan 15;148(1):47–57. doi: 10.1016/0042-6822(86)90402-2. [DOI] [PubMed] [Google Scholar]
- Sturgess A. Recently characterised autoantibodies and their clinical significance. Aust N Z J Med. 1992 Jun;22(3):279–289. doi: 10.1111/j.1445-5994.1992.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Thompson D., Larson G. Western blots using stained protein gels. Biotechniques. 1992 May;12(5):656–658. [PubMed] [Google Scholar]
- Verma M., Dutta S. K. DNA sequences encoding enolase are remarkably conserved from yeast to mammals. Life Sci. 1994;55(12):893–899. doi: 10.1016/0024-3205(94)00534-6. [DOI] [PubMed] [Google Scholar]
- Yeo J. P., Toh B. H. Cell cycle-associated autoantibodies: markers for autoimmunity and probes for molecular cell biology. Autoimmunity. 1994;18(4):291–300. doi: 10.3109/08916939409009531. [DOI] [PubMed] [Google Scholar]