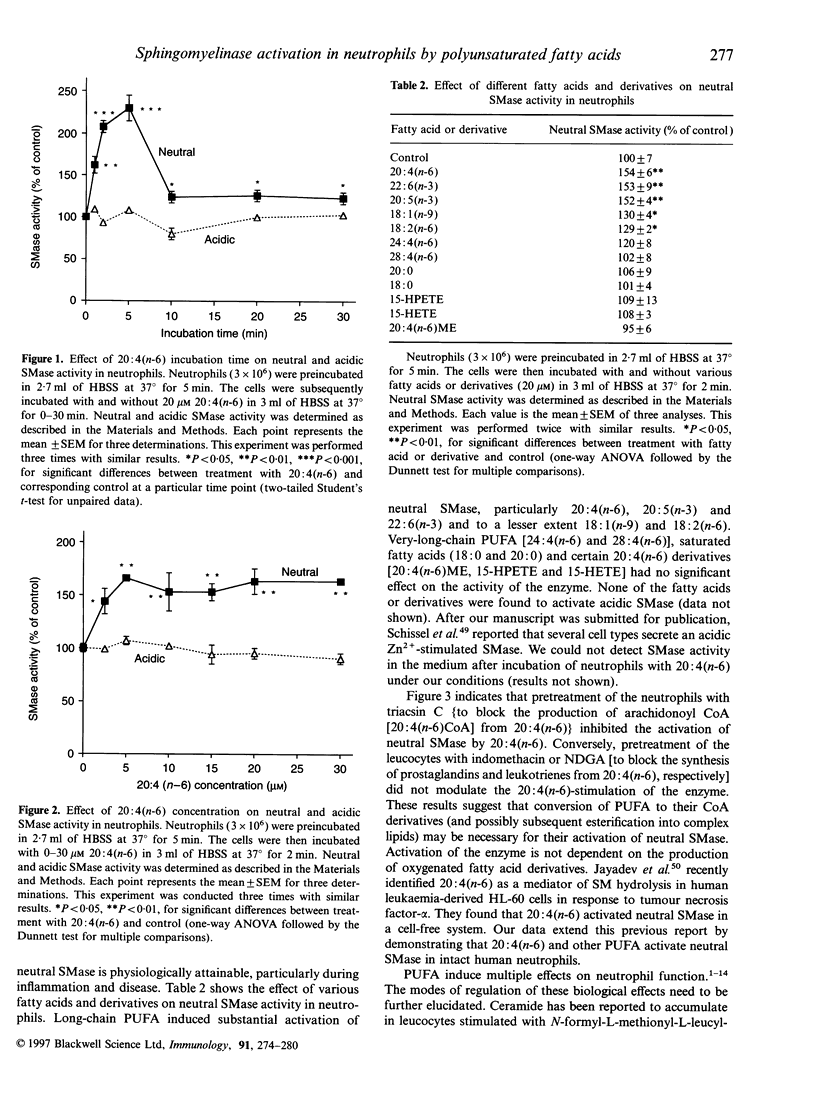
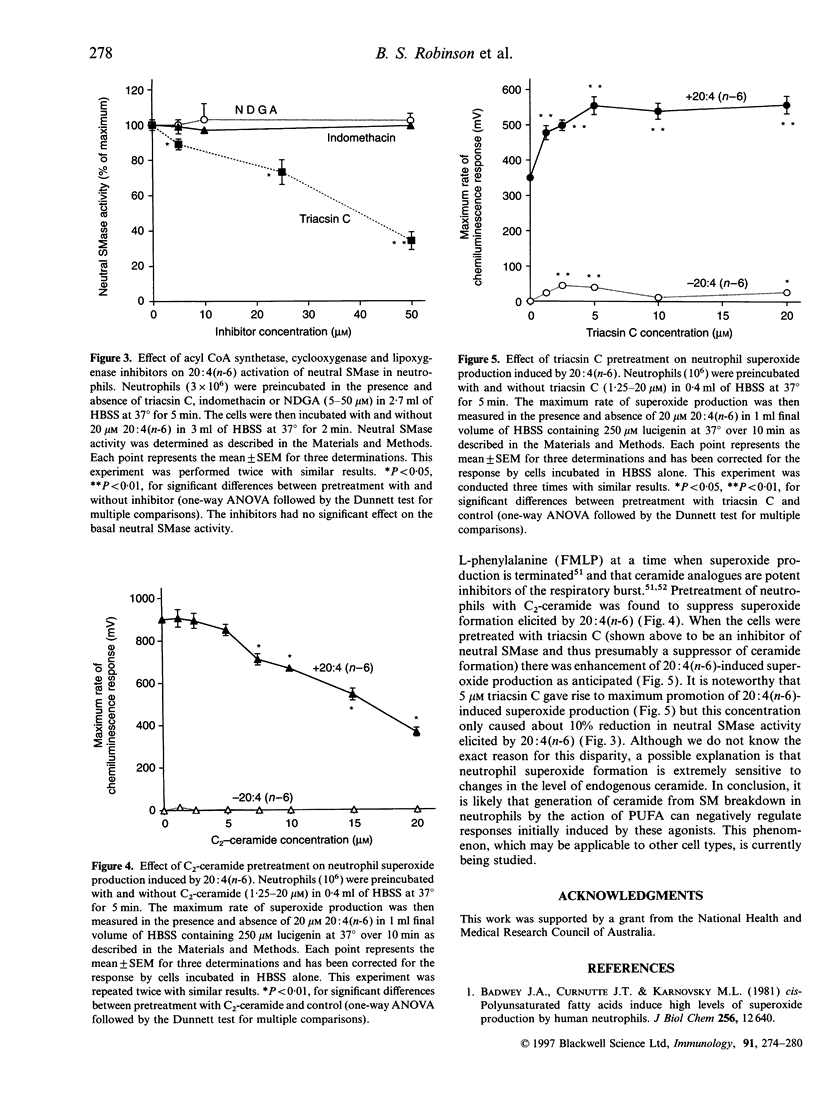
Abstract
Although unesterified polyunsaturated fatty acids (PUFA) have been shown to elicit marked changes in neutrophil function, the associated signal transduction processes require clarification. In this study we examined the effect of PUFA on the sphingomyelin (SM)-signalling cycle in human neutrophils. Treatment of neutrophils with eicosatetraenoic acid [arachidonic acid, 20:4(n-6)] caused a decrease in the mass of cellular SM and an increase in the level of ceramide. 20:4(n-6)-stimulated neutral sphingomyelinase (SMase) activity of the leucocytes in a time- and concentration-dependent manner. Other unsaturated fatty acids, docosahexaenoic [22:6(n-3)], eicosapentaenoic [20:5(n-3)], octadecenoic [oleic, 18:1(n-9)] and octadecadienoic [linoleic, 18:2(n-6)] acids also had the capacity to activate neutral SMase; however, certain 20:4(n-6) derivatives ¿20:4(n-6) methyl ester [20:4(n-6)ME], 15-hydroperoxyeicosatetraenoic (15-HPETE) and 15-hydroxyeicosatetraenoic (15-HETE) acids¿, very-long-chain PUFA ¿tetracosatetraenoic [24:4(n-6)] and octacosatetraenoic [28:4(n-6)] acids¿ and saturated fatty acids [octadecanoic (stearic, 18:0) and eicosanoic (arachidic, 20:0) acids] had no significant effect. Activation of neutral SMase by 20:4(n-6) appeared to involve metabolism via 20:4(n-6)CoA (arachidonoyl CoA) and was not dependent on prostaglandin and leukotriene synthesis. All of the fatty acids and derivatives tested failed to activate acidic SMase of neutrophils. Ceramide was found to inhibit 20:4(n-6)-induced superoxide generation by the cells. It is envisaged that the PUFA-induced ceramide production in neutrophils plays a role in the regulation of biological responses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson G. A. Structural and metabolic heterogeneity of rat liver glycerophosphatides. Eur J Biochem. 1968 May;4(4):478–486. doi: 10.1111/j.1432-1033.1968.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Atkinson Y. H., Murray A. W., Krilis S., Vadas M. A., Lopez A. F. Human tumour necrosis factor-alpha (TNF-alpha) directly stimulates arachidonic acid release in human neutrophils. Immunology. 1990 May;70(1):82–87. [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Robinson J. M., Berde C. B., Karnovsky M. J., Karnovsky M. L. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984 Jun 25;259(12):7870–7877. [PubMed] [Google Scholar]
- Ballou L. R., Laulederkind S. J., Rosloniec E. F., Raghow R. Ceramide signalling and the immune response. Biochim Biophys Acta. 1996 Jun 11;1301(3):273–287. doi: 10.1016/0005-2760(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Ballou L. R. Sphingolipids and cell function. Immunol Today. 1992 Sep;13(9):339–341. doi: 10.1016/0167-5699(92)90167-6. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Ferrante A., Harvey D. P., Poulos A. Polyunsaturated fatty acids increase neutrophil adherence and integrin receptor expression. J Leukoc Biol. 1993 Apr;53(4):420–426. doi: 10.1002/jlb.53.4.420. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Ferrante A., Poulos A., Smithers L., Rathjen D. A., Robinson B. S. Inhibitory effects of arachidonic acid (20:4,n-6) and its monohydroperoxy- and hydroxy-metabolites on procoagulant activity in endothelial cells. Atherosclerosis. 1995 Jul;116(1):125–133. doi: 10.1016/0021-9150(95)05538-8. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem. 1982 May 10;257(9):5196–5200. [PubMed] [Google Scholar]
- Chatterjee S. Neutral sphingomyelinase. Adv Lipid Res. 1993;26:25–48. [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985 May;75(5):1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D. V., Cook H. W., Spence M. W. Evidence that neutral sphingomyelinase of cultured murine neuroblastoma cells is oriented externally on the plasma membrane. Biochim Biophys Acta. 1984 Nov 7;777(2):339–342. doi: 10.1016/0005-2736(84)90437-1. [DOI] [PubMed] [Google Scholar]
- Deby-Dupont G., Ducarne H., de Landsheere C., Ancion J. C., Noël F. X., Radoux L., Deby C. Intense rises of unesterified arachidonate plasma levels in stressed humans. Biomed Pharmacother. 1983;37(8):386–391. [PubMed] [Google Scholar]
- Doerfler M. E., Weiss J., Clark J. D., Elsbach P. Bacterial lipopolysaccharide primes human neutrophils for enhanced release of arachidonic acid and causes phosphorylation of an 85-kD cytosolic phospholipase A2. J Clin Invest. 1994 Apr;93(4):1583–1591. doi: 10.1172/JCI117138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstin M., Durstin S., Molski T. F., Becker E. L., Sha'afi R. I. Cytoplasmic phospholipase A2 translocates to membrane fraction in human neutrophils activated by stimuli that phosphorylate mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3142–3146. doi: 10.1073/pnas.91.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Goh D., Harvey D. P., Robinson B. S., Hii C. S., Bates E. J., Hardy S. J., Johnson D. W., Poulos A. Neutrophil migration inhibitory properties of polyunsaturated fatty acids. The role of fatty acid structure, metabolism, and possible second messenger systems. J Clin Invest. 1994 Mar;93(3):1063–1070. doi: 10.1172/JCI117056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Separation of mononuclear and polymorphonuclear leucocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48(1):81–85. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- Graber R., Sumida C., Nunez E. A. Fatty acids and cell signal transduction. J Lipid Mediat Cell Signal. 1994 Mar;9(2):91–116. [PubMed] [Google Scholar]
- Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1987 Mar 12;97(2):209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Linardic C. M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993 Dec 21;1154(3-4):223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Hardy S. J., Ferrante A., Poulos A., Robinson B. S., Johnson D. W., Murray A. W. Effect of exogenous fatty acids with greater than 22 carbon atoms (very long chain fatty acids) on superoxide production by human neutrophils. J Immunol. 1994 Aug 15;153(4):1754–1761. [PubMed] [Google Scholar]
- Hardy S. J., Ferrante A., Robinson B. S., Johnson D. W., Poulos A., Clark K. J., Murray A. W. In vitro activation of rat brain protein kinase C by polyenoic very-long-chain fatty acids. J Neurochem. 1994 Apr;62(4):1546–1551. doi: 10.1046/j.1471-4159.1994.62041546.x. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Robinson B. S., Ferrante A., Hii C. S., Johnson D. W., Poulos A., Murray A. W. Polyenoic very-long-chain fatty acids mobilize intracellular calcium from a thapsigargin-insensitive pool in human neutrophils. The relationship between Ca2+ mobilization and superoxide production induced by long- and very-long-chain fatty acids. Biochem J. 1995 Oct 15;311(Pt 2):689–697. doi: 10.1042/bj3110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Robinson B. S., Poulos A., Harvey D. P., Ferrante A., Murray A. W. The neutrophil respiratory burst. Responses to fatty acids, N-formylmethionylleucylphenylalanine and phorbol ester suggest divergent signalling mechanisms. Eur J Biochem. 1991 Jun 15;198(3):801–806. doi: 10.1111/j.1432-1033.1991.tb16084.x. [DOI] [PubMed] [Google Scholar]
- Hii C. S., Ferrante A., Edwards Y. S., Huang Z. H., Hartfield P. J., Rathjen D. A., Poulos A., Murray A. W. Activation of mitogen-activated protein kinase by arachidonic acid in rat liver epithelial WB cells by a protein kinase C-dependent mechanism. J Biol Chem. 1995 Mar 3;270(9):4201–4204. doi: 10.1074/jbc.270.9.4201. [DOI] [PubMed] [Google Scholar]
- Jayadev S., Linardic C. M., Hannun Y. A. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994 Feb 25;269(8):5757–5763. [PubMed] [Google Scholar]
- Khan W. A., Blobe G. C., Hannun Y. A. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell Signal. 1995 Mar;7(3):171–184. doi: 10.1016/0898-6568(94)00089-t. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N. Sphingomyelin and derivatives as cellular signals. Prog Lipid Res. 1991;30(1):1–38. doi: 10.1016/0163-7827(91)90005-p. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. Ceramide: a novel second messenger. Trends Cell Biol. 1992 Aug;2(8):232–236. doi: 10.1016/0962-8924(92)90310-j. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Hannun Y. A., Bell R. M. Introduction: sphingolipids and their metabolites in cell regulation. Adv Lipid Res. 1993;25:1–24. [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Volpi M., Sha'afi R. I. Modulation of rabbit neutrophil aggregation and degranulation by free fatty acids. J Leukoc Biol. 1984 Sep;36(3):333–340. doi: 10.1002/jlb.36.3.333. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A., Becker E. L. A possible role of arachidonic acid in human neutrophil aggregation and degranulation. Am J Pathol. 1979 Sep;96(3):799–810. [PMC free article] [PubMed] [Google Scholar]
- Poulos A., Robinson B. S., Ferrante A., Harvey D. P., Hardy S. J., Murray A. W. Effect of 22-32 carbon n-3 polyunsaturated fatty acids on superoxide production in human neutrophils: synergism of docosahexaenoic acid with f-met-leu-phe and phorbol ester. Immunology. 1991 May;73(1):102–108. [PMC free article] [PubMed] [Google Scholar]
- Robinson B. S., Johnson D. W., Poulos A. Unique molecular species of phosphatidylcholine containing very-long-chain (C24-C38) polyenoic fatty acids in rat brain. Biochem J. 1990 Feb 1;265(3):763–767. doi: 10.1042/bj2650763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schissel S. L., Schuchman E. H., Williams K. J., Tabas I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J Biol Chem. 1996 Aug 2;271(31):18431–18436. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Waite M. Phosphatidylinositol hydrolysis by phospholipase A2 and C activities in human peripheral blood neutrophils. J Leukoc Biol. 1992 Dec;52(6):670–678. doi: 10.1002/jlb.52.6.670. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Sam L. M., Justen J. M., Leach K. L., Epps D. E. Human polymorphonuclear neutrophil activation with arachidonic acid. Br J Pharmacol. 1987 Jul;91(3):641–649. doi: 10.1111/j.1476-5381.1987.tb11258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M. W. Sphingomyelinases. Adv Lipid Res. 1993;26:3–23. [PubMed] [Google Scholar]
- Street J. M., Johnson D. W., Singh H., Poulos A. Metabolism of saturated and polyunsaturated fatty acids by normal and Zellweger syndrome skin fibroblasts. Biochem J. 1989 Jun 15;260(3):647–655. doi: 10.1042/bj2600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testi R. Sphingomyelin breakdown and cell fate. Trends Biochem Sci. 1996 Dec;21(12):468–471. doi: 10.1016/s0968-0004(96)10056-6. [DOI] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Machleidt T., Witte D., Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994 Sep 23;78(6):1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Wong K., Li X. B., Hunchuk N. N-acetylsphingosine (C2-ceramide) inhibited neutrophil superoxide formation and calcium influx. J Biol Chem. 1995 Feb 17;270(7):3056–3062. doi: 10.1074/jbc.270.7.3056. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S., Yoshimoto T., Tsubura E. Arachidonic acid-induced chemiluminescence of human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1982 Aug;107(3):779–784. doi: 10.1016/0006-291x(82)90591-5. [DOI] [PubMed] [Google Scholar]



