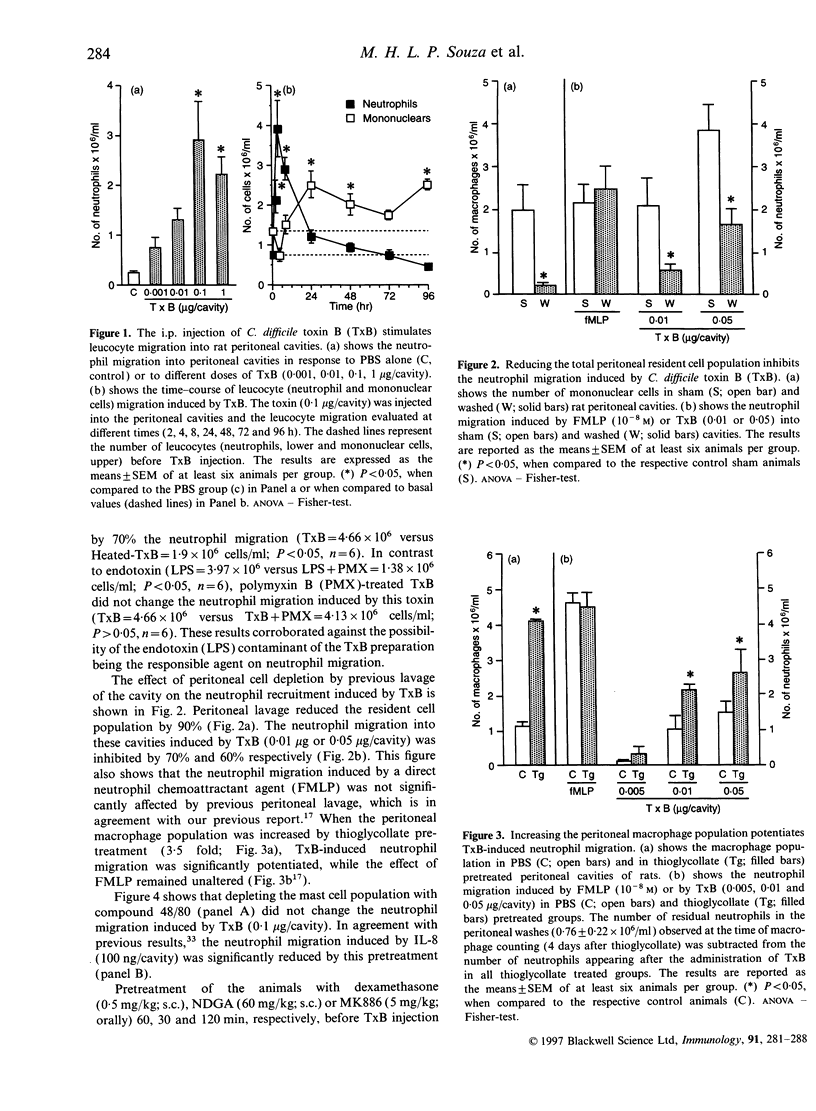
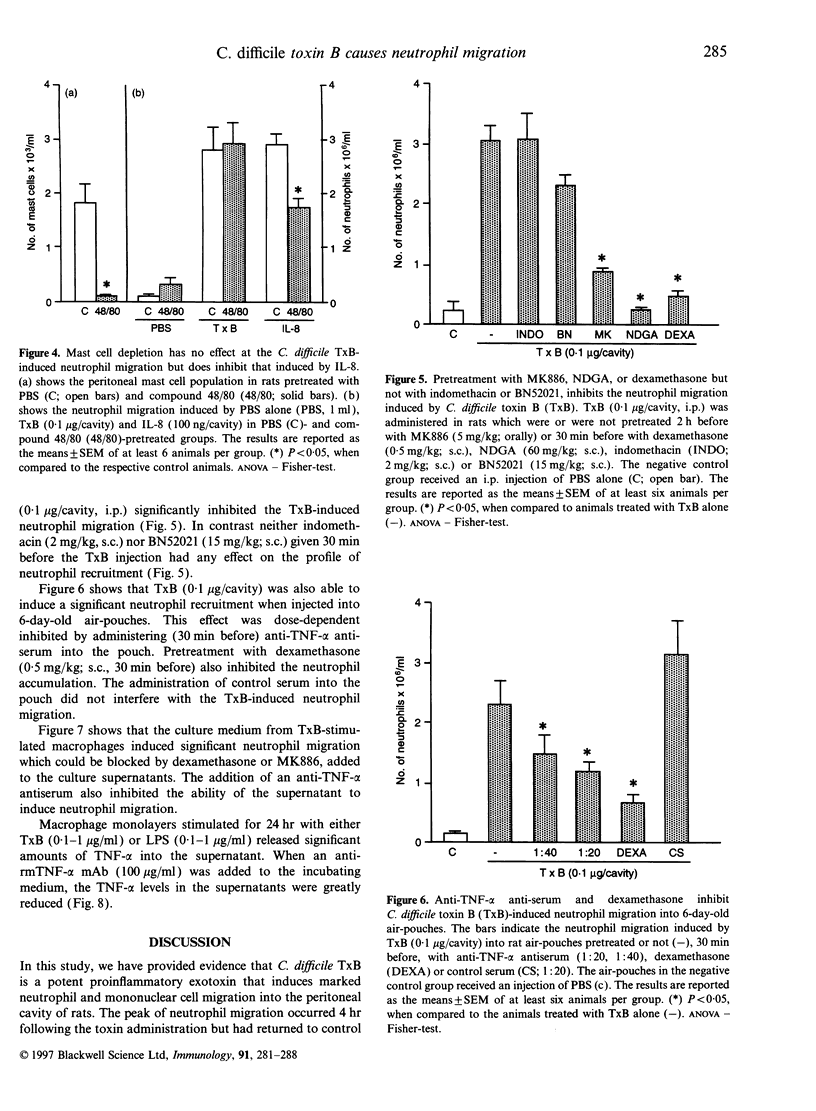
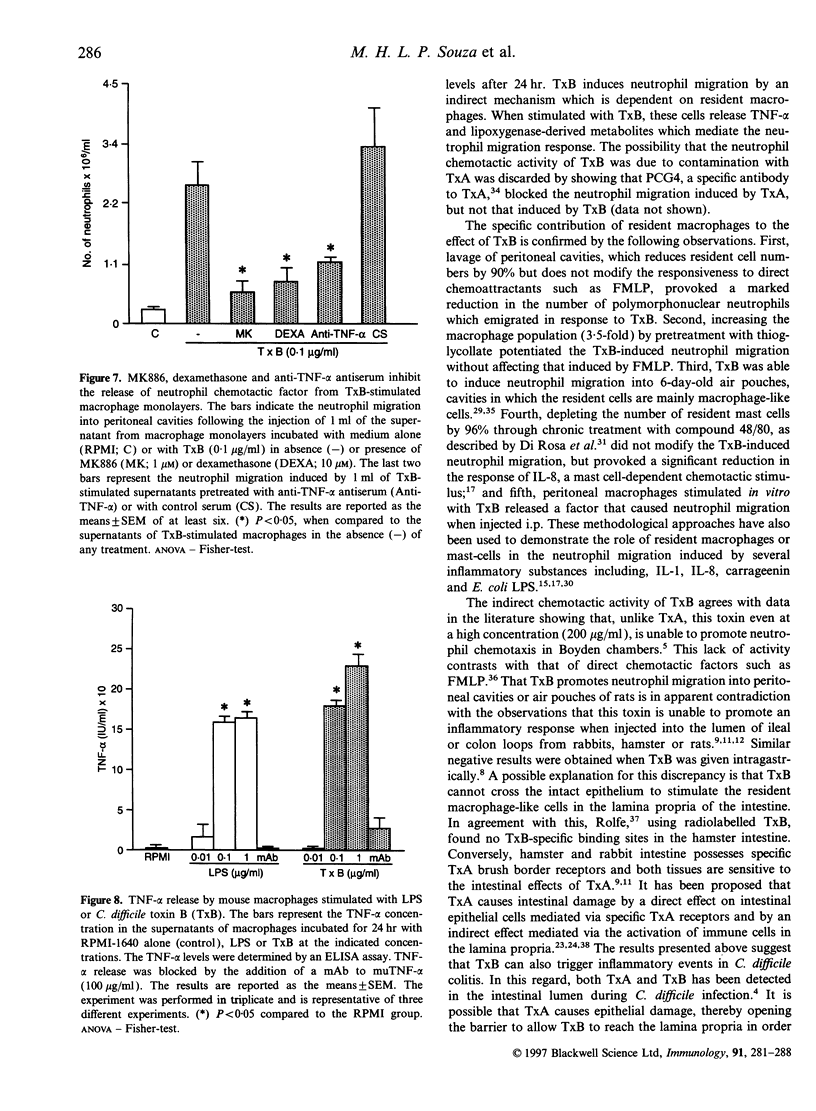
Abstract
Clostridium difficile (Cd) toxins appear to mediate the inflammatory response in pseudomembranous colitis and/or colitis associated with the use of antibiotics. In contrast to Cd Toxin A (TxA), Cd Toxin B (TxB) has been reported not to promote fluid secretion or morphological damage in rabbits and hamsters and also does not induce neutrophil chemotaxis in vitro. However, TxB is about 1000 times more potent than TxA in stimulating the release of tumour necrosis factor-alpha (TNF-alpha) by cultured monocytes. In the present study, we investigated the ability of TxB to promote neutrophil migration into peritoneal cavities and subcutaneous air-pouches of rats. We also examined the role of resident peritoneal cells in this process as well as the inflammatory mediators involved. TxB caused a significant and dose-dependent neutrophil influx with a maximal response at 0.1 microgram/cavity after 4 hr. Depleting the peritoneal resident cell population by washing the peritoneal cavity or increasing this population by pretreating the animals with thioglycollate blocked and amplified the TxB-induced neutrophil migration, respectively. Pretreating the animals with MK886 (a lipoxygenase inhibitor), NDGA (a dual cyclo- and lipoxygenase inhibitor) or the glucocorticoid, dexamethasone, but not with indomethacin (a cyclo-oxygenase inhibitor), or BN52021 (a platelet-activating factor antagonist), inhibited the neutrophil migration evoked by TxB. Pretreatment with dexamethasone or the administration of anti-TNF-alpha serum into the air-pouches also significantly reduced the TxB-induced neutrophil migration. Supernatants from TxB-stimulated macrophages induced neutrophil migration when injected into the rat peritoneal cavity. This effect was attenuated by the addition of either MK886 or dexamethasone to the macrophage monolayer and by preincubating the supernatants with anti-TNF-alpha serum. TxB also stimulated the release of TNF-alpha by macrophages. Overall, these results suggest that TxB induces an intense neutrophil migration which is mediated by macrophage-derived TNF-alpha and lipoxygenase products.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barroso L. A., Wang S. Z., Phelps C. J., Johnson J. L., Wilkins T. D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990 Jul 11;18(13):4004–4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Korom S., Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989 Nov;57(11):3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Boukili M. A., da Motta J. I., Vargaftig B. B., Ferreira S. H. Blockade by fenspiride of endotoxin-induced neutrophil migration in the rat. Eur J Pharmacol. 1993 Jul 6;238(1):47–52. doi: 10.1016/0014-2999(93)90503-a. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., McComb D. J., Movat H. Z. Protein synthesis dependent and independent mechanisms of neutrophil emigration. Different mechanisms of inflammation in rabbits induced by interleukin-1, tumor necrosis factor alpha or endotoxin versus leukocyte chemoattractants. Am J Pathol. 1989 Jul;135(1):227–237. [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981 Jun;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Ehrich M. Biochemical and pathological effects of Clostridium difficile toxins in mice. Toxicon. 1982;20(6):983–989. doi: 10.1016/0041-0101(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Faccioli L. H., Souza G. E., Cunha F. Q., Poole S., Ferreira S. H. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration "in vivo" by indirect mechanisms. Agents Actions. 1990 Jun;30(3-4):344–349. doi: 10.1007/BF01966298. [DOI] [PubMed] [Google Scholar]
- Flegel W. A., Müller F., Däubener W., Fischer H. G., Hadding U., Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991 Oct;59(10):3659–3666. doi: 10.1128/iai.59.10.3659-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Goodman S. A., Vukajlovich S. W., Munkenbeck P., Morrison D. C. Selective interaction between lymphocytes and lipid A subunits in lipopolysaccharide macromolecular aggregates. Rev Infect Dis. 1984 Jul-Aug;6(4):511–518. doi: 10.1093/clinids/6.4.511. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C. P., Becker S., Linevsky J. K., Joshi M. A., O'Keane J. C., Dickey B. F., LaMont J. T., Pothoulakis C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest. 1994 Mar;93(3):1257–1265. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C. P., Pothoulakis C., LaMont J. T. Clostridium difficile colitis. N Engl J Med. 1994 Jan 27;330(4):257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- Kim P. H., Iaconis J. P., Rolfe R. D. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987 Dec;55(12):2984–2992. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanko I. C., Gordon T. P., Rozenbilds M. A., Brooks P. M., Roberts-Thomson P. J. The subcutaneous air pouch model of synovium and the inflammatory response to heat aggregated gammaglobulin. Agents Actions. 1986 Jun;18(3-4):421–428. doi: 10.1007/BF01965007. [DOI] [PubMed] [Google Scholar]
- Lima A. A., Lyerly D. M., Wilkins T. D., Innes D. J., Guerrant R. L. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect Immun. 1988 Mar;56(3):582–588. doi: 10.1128/iai.56.3.582-588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. D., Pothoulakis C., Baeker T. R., LaMont J. T., Rothstein T. L. Macrophage-dependent stimulation of T cell-depleted spleen cells by Clostridium difficile toxin A and calcium ionophore. Cell Immunol. 1990 Mar;126(1):155–163. doi: 10.1016/0008-8749(90)90308-e. [DOI] [PubMed] [Google Scholar]
- Misfeldt M. L., Legaard P. K., Howell S. E., Fornella M. H., LeGrand R. D. Induction of interleukin-1 from murine peritoneal macrophages by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990 Apr;58(4):978–982. doi: 10.1128/iai.58.4.978-982.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. J., Ketley J. M., Haslam S. C., Stephen J., Burdon D. W., Candy D. C., Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986 Jan;27(1):78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Harris J. G., Flower R. J. A role for endogenous histamine in interleukin-8-induced neutrophil infiltration into mouse air-pouch: investigation of the modulatory action of systemic and local dexamethasone. Br J Pharmacol. 1994 Jul;112(3):801–808. doi: 10.1111/j.1476-5381.1994.tb13150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Castagliuolo I., LaMont J. T., Jaffer A., O'Keane J. C., Snider R. M., Leeman S. E. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Karmeli F., Kelly C. P., Eliakim R., Joshi M. A., O'Keane C. J., Castagliuolo I., LaMont J. T., Rachmilewitz D. Ketotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileum. Gastroenterology. 1993 Sep;105(3):701–707. doi: 10.1016/0016-5085(93)90886-h. [DOI] [PubMed] [Google Scholar]
- Pothoulakis C., Sullivan R., Melnick D. A., Triadafilopoulos G., Gadenne A. S., Meshulam T., LaMont J. T. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J Clin Invest. 1988 Jun;81(6):1741–1745. doi: 10.1172/JCI113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. B., Davies D. R. Pseudomembranous colitis. J Clin Pathol. 1977 Jan;30(1):1–12. doi: 10.1136/jcp.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro R. A., Flores C. A., Cunha F. Q., Ferreira S. H. IL-8 causes in vivo neutrophil migration by a cell-dependent mechanism. Immunology. 1991 Aug;73(4):472–477. [PMC free article] [PubMed] [Google Scholar]
- Riegler M., Sedivy R., Pothoulakis C., Hamilton G., Zacherl J., Bischof G., Cosentini E., Feil W., Schiessel R., LaMont J. T. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995 May;95(5):2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D. Binding kinetics of Clostridium difficile toxins A and B to intestinal brush border membranes from infant and adult hamsters. Infect Immun. 1991 Apr;59(4):1223–1230. doi: 10.1128/iai.59.4.1223-1230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza G. E., Cunha F. Q., Mello R., Ferreira S. H. Neutrophil migration induced by inflammatory stimuli is reduced by macrophage depletion. Agents Actions. 1988 Jul;24(3-4):377–380. doi: 10.1007/BF02028296. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J., Jennische E., Lange S., Lönnroth I. Enterotoxins from Clostridium difficile; diarrhoeogenic potency and morphological effects in the rat intestine. Gut. 1990 Jul;31(7):781–785. doi: 10.1136/gut.31.7.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., O'Brien M. J., LaMont J. T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987 Aug;93(2):273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]



