Abstract
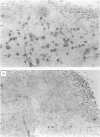
Following allergen exposure, sensitized Brown-Norway rats develop airway hyperresponsiveness (AHR) and eosinophilic inflammation together with an increase in activated T cells (CD25+) in the airways. We tested the hypothesis that CD4+ T cells are involved directly in the acquisition of AHR. Spleen T cells from animals that were injected intraperitoneally on three consecutive days with ovalbumin/Al(OH)3, showed a dose-dependent proliferative response in vitro to ovalbumin, but not to bovine serum albumin, as measured by [3H]thymidine uptake. For total T-cell transfer, spleen cells obtained from donor rats 4 days after sensitization were depleted of adherent cells by a nylon wool column separation. CD4+ and CD8+ T cells were purified by immunomagnetic beads cell separation. Recipient naive rats were injected intravenously with 50 x 10(6) total T cells, 20 x 10(6) and 5 x 10(6) CD4+ cells, and 5 x 10(6) CD8+ cells, and were exposed to ovalbumin aerosol 24 hr afterwards. After a further 24 hr, airway responsiveness to acetylcholine (ACh) was measured and provocative concentration (PC) values PC100, PC200 and PC300) (the ACh concentration needed to achieve 100, 200 and 300% increase in lung resistance above baseline) were calculated. Airway responsiveness was significantly increased in recipients of sensitized total T cells compared with recipients of cells from saline-injected donor rats (P < 0.05). There were significantly increased eosinophil major basic protein (MBP)+ cell counts/mm2 in airway submucosal tissue in the hyperreactive rats and a significant correlation was found between the number of MBP+ cells and PC100 (r = 0.75; P < 0.03) in recipients of sensitized total T cells. Purified CD4+ T cells from sensitized donors induced AHR in naive recipients (P < 0.05), while sensitized CD8+ and naive CD4+ cells failed to do so. Our data indicate that T cells may induce AHR through an eosinophilic airway inflammation and that CD4+ T cells may have a direct effect in this process in Brown-Norway rats.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley A. M., Menz G., Storz C., Robinson D. S., Bradley B., Jeffery P. K., Durham S. R., Kay A. B. Identification of T lymphocytes, macrophages, and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial responsiveness. Am Rev Respir Dis. 1992 Aug;146(2):500–506. doi: 10.1164/ajrccm/146.2.500. [DOI] [PubMed] [Google Scholar]
- Berman J. S., Weller P. F. Airway eosinophils and lymphocytes in asthma. Birds of a feather? Am Rev Respir Dis. 1992 Jun;145(6):1246–1248. doi: 10.1164/ajrccm/145.6.1246. [DOI] [PubMed] [Google Scholar]
- Bradley B. L., Azzawi M., Jacobson M., Assoufi B., Collins J. V., Irani A. M., Schwartz L. B., Durham S. R., Jeffery P. K., Kay A. B. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991 Oct;88(4):661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Becker A. B., Lazarus S. C., Frick O. L., Nadel J. A., Gold W. M. Antigen-induced airway hyperresponsiveness and pulmonary inflammation in allergic dogs. J Appl Physiol (1985) 1985 Apr;58(4):1347–1353. doi: 10.1152/jappl.1985.58.4.1347. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E. J., Hirst E. M., Sanderson C. J. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989 May 1;73(6):1504–1512. [PubMed] [Google Scholar]
- Corrigan C. J., Haczku A., Gemou-Engesaeth V., Doi S., Kikuchi Y., Takatsu K., Durham S. R., Kay A. B. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. Am Rev Respir Dis. 1993 Mar;147(3):540–547. doi: 10.1164/ajrccm/147.3.540. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- DUNNILL M. S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960 Jan;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Lee T. H., Kemeny D. M. Ricin enhances IgE responses by inhibiting a subpopulation of early-activated IgE regulatory CD8+ T cells. Immunology. 1993 Feb;78(2):226–236. [PMC free article] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Eidelman D. H., Minshall E., Dandurand R. J., Schotman E., Song Y. L., Yasruel Z., Moqbel R., Hamid Q. Evidence for major basic protein immunoreactivity and interleukin 5 gene activation during the late phase response in explanted airways. Am J Respir Cell Mol Biol. 1996 Nov;15(5):582–589. doi: 10.1165/ajrcmb.15.5.8918365. [DOI] [PubMed] [Google Scholar]
- Elwood W., Lötvall J. O., Barnes P. J., Chung K. F. Characterization of allergen-induced bronchial hyperresponsiveness and airway inflammation in actively sensitized brown-Norway rats. J Allergy Clin Immunol. 1991 Dec;88(6):951–960. doi: 10.1016/0091-6749(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Garssen J., Van Loveren H., Van Der Vliet H., Bot H., Nijkamp F. P. T cell-mediated induction of airway hyperresponsiveness and altered lung functions in mice are independent of increased vascular permeability and mononuclear cell infiltration. Am Rev Respir Dis. 1993 Feb;147(2):307–313. doi: 10.1164/ajrccm/147.2.307. [DOI] [PubMed] [Google Scholar]
- Gerblich A. A., Campbell A. E., Schuyler M. R. Changes in T-lymphocyte subpopulations after antigenic bronchial provocation in asthmatics. N Engl J Med. 1984 May 24;310(21):1349–1352. doi: 10.1056/NEJM198405243102103. [DOI] [PubMed] [Google Scholar]
- Gundel R. H., Letts L. G., Gleich G. J. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991 Apr;87(4):1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSTON J. C., DE NAVASQUEZ S., TROUNCE J. R. A clinical and pathological study of fatal cases of status asthmaticus. Thorax. 1953 Sep;8(3):207–213. doi: 10.1136/thx.8.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A., Chung K. F., Sun J., Barnes P. J., Kay A. B., Moqbel R. Airway hyperresponsiveness, elevation of serum-specific IgE and activation of T cells following allergen exposure in sensitized Brown-Norway rats. Immunology. 1995 Aug;85(4):598–603. [PMC free article] [PubMed] [Google Scholar]
- Haczku A., Macary P., Haddad E. B., Huang T. J., Kemeny D. M., Moqbel R., Chung K. F. Expression of Th-2 cytokines interleukin-4 and -5 and of Th-1 cytokine interferon-gamma in ovalbumin-exposed sensitized Brown-Norway rats. Immunology. 1996 Jun;88(2):247–251. doi: 10.1111/j.1365-2567.1996.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A., Moqbel R., Elwood W., Sun J., Kay A. B., Barnes P. J., Chung K. F. Effects of prolonged repeated exposure to ovalbumin in sensitized brown Norway rats. Am J Respir Crit Care Med. 1994 Jul;150(1):23–27. doi: 10.1164/ajrccm.150.1.8025754. [DOI] [PubMed] [Google Scholar]
- Haczku A., Moqbel R., Jacobson M., Kay A. B., Barnes P. J., Chung K. F. T-cells subsets and activation in bronchial mucosa of sensitized Brown-Norway rats after single allergen exposure. Immunology. 1995 Aug;85(4):591–597. [PMC free article] [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Oliver J., McMenamin C., Schon-Hegrad M. A. Studies on the surface phenotype and functions of dendritic cells in parenchymal lung tissue of the rat. Immunology. 1992 Apr;75(4):582–587. [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Noble A., Holmes B. J., Diaz-Sanchez D. Immune regulation: a new role for the CD8+ T cell. Immunol Today. 1994 Mar;15(3):107–110. doi: 10.1016/0167-5699(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., Renz H., Loader J. E., Bradley K. L., Gelfand E. W. Airway response to electrical field stimulation in sensitized inbred mice. Passive transfer of increased responsiveness with peribronchial lymph nodes. J Clin Invest. 1992 Mar;89(3):747–752. doi: 10.1172/JCI115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti R., Annunziato F., Biagiotti R., Giudizi M. G., Piccinni M. P., Giannarini L., Sampognaro S., Parronchi P., Vinante F., Pizzolo G. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994 Dec 1;180(6):2407–2411. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin C., Schon-Hegrad M., Oliver J., Girn B., Holt P. G. Regulation of IgE responses to inhaled antigens: cellular mechanisms underlying allergic sensitization versus tolerance induction. Int Arch Allergy Appl Immunol. 1991;94(1-4):78–82. doi: 10.1159/000235331. [DOI] [PubMed] [Google Scholar]
- Moqbel R., Barkans J., Bradley B. L., Durham S. R., Kay A. B. Application of monoclonal antibodies against major basic protein (BMK-13) and eosinophil cationic protein (EG1 and EG2) for quantifying eosinophils in bronchial biopsies from atopic asthma. Clin Exp Allergy. 1992 Feb;22(2):265–273. doi: 10.1111/j.1365-2222.1992.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996 Mar;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Müller K. M., Jaunin F., Masouyé I., Saurat J. H., Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol. 1993 Jun 15;150(12):5576–5584. [PubMed] [Google Scholar]
- Nakajima H., Iwamoto I., Tomoe S., Matsumura R., Tomioka H., Takatsu K., Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992 Aug;146(2):374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Motojima S., Fukuda T., Makino S. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis. 1992 Jun;145(6):1469–1476. doi: 10.1164/ajrccm/145.6.1469. [DOI] [PubMed] [Google Scholar]
- Pabst R., Binns R. M., Licence S. T., Peter M. Evidence of a selective major vascular marginal pool of lymphocytes in the lung. Am Rev Respir Dis. 1987 Nov;136(5):1213–1218. doi: 10.1164/ajrccm/136.5.1213. [DOI] [PubMed] [Google Scholar]
- Renz H., Lack G., Saloga J., Schwinzer R., Bradley K., Loader J., Kupfer A., Larsen G. L., Gelfand E. W. Inhibition of IgE production and normalization of airways responsiveness by sensitized CD8 T cells in a mouse model of allergen-induced sensitization. J Immunol. 1994 Jan 1;152(1):351–360. [PubMed] [Google Scholar]
- Robinson D., Hamid Q., Bentley A., Ying S., Kay A. B., Durham S. R. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993 Aug;92(2):313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Saloga J., Renz H., Lack G., Bradley K. L., Greenstein J. L., Larsen G., Gelfand E. W. Development and transfer of immediate cutaneous hypersensitivity in mice exposed to aerosolized antigen. J Clin Invest. 1993 Jan;91(1):133–140. doi: 10.1172/JCI116162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler M., Gott K., Shopp G., Crooks L. CD3+ and CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Am Rev Respir Dis. 1992 Dec;146(6):1582–1588. doi: 10.1164/ajrccm/146.6.1582. [DOI] [PubMed] [Google Scholar]
- Uchida D. A., Ackerman S. J., Coyle A. J., Larsen G. L., Weller P. F., Freed J., Irvin C. G. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am Rev Respir Dis. 1993 Apr;147(4):982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Garssen J., Nijkamp F. P. T cell-mediated airway hyperreactivity in mice. Eur Respir J Suppl. 1991 Apr;13:16s–26s. [PubMed] [Google Scholar]
- Walker C., Bode E., Boer L., Hansel T. T., Blaser K., Virchow J. C., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992 Jul;146(1):109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- Walker C., Kaegi M. K., Braun P., Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991 Dec;88(6):935–942. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Rambaldi A., Biondi A., Chen Z. G., Sanderson C. J., Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989 Apr;19(4):701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- Wanner A., Abraham W. M., Douglas J. S., Drazen J. M., Richerson H. B., Ram J. S. NHLBI Workshop Summary. Models of airway hyperresponsiveness. Am Rev Respir Dis. 1990 Jan;141(1):253–257. doi: 10.1164/ajrccm/141.1.253. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Mishima H., Renzi P. M., Xu L. J., Hamid Q., Martin J. G. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T cells in brown Norway rats. J Clin Invest. 1995 Sep;96(3):1303–1310. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Hayashi Y., Sugama Y., Miura Y., Kasahara T., Kitamura S., Torisu M., Mita S., Tominaga A., Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988 May 1;167(5):1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]