Abstract
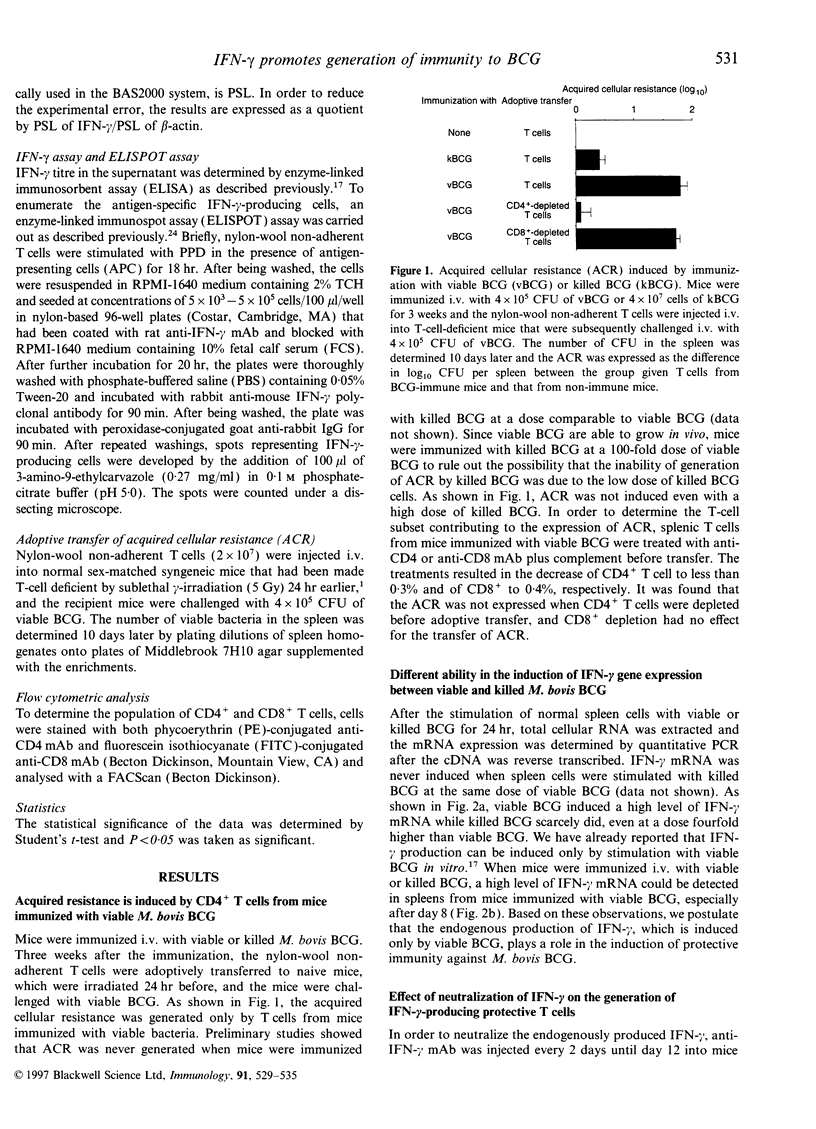
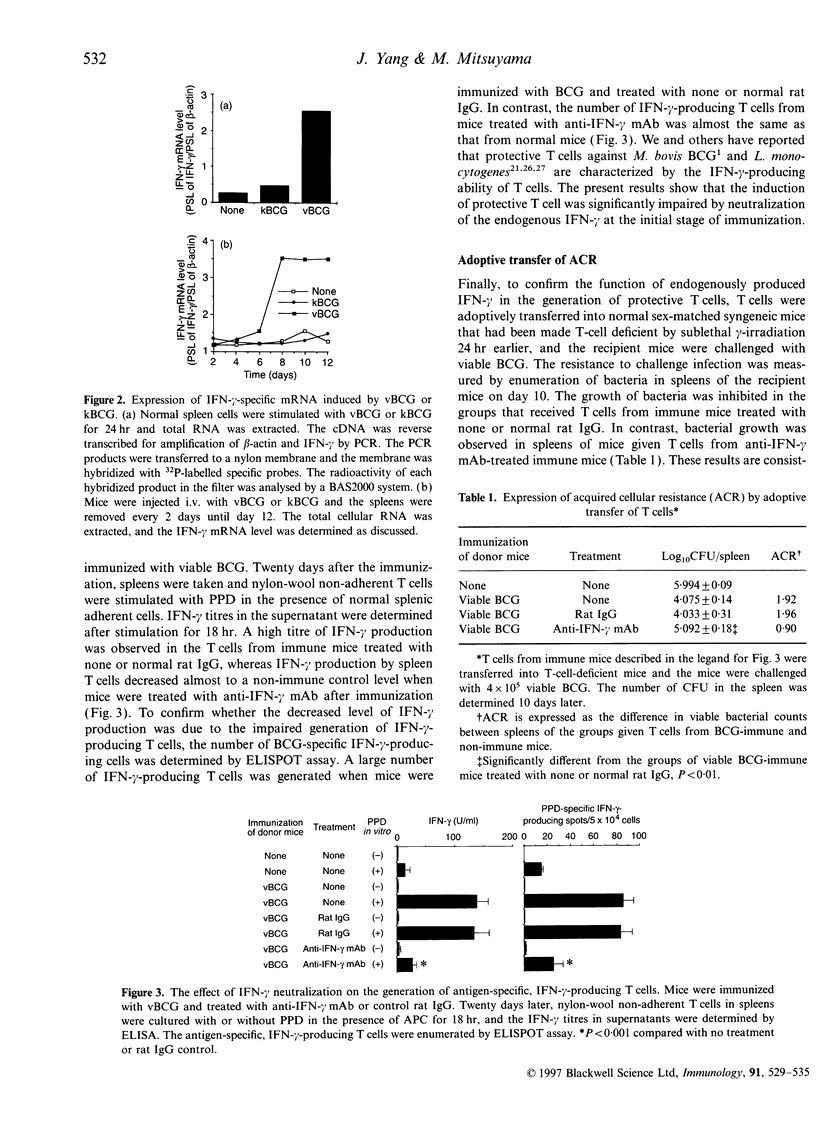
Protective CD4+ T cells against Mycobacterium bovis bacillus Calmette-Guérin (BCG), which are characterized by the ability to produce interferon-gamma (IFN-gamma), could be induced by immunization of mice with viable BCG but not with killed BCG. A high level of IFN-gamma mRNA was observed when normal spleen cells were stimulated with viable but not killed BCG. By comparing mice immunized with either viable or killed M. bovis BCG, it was found that a high level of IFN-gamma mRNA was expressed only after immunization with viable BCG. This finding prompted us to investigate the effect of neutralizing the IFN-gamma on the final generation of protective T cells against M. bovis BCG. When endogenous IFN-gamma was neutralized by administration of anti-IFN-gamma monoclonal antibody in mice immunized with viable BCG, the generation of protective T cells was significantly impaired, as revealed by the adoptive transfer of spleen T cells. The generation of BCG-specific, IFN-gamma-producing T cells was also abolished. These results clearly demonstrate that endogenous IFN-gamma actually plays a critical role in the generation of protective T cells against M. bovis BCG in vivo. Moreover, this study suggests that the lack of IFN-gamma-inducing ability is responsible for the inability of killed M. bovis BCG to induce protective T cells in mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley L. M., Dalton D. K., Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996 Aug 15;157(4):1350–1358. [PubMed] [Google Scholar]
- Chan J., Tanaka K., Carroll D., Flynn J., Bloom B. R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995 Feb;63(2):736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Roberts A. D., Rhoades E. R., Callahan J. E., Getzy D. M., Orme I. M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995 Mar;84(3):423–432. [PMC free article] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Ferrick D. A., Schrenzel M. D., Mulvania T., Hsieh B., Ferlin W. G., Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995 Jan 19;373(6511):255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L., Patel P. J. Enhanced production of murine interferon gamma by T cells generated in response to bacterial infection. J Exp Med. 1982 Jul 1;156(1):112–127. doi: 10.1084/jem.156.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday B. J., Sadick M. D., Wang Z. E., Reiner S. L., Heinzel F. P., Parslow T. G., Locksley R. M. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991 Sep 1;147(5):1653–1658. [PubMed] [Google Scholar]
- Hubbard R. D., Flory C. M., Collins F. M. Memory T cell-mediated resistance to Mycobacterium tuberculosis infection in innately susceptible and resistant mice. Infect Immun. 1991 Jun;59(6):2012–2016. doi: 10.1128/iai.59.6.2012-2016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Mitsuyama M., Muramori K., Tsukada H., Nomoto K. Interleukin-1-induced promotion of T-cell differentiation in mice immunized with killed Listeria monocytogenes. Infect Immun. 1990 Dec;58(12):3973–3979. doi: 10.1128/iai.58.12.3973-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo R., Le J., Shapiro D., Havell E. A., Huang S., Aguet M., Bosland M., Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993 Oct 1;178(4):1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura I., Tsukada H., Yoshikawa H., Fujita M., Nomoto K., Mitsuyama M. IFN-gamma-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J Immunol. 1992 May 1;148(9):2887–2893. [PubMed] [Google Scholar]
- Kawamura I., Yang J., Takaesu Y., Fujita M., Nomoto K., Mitsuyama M. Antigen provoking gamma interferon production in response to Mycobacterium bovis BCG and functional difference in T-cell responses to this antigen between viable and killed BCG-immunized mice. Infect Immun. 1994 Oct;62(10):4396–4403. doi: 10.1128/iai.62.10.4396-4403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995 Aug;16(8):374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Ladel C. H., Daugelat S., Kaufmann S. H. Immune response to Mycobacterium bovis bacille Calmette Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995 Feb;25(2):377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Hsieh C. S., Murphy K. M., O'Garra A. Dendritic cells and macrophages are required for Th1 development of CD4+ T cells from alpha beta TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-gamma production is IFN-gamma-dependent. Int Immunol. 1993 Sep;5(9):1119–1128. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M., Igarashi K., Kawamura I., Ohmori T., Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990 May;58(5):1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996 Mar;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibori T., Xiong H., Kawamura I., Arakawa M., Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun. 1996 Aug;64(8):3188–3195. doi: 10.1128/iai.64.8.3188-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The immune response to the cell wall of Mycobacterium bovis BCG. Clin Exp Immunol. 1988 Mar;71(3):388–393. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Pedrazzini T., Hug K., Louis J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987 Sep 15;139(6):2032–2037. [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E., Hoehn P., Huels C., Goedert S., Palm N., Rüde E., Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol. 1994 Apr;24(4):793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–3155. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serushago B. A., Mitsuyama M., Handa T., Muramori K., Koga T., Nomoto K. Difference in the functional maturation of T cells against Listeria monocytogenes in lymph nodes and spleen. Immunology. 1992 Feb;75(2):238–244. [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H., Kawamura I., Arakawa M., Nomoto K., Mitsuyama M. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect Immun. 1991 Oct;59(10):3589–3595. doi: 10.1128/iai.59.10.3589-3595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Kawamura I., Mitsuyama M. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect Immun. 1997 Jan;65(1):72–77. doi: 10.1128/iai.65.1.72-77.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Kawamura I., Zhu H., Mitsuyama M. Involvement of natural killer cells in nitric oxide production by spleen cells after stimulation with Mycobacterium bovis BCG. Study of the mechanism of the different abilities of viable and killed BCG. J Immunol. 1995 Dec 15;155(12):5728–5735. [PubMed] [Google Scholar]



