Abstract
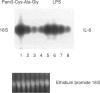
Monocytes (MO) and macrophages (MAC) are important producers of cytokines involved in the pathophysiology of bacterial sepsis. Most studies concentrate on the effects of bacterial lipopolysaccharides (LPS) regarding the induction of cytokine gene expression and secretion in MO/MAC. Here we report that besides LPS, the synthetic lipoprotein analogue lipopeptide N-palmitoyl-S-(2,3-bis(palmitoyl)-(2RS)-propyl)-(R)-cysteinyl-alanyl- glycine (Pam3-Cys-Ala-Gly), another component of the outer membrane of Gram-negative bacteria, as well as heat-killed Staphyloccocus aureus (S. aureus/SAC) are potent stimuli for cytokines in human MO. For all three investigated stimuli we found an individual pattern of cytokine induction: LPS was most potent in inducing interleukin-6 (IL-6) synthesis, whereas for tumour necrosis factor-alpha (TNF-alpha) secretion SAC was the best stimulus. Comparable amounts of IL-8 were induced by either LPS or Pam3-Cys-Ala-Gly, with SAC being less effective even at higher concentrations. The addition of serum led to an increase in LPS-, SAC- and Pam3-Cys-Ala-Gly-stimulated TNF-alpha secretion, indicating that the presence of serum is critical not just for LPS stimulation. Furthermore, as is known for LPS, Pam3-Cys-Ala-Gly and SAC rendered MO refractory to a second bacterial stimulus. Pam3-Cys-Ala-Gly and SAC induced tolerance for itself, but LPS could partially overcome this effect. As the CD14 molecule is discussed as a common receptor for different bacterial components, we investigated whether the TNF-alpha response of MO could be blocked by anti-CD14 antibodies. MY4, a CD14 antibody, selectively blocked the TNF-alpha secretion induced by LPS but not by Pam3-Cys-Ala-Gly or SAC. In summary, we conclude that besides LPS, lipopeptide Pam3-Cys-Ala-Gly and SAC are potent stimuli for human MO, while the mechanisms of activation seem to be partially different from LPS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Brugger W., Scheibenbogen C., Kreutz M., Leser H. G., Rehm A., Löhr G. W. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990 Jun;47(6):490–497. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- Busam K., Gieringer C., Freudenberg M., Hohmann H. P. Staphylococcus aureus and derived exotoxins induce nuclear factor kappa B-like activity in murine bone marrow macrophages. Infect Immun. 1992 May;60(5):2008–2015. doi: 10.1128/iai.60.5.2008-2015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M. The nonspecific nature of endotoxin tolerance. Trends Microbiol. 1995 Aug;3(8):320–324. doi: 10.1016/s0966-842x(00)88963-5. [DOI] [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentener M. A., Bazil V., Von Asmuth E. J., Ceska M., Buurman W. A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993 Apr 1;150(7):2885–2891. [PubMed] [Google Scholar]
- Fidler I. J., Nii A., Utsugi T., Brown D., Bakouche O., Kleinerman E. S. Differential release of TNF-alpha, IL 1, and PGE2 by human blood monocytes subsequent to interaction with different bacterial derived agents. Lymphokine Res. 1990 Winter;9(4):449–463. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granowitz E. V., Porat R., Mier J. W., Orencole S. F., Kaplanski G., Lynch E. A., Ye K., Vannier E., Wolff S. M., Dinarello C. A. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993 Aug 1;151(3):1637–1645. [PubMed] [Google Scholar]
- Hauschildt S., Bassenge E., Bessler W., Busse R., Mülsch A. L-arginine-dependent nitric oxide formation and nitrite release in bone marrow-derived macrophages stimulated with bacterial lipopeptide and lipopolysaccharide. Immunology. 1990 Jul;70(3):332–337. [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S., Hoffmann P., Beuscher H. U., Dufhues G., Heinrich P., Wiesmüller K. H., Jung G., Bessler W. G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990 Jan;20(1):63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- Heumann D., Barras C., Severin A., Glauser M. P., Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994 Jul;62(7):2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Hoffmann P., Heinle S., Schade U. F., Loppnow H., Ulmer A. J., Flad H. D., Jung G., Bessler W. G. Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology. 1988 May;177(2):158–170. doi: 10.1016/S0171-2985(88)80036-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann P., Wiesmüller K. H., Metzger J., Jung G., Bessler W. G. Induction of tumor cytotoxicity in murine bone marrow-derived macrophages by two synthetic lipopeptide analogues. Biol Chem Hoppe Seyler. 1989 Jun;370(6):575–582. doi: 10.1515/bchm3.1989.370.1.575. [DOI] [PubMed] [Google Scholar]
- Kusunoki T., Hailman E., Juan T. S., Lichenstein H. S., Wright S. D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995 Dec 1;182(6):1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki T., Wright S. D. Chemical characteristics of Staphylococcus aureus molecules that have CD14-dependent cell-stimulating activity. J Immunol. 1996 Dec 1;157(11):5112–5117. [PubMed] [Google Scholar]
- LaRue K. E., McCall C. E. A labile transcriptional repressor modulates endotoxin tolerance. J Exp Med. 1994 Dec 1;180(6):2269–2275. doi: 10.1084/jem.180.6.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann R., Wesp M., Dukor P. Modulation of interferon-gamma-induced major histocompatibility (MHC) and CD14 antigen changes by lipophilic muramyltripeptide MTP-PE in human monocytes. Cell Immunol. 1988 Nov;117(1):45–55. doi: 10.1016/0008-8749(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Tobias P. S., Wolfson E., Ulevitch R. J. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992 Jul 1;149(1):200–206. [PubMed] [Google Scholar]
- Mathison J. C., Virca G. D., Wolfson E., Tobias P. S., Glaser K., Ulevitch R. J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990 Apr;85(4):1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J., Wolfson E., Steinemann S., Tobias P., Ulevitch R. Lipopolysaccharide (LPS) recognition in macrophages. Participation of LPS-binding protein and CD14 in LPS-induced adaptation in rabbit peritoneal exudate macrophages. J Clin Invest. 1993 Oct;92(4):2053–2059. doi: 10.1172/JCI116801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C. E., Grosso-Wilmoth L. M., LaRue K., Guzman R. N., Cousart S. L. Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest. 1993 Mar;91(3):853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Arndt L. L., Akins D. R., Curetty L. L., Harrich D. A., Radolf J. D. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-kappa B. Infect Immun. 1996 Sep;64(9):3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitermann A., Metzger J., Wiesmüller K. H., Jung G., Bessler W. G. Lipopeptide derivatives of bacterial lipoprotein constitute potent immune adjuvants combined with or covalently coupled to antigen or hapten. Biol Chem Hoppe Seyler. 1989 Apr;370(4):343–352. doi: 10.1515/bchm3.1989.370.1.343. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Timmerman C. P., Mattsson E., Martinez-Martinez L., De Graaf L., Van Strijp J. A., Verbrugh H. A., Verhoef J., Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993 Oct;61(10):4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Feist W., Heine H., Kirikae T., Kirikae F., Kusumoto S., Kusama T., Brade H., Schade U., Rietschel E. T. Modulation of endotoxin-induced monokine release in human monocytes by lipid A partial structures that inhibit binding of 125I-lipopolysaccharide. Infect Immun. 1992 Dec;60(12):5145–5152. doi: 10.1128/iai.60.12.5145-5152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann B., Brade H., Rietschel E. T., Dziarski R., Bazil V., Kusumoto S., Flad H. D., Ulmer A. J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994 Nov;62(11):4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Blumenstein M., Käfferlein E., Kieper D., Petersmann I., Endres S., Flegel W. A., Northoff H., Riethmüller G., Haas J. G. In vitro desensitization to lipopolysaccharide suppresses tumour necrosis factor, interleukin-1 and interleukin-6 gene expression in a similar fashion. Immunology. 1992 Feb;75(2):264–268. [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Wedel A., Schraut W., Ströbel M., Wendelgass P., Sternsdorf T., Bäuerle P. A., Haas J. G., Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994 Jun 24;269(25):17001–17004. [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Snyder Y. M., Roeder W. D. Endotoxin-macrophage interaction: post-translational regulation of tumor necrosis factor expression. J Immunol. 1989 Aug 15;143(4):1223–1227. [PubMed] [Google Scholar]