Abstract
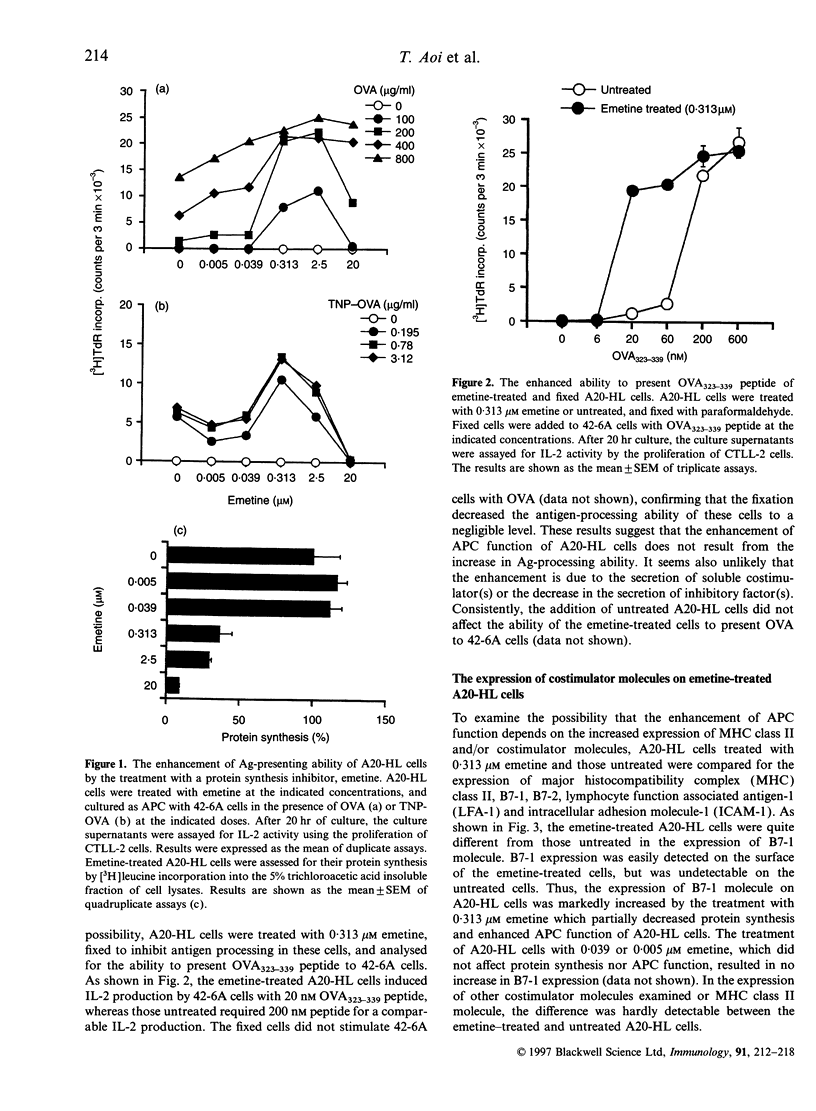
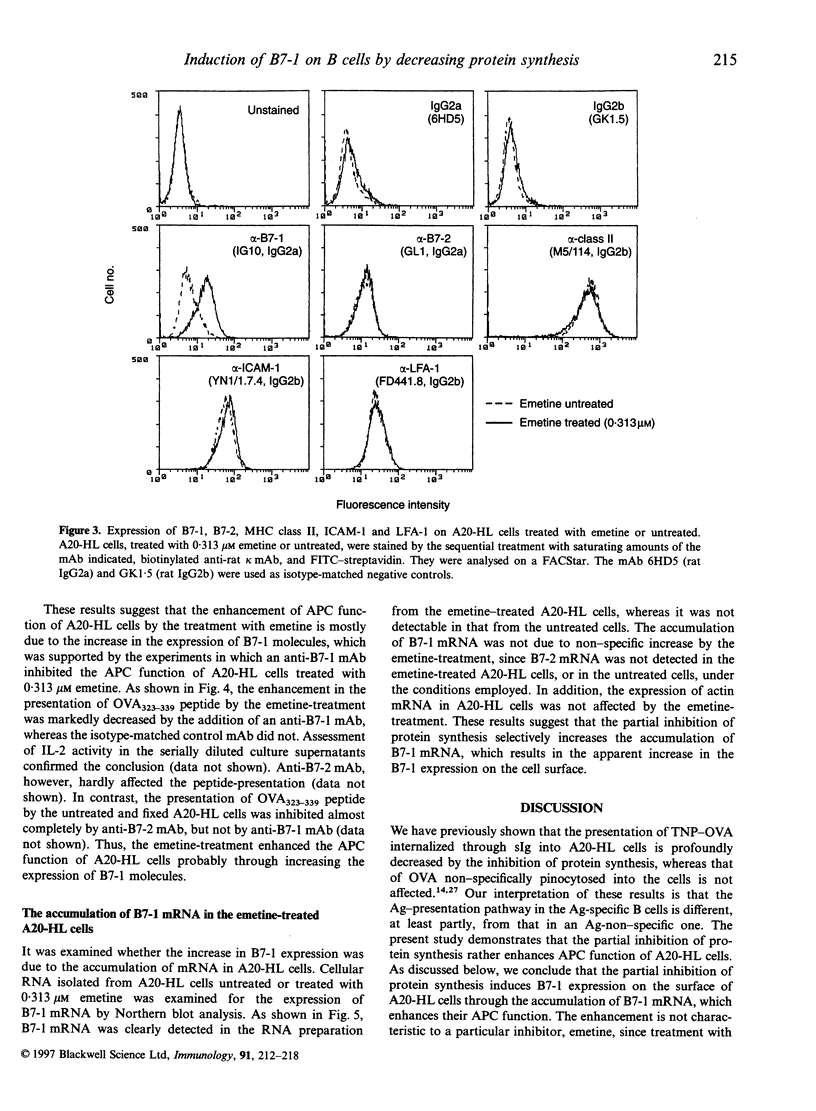
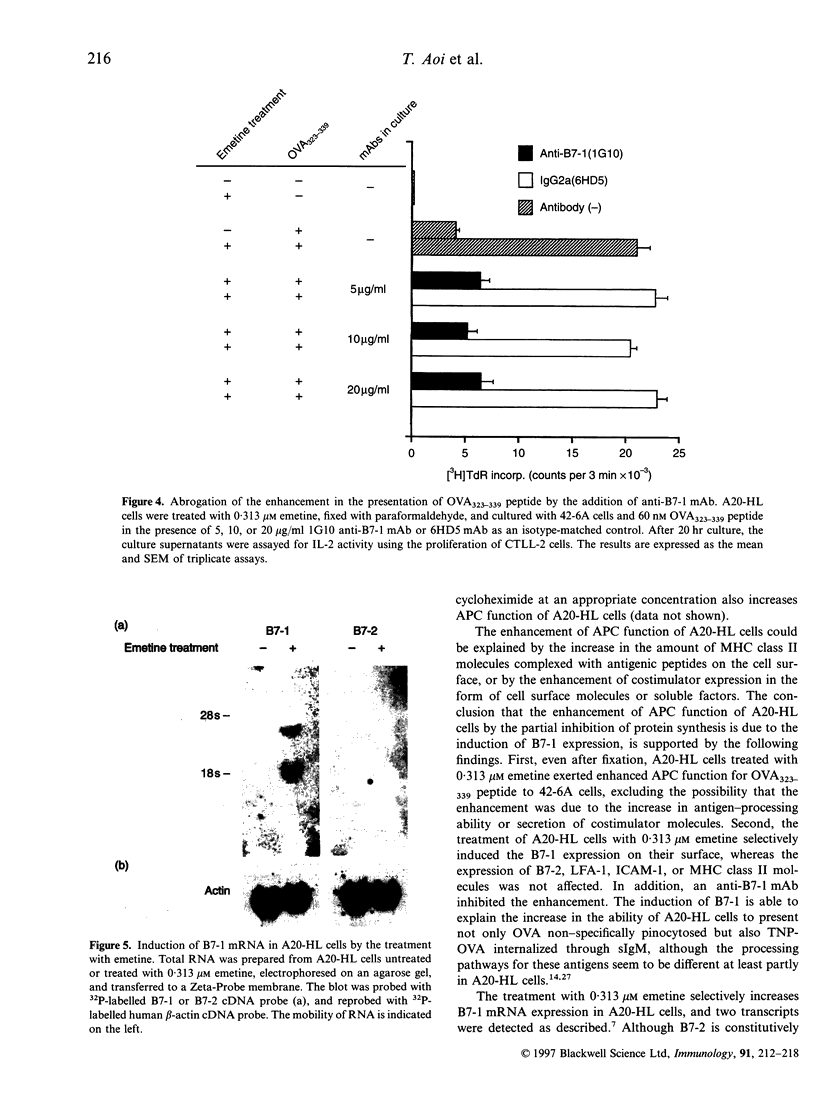
During the investigation of the role of protein synthesis in antigen-presenting cell (APC) function of A20-HL B lymphoma cells, we found that partial inhibition of protein synthesis enhanced their APC function. The treatment of A20-HL cells with 0.313-2.5 microM emetine, an irreversible inhibitor of protein synthesis, decreased protein synthesis by 60-70%, and enhanced their APC function to stimulate I-Ad/OVA323-339-specific T cells to produce interleukin-2 in response to ovalbumin (OVA). The emetine-treated and paraformaldehyde-fixed A20-HL cells required only 20 nM OVA323-339 peptide to stimulate the T cells, whereas those untreated and fixed required 200 nM peptide. This enhancement of APC function was mostly because of the induction of B7-1 expression on A20-HL cells by the emetine treatment, since B7-1 molecules were detected on the emetine-treated A20-HL cells, but only negligibly, if at all, on the untreated cells, and an anti-B7-1 monoclonal antibody, 1G10, inhibited the enhanced APC function of the emetine-treated A20-HL cells. The emetine-treatment also increased B7-1 mRNA expression in A20-HL cells, suggesting that the induction of B7-1 expression was due to the increase in the accumulation of mRNA and the translation with residual ability to synthesize protein. Thus, partial inhibition of protein synthesis in A20-HL cells increases B7-1 mRNA accumulation and its expression on the cell surface, which results in the enhancement of their APC function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskar S., Clements V. K., Glimcher L. H., Nabavi N., Ostrand-Rosenberg S. Rejection of MHC class II-transfected tumor cells requires induction of tumor-encoded B7-1 and/or B7-2 costimulatory molecules. J Immunol. 1996 May 15;156(10):3821–3827. [PubMed] [Google Scholar]
- Baskar S., Glimcher L., Nabavi N., Jones R. T., Ostrand-Rosenberg S. Major histocompatibility complex class II+B7-1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor-bearing mice. J Exp Med. 1995 Feb 1;181(2):619–629. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Edwards D. R., Mahadevan L. C. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992 Jul;11(7):2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Gribben J. G., Ng J. W., Kim J., Goldberg J. M., Hathcock K., Laszlo G. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993 Dec 1;178(6):2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fallarino F., Uyttenhove C., Boon T. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor: superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996 Apr 15;156(8):2909–2917. [PubMed] [Google Scholar]
- Galvin F., Freeman G. J., Razi-Wolf Z., Hall W., Jr, Benacerraf B., Nadler L., Reiser H. Murine B7 antigen provides a sufficient costimulatory signal for antigen-specific and MHC-restricted T cell activation. J Immunol. 1992 Dec 15;149(12):3802–3808. [PubMed] [Google Scholar]
- Ghersa P., Hooft van Huijsduijnen R., Whelan J., DeLamarter J. F. Labile proteins play a dual role in the control of endothelial leukocyte adhesion molecule-1 (ELAM-1) gene regulation. J Biol Chem. 1992 Sep 25;267(27):19226–19232. [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Inobe M., Linsley P. S., Ledbetter J. A., Nagai Y., Tamakoshi M., Uede T. Identification of an alternatively spliced form of the murine homologue of B7. Biochem Biophys Res Commun. 1994 Apr 15;200(1):443–449. doi: 10.1006/bbrc.1994.1469. [DOI] [PubMed] [Google Scholar]
- Kakiuchi T., Taira S., Nariuchi H. B cells as antigen-presenting cells: antibody production in vitro against a T-dependent antigen. Cell Immunol. 1986 Jul;100(2):515–524. doi: 10.1016/0008-8749(86)90049-3. [DOI] [PubMed] [Google Scholar]
- Kakiuchi T., Watanabe M., Hozumi N., Nariuchi H. Differential sensitivity of specific and nonspecific antigen-presentation by B cells to a protein synthesis inhibitor. J Immunol. 1990 Sep 15;145(6):1653–1658. [PubMed] [Google Scholar]
- Krummel M. F., Allison J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995 Aug 1;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulonis U., Dosiou C., Freeman G., Lamont C., Mauch P., Nadler L. M., Griffin J. D. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J Immunol. 1996 Feb 1;156(3):1126–1131. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Oguchi S., Weisz A., Esumi H. Enhancement of inducible-type NO synthase gene transcription by protein synthesis inhibitors. Activation of an intracellular signal transduction pathway by low concentrations of cycloheximide. FEBS Lett. 1994 Feb 7;338(3):326–330. doi: 10.1016/0014-5793(94)80293-9. [DOI] [PubMed] [Google Scholar]
- Pontecorvi A., Tata J. R., Phyillaier M., Robbins J. Selective degradation of mRNA: the role of short-lived proteins in differential destabilization of insulin-induced creatine phosphokinase and myosin heavy chain mRNAs during rat skeletal muscle L6 cell differentiation. EMBO J. 1988 May;7(5):1489–1495. doi: 10.1002/j.1460-2075.1988.tb02967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers G. D., Faherty D. A., Connaughton S. E., Biondi D. A., Godfrey D. I., Gault A., Chen C. Y., Nabavi N. Expression and functional analysis of murine B7 delineated by a novel monoclonal antibody. Cell Immunol. 1994 Feb;153(2):298–311. doi: 10.1006/cimm.1994.1030. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- Tivol E. A., Borriello F., Schweitzer A. N., Lynch W. P., Bluestone J. A., Sharpe A. H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995 Nov;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Wall R., Briskin M., Carter C., Govan H., Taylor A., Kincade P. A labile inhibitor blocks immunoglobulin kappa-light-chain-gene transcription in a pre-B leukemic cell line. Proc Natl Acad Sci U S A. 1986 Jan;83(2):295–298. doi: 10.1073/pnas.83.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Wegmann D. R., Ochi A., Hozumi N. Antigen presentation by a B-cell line transfected with cloned immunoglobulin heavy- and light-chain genes specific for a defined hapten. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5247–5251. doi: 10.1073/pnas.83.14.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]