Introduction
This review presents a comprehensive approach to children and adults with a first seizure, an event that may have profound emotional, social, and vocational consequences.
A first “grand mal” convulsion is frightening, yet prospective, population-based studies indicate that we all face an 8-10% lifetime risk of one seizure1 and a 3% chance of epilepsy.2 It seems likely that everyone could have a seizure if a particular set of circumstances occur—but some people have a lower seizure threshold than others. A first seizure caused by an acute disturbance of brain function (acute symptomatic or provoked) is unlikely to recur (3-10%). If a first seizure is unprovoked, however, meta-analyses suggest that 30-50% will recur; and after a second unprovoked seizure, 70-80% will recur, justifying the diagnosis of epilepsy (a tendency for recurrent seizures).3-5
When a person presents to the healthcare system with a first seizure, it is almost always a convulsive seizure, either generalised or focal. Other seizure types such as absence or complex partial seizures typically occur several times before the person or family become concerned.
Methods
We reviewed all literature listed in PubMed under the headings “first seizure” and “initial seizure.” Two of us have helped to develop a practice parameter on treatment of a first seizure in children, and all of us have conducted prospective studies of first seizures. We are unaware of any systematic (Cochrane) review of this topic. All references cited were judged to give strong evidence.
Is it a seizure?
The differential diagnosis for a first seizure is wide. Most important in our experience are syncope (including breath holding and pallid syncope), transient ischaemic attacks, metabolic encephalopathy (including hypoglycaemia or electrolyte disturbance), sleep walking, night terrors, complex migraines, cardiac arrhythmias, and pseudoseizures. “Convulsive syncope” presents a particular challenge when syncope provokes a post-anoxic convulsion. A detailed history from both patient and witness is paramount, but no single feature is diagnostic. Tongue biting is not common but is fairly specific for a convulsive seizure, while postictal confusion suggests a seizure. If the first event is ambiguous, we advocate waiting for a recurrence for clarification. In our experience, and as outlined in a thoughtful review, misdiagnosis of an “epileptic” seizure may be more stigmatising than a delayed diagnosis of epilepsy.6
Summary points
The differential diagnosis of a first seizure is wide
A first seizure mandates individual counselling about the risk of recurrence, the pros and cons of drug treatment, and the impact on lifestyle
A first seizure provoked by an acute brain disturbance is unlikely to recur (3-10%), whereas a first unprovoked seizure has a recurrence risk of 30-50% over the next two years
Many people presenting for the first time with a convulsive seizure have had prior unrecognised seizures
A seizure can be diagnosed only by the history, but investigations should include prompt electroencephalography and usually magnetic resonance imaging
After counselling, most patients do not choose anti-epileptic drug treatment after a first seizure
Restrictions on activities after a first seizure should be individualised. Restrictions on driving vehicles vary between countries: in the United Kingdom non-commercial driving is not permitted for 12 months after an unprovoked seizure
The “first” seizure may not be the first
Large consecutive case series indicate that many people presenting with a dramatic first generalised tonic-clonic “grand-mal” seizure have had previous, undiagnosed simple or complex partial seizures (such as intense “deja-vu”, a sudden feeling of fear, a bad smell or taste, or brief language difficulties), absence seizures, or epileptic myoclonus.7 The first convulsive seizure may simply be the first recognised seizure pointing to the diagnosis of epilepsy.
Box 1: Essential diagnostic procedures in patients with a first seizure
Clinical examination
Assessment of seizure semiology
Routine laboratory tests (depending on clinical circumstances)
Cerebrospinal fluid (if encephalitis or subarachnoid haemorrhage is suspected)
Drug screening (depending on clinical circumstances)
Early standard electroencephalography, if possible within 24 hours
Sleep deprived electroencephalography within 1 week
High resolution magnetic resonance imaging, if possible
In all adults
In all children except those with idiopathic (genetic) focal or generalised epilepsy syndromes
What has provoked the first seizure?
Population based studies indicate that 25-30% of first seizures are “acute symptomatic” or “provoked” by a brain insult or a metabolic or toxic disturbance of brain function.8-10 Provoking factors include fever, head injury, excessive alcohol intake, withdrawal from alcohol or drugs, hypoglycaemia, electrolyte disturbance, brain infection, ischaemic stroke, intracranial haemorrhage, and proconvulsive drugs (such as clozapine, maprotiline, tramadol, theophylline, baclofen). Seizures associated with reversible metabolic or toxic disturbances are associated with a minor risk of subsequent epilepsy (≤ 3% based on large case series). Those provoked by disorders that cause permanent damage to the brain, such as brain abscess, have a higher risk of recurrence (≥ 10%).
Seizures that follow severe psychological stress or considerable sleep deprivation are not considered “acute symptomatic” but instead “triggered” by these factors in susceptible individuals with an underlying epilepsy disorder. Rarely, seizures are triggered by specific stimuli such as stroboscopic lights or reading. These reflex epilepsies can rarely be diagnosed with the first seizure, although identifying specific triggers may assist treatment for those with recurrences.
What investigations are needed?
A practice parameter noted little justification for routine investigations of blood, urine, and cerebrospinal fluid in children; however, the circumstances of a first seizure should direct investigations.11 For example, a child with insulin dependent diabetes must be assessed for hypoglycaemia, while an adult with fever and headache is a candidate for a lumbar puncture to exclude encephalitis.
If a first seizure is unprovoked, large case series support the value of electroencephalography (EEG), and often magnetic resonance imaging (MRI), to identify the cause (box 1).11,12 Such images cannot be used to diagnose the event—the diagnosis can only be made from the patient's history. The value of EEG is to point to focal lesions (especially localised slow waves), predict recurrence (see below), and indicate a specific epilepsy syndrome (spike pattern). When performed within 24-48 hours of a first seizure EEG shows substantial abnormalities in about 70% of cases.7,13 The yield may be lower with longer delays after the seizure. When standard EEG is negative, systematic case series have shown that sleep deprived EEG will detect epileptiform (spike) discharges in an additional 13-31% of cases.7,13 Sleep deprived EEG may be carried out in any routine EEG laboratory.
While not always available, MRI is the best method for structural imaging. Several case series comparing it with computed tomography in the same patient indicate that the latter may not detect small tumours or other subtle pathologies (figure).7 After a first seizure, abnormalities detected by MRI that lead directly to intervention are more common in adults than children.14 In a series of 166 adults with a first seizure, the most common aetiologies diagnosed with both computed tomography and MRI were cerebrovascular lesions (26%), brain tumours (12%), traumatic scar formations (5%), and other conditions (4%).15 Subcortical vascular encephalopathy itself is also associated with an increased risk for seizures.16 In elderly people, a first seizure may be caused by a silent stroke only recognisable by MRI.
Figure 1.
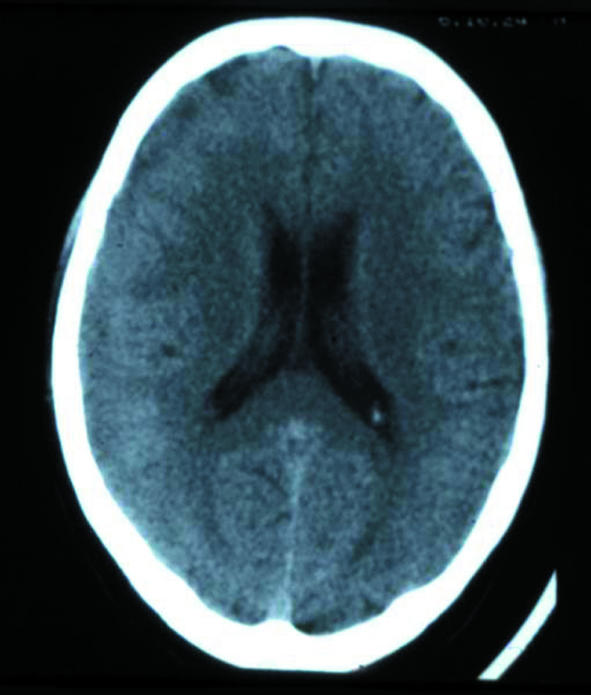
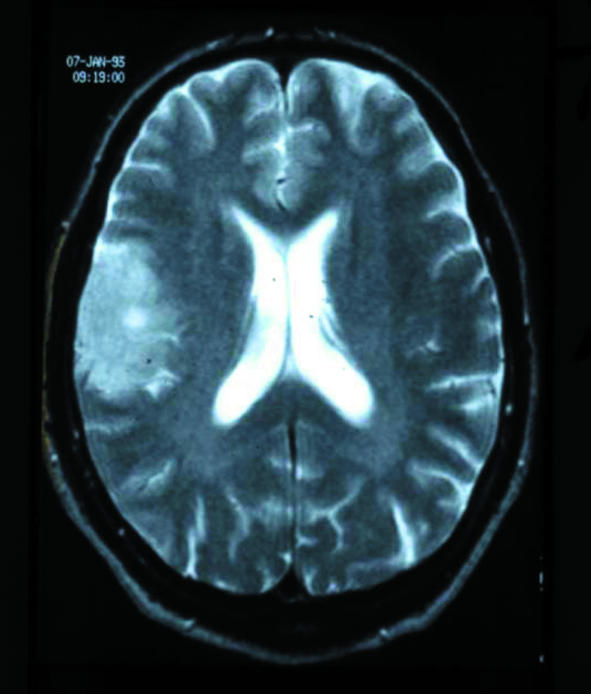
A 44 year old woman with a first seizure had an apparently normal computed tomogram (left), whereas the corresponding magnetic resonance image (right) was obviously pathological, revealing a right hemispheric glioma—and showing the superiority of MRI for structural imaging
If the seizure was unprovoked, does the person have an epilepsy syndrome?
Once an acute provoking cause has been excluded, the next step is to decide if the first seizure indicated a focal or generalised epilepsy syndrome—a critical distinction if drug treatment is considered. An epilepsy syndrome can be diagnosed after one seizure, even though a single seizure is insufficient for the diagnosis of epilepsy.7 The diagnosis of epilepsy addresses recurrence risk, whereas epilepsy syndrome is a broader concept encompassing age of onset, aetiology, prognosis, and response to treatment. For example, a child with a first nocturnal seizure and typical EEG spikes can be diagnosed as having benign rolandic epilepsy, a disorder of genetic aetiology that constitutes 15% of childhood epilepsy and nearly always remits.
In a prospective study of 300 older children and adults with a first seizure a syndrome diagnosis could be made in 80%: clinical details plus family history allowed diagnosis in 47%, EEG allowed diagnosis in an additional 30%, and plus MRI allowed diagnosis in another 4%.7
What is the recurrence risk and what sorts of activity restrictions are needed?
People with a first seizure may cope more successfully once they understand the issues. Although not systematically studied, it seems intuitively correct that avoiding provoking or triggering factors should reduce recurrences. For example, a university student with a first seizure after studying all night would be best to avoid sleep deprivation.
A meta-analysis concluded that the risk of recurrence after a first unprovoked seizure was 42% over the next two years.17 The significance of two definite unprovoked seizures within 24 hours is uncertain. One prospective study suggested that these two attacks should be viewed as a single, first seizure,5 whereas another concluded they should be viewed as separate events, permitting a diagnosis of epilepsy.18
Meta-analysis of case series17 shows that about 60-70% of recurrences are within six months of the first seizure, with an exponential decrease in risk thereafter. The strongest risk factors for recurrence are aetiology (pre-existing brain abnormalities indicate “remote symptomatic” epilepsy) and EEG abnormalities, especially focal spikes (box 2).3,13,17
We suggest that restrictions to recreational activity after a first untreated, unprovoked seizure should be individualised and limited to two or three months for children and adults.19 It seems likely, but unproved, that swimming, scuba diving, and climbing carry a higher risk for injury than do cross-country skiing, long distance running, or soccer. Individuals should probably be suspended from working with dangerous machines for at least six months.
Laws regarding the suspension of a driving license after a first seizure vary between countries from no restriction to one year. In the United Kingdom the right to drive is granted by the Driving and Vehicle Licensing Authority. Non-commercial driving is permitted after one year's freedom from seizures after an unprovoked seizure and on a case-by-case basis for provoked seizures. Commercial driving after an unprovoked seizure is usually not permitted until 10 years' freedom from seizure with antiepileptic drug treatment.
Box 2: Reported risk factors for seizure recurrence
Remote symptomatic aetiology (pre-existing static brain abnormalities that are, by implication, causative)
Focal neurological findings
Focal seizure phenomenology (including Todd's paresis)
Focal or generalised epileptiform activity on EEG
Tumours or other progressive lesions as the underlying pathology
Status epilepticus
Family history of epilepsy
Previous febrile seizures
Among neurologists there is a growing consensus that non-commercial drivers with a first unprovoked seizure should stop driving only for three to six months, especially those with favourable prognostic factors. If a first seizure was acute symptomatic, then most patients should be able to drive within three months. Commercial drivers with an unprovoked seizure should be subject to a more restrictive rule (such as at least two years seizure-free without medication).20
Are antiepileptic drugs needed after a first seizure?
Drug treatment after a first seizure is controversial.21-24 A practice parameter about first seizures in children concluded that antiepileptic drugs decrease but do not eliminate seizure recurrence and have no effect on long term remission.23 Two large recent randomised studies of children and adults compared antiepileptic drugs with no treatment after a first seizure and came to an identical conclusion.22,23 Any decision to start treatment must weigh the risk of another seizure against the risks of side effects from chronic drug treatment.21-23
Treatment may be justified when the risk of recurrence is high, such as with a focal structural brain deficit and corresponding EEG epileptiform activity (as after a stroke or brain abscess); when the risk of injury from a recurrent seizure is high (such as for those with a spinal cervical fracture, with severe osteoporosis, or taking anticoagulants); or when the risk of economic hardship from a recurrence is high (such as loss of employment).
If drug treatment is considered, which drug is preferred?
If drug treatment is considered after a first seizure, the chosen antiepileptic drug should have high efficacy, long term safety, good tolerability, and low interaction potential and allow a good quality of life, especially since half of all patients would never have another seizure without treatment. The starting dose should be in the lower range. Phenytoin and barbiturates should be avoided because of neurotoxic and cognitive side effects.
If an underlying epilepsy syndrome has been established, the following antiepileptic drugs are available (listed alphabetically because there are no available comparative trials after a first seizure):
For focal seizures—carbamazepine, clobazam (especially children), gabapentin, lamotrigine, oxcarbazepine, topiramate, valproate
For generalised seizures—lamotrigine, topiramate, valproate.
Drug choice should be individualised, and consideration given to factors such as teratogenicity, the patient's cognitive abilities, drug interactions, the doctor's familiarity with the drug, and cost.
Box 3: Steps for the family doctor
On the basis of the history and physical examination, be sure that the event was a first seizure
Exclude acute provoking factors by history and screening laboratory tests
Arrange electroencephalography and magnetic resonance imaging (if available)
Review on an individual basis the risk of a recurrence and the potential social and psychological consequences of a recurrent seizure
Review restrictions for the person's activities, especially for driving
For unprovoked seizures, discuss but usually do not prescribe antiepileptic drug treatment
Seek expert consultation for diagnosis of epilepsy syndrome and management of provoking factors
How long should drug treatment be continued?
In childhood epilepsy (as opposed to first seizure) drug treatment is usually continued until the child has been free of seizures for one to two years. If a child starts drug treatment after a first seizure, there is little justification for continuing treatment beyond one year free from seizures, except in the case of a few epilepsy syndromes, such as juvenile myoclonic epilepsy, that usually require long term treatment.
There are no published data to guide length of treatment after a first seizure in adults. Each case must be viewed individually, including consideration of the medical and social consequences of another seizure. It is tempting to use EEG and neuroimaging to help with this decision because persistent EEG abnormalities, and a documented aetiology, are associated with a higher risk of relapse when antiepileptic drugs are withdrawn after several years of remission (affirmed by a meta-analysis).25 It would seem prudent for adult patients to decide the parameters for discontinuing before starting treatment. If drug treatment is started after a first seizure in adults, we suggest at least one year of treatment, except for those at low risk for recurrence, when six months without seizures may be sufficient.
Information sources for patients
Epilepsy Action (epilepsy.org.uk/)—Sponsored by the British Epilepsy Association, this site provides information about many aspects of epilepsy for patients of all ages
DVLA. Medical rules. Chapter 1: neurological disorders (dvla.gov.uk/at_a_glance/ch1_neurological.htm)— Outlines the regulations for a driving permit for people in the UK with one or more seizures, provoked or unprovoked
Epilepsy.com (www.epilepsy.com)—This US based website includes information written by international contributors for both healthcare providers and patients
Epilepsy Foundation (epilepsyfoundation.org)—This site is sponsored by the American Epilepsy Foundation
International League Against Epilepsy (ilae-epilepsy.org)— Provides some direct information, points out other educational material, and directs patients to local organisations for additional information and support
Conclusions
A first seizure means an uncertain future for the individual, but the consequences of a recurrence vary between individuals in different geographical areas and social situations. We agree with a practice parameter that treatment decisions must take into account medical issues and patient and family preference.23 The ultimate goal of assessment and treatment is to optimise quality of life and achieve a good balance between feeling almost healthy and yet practising some caution for at least a year. Hopefully, individualised coping strategies will be improved by careful counselling (box 3).
Contributors: BP-E and PC developed the first draft of the paper. Revisions were undertaken by PC, taking into account several critical reviews by CC and EB. The final manuscript was carefully reviewed and approved by all authors. PC is guarantor for this review.
Competing interests: None declared.
References
- 1.Hauser WA, Rich SS, Annegers JF, Anderson VE. Seizure recurrence after a 1st unprovoked seizure: an extended follow-up. Neurology 1990;40: 1163-70. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993;34: 453-68. [DOI] [PubMed] [Google Scholar]
- 3.Camfield PR, Camfield CS, Dooley JM, Tibbles J, Fung T, Garner B. Epilepsy after a first unprovoked seizure in childhood. Neurology 1985;35: 1657-60. [DOI] [PubMed] [Google Scholar]
- 4.Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med 1998;338: 429-34. [DOI] [PubMed] [Google Scholar]
- 5.Shinnar S, Berg AT, O'Dell C, Newstein D, Moshe SL, Hauser WA. Predictors of multiple seizures in a cohort of children prospectively followed from the time of their first unprovoked seizure. Ann Neurol 2000;48: 140-7. [PubMed] [Google Scholar]
- 6.Jacoby A, Snape D, Baker GA. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol 2005;4: 171-8. [DOI] [PubMed] [Google Scholar]
- 7.King MA, Newton MR, Jackson MD. Epileptology of the first seizure presentation. Lancet 1998;352: 1007-11. [DOI] [PubMed] [Google Scholar]
- 8.Hart YM, Sander JW, Johnson AL, Shorvon SD. National general practice study of epilepsy: recurrence after a first seizure. Lancet 1990;336: 1271-4. [DOI] [PubMed] [Google Scholar]
- 9.Chadwick D. Diagnosis of epilepsy. Lancet 1990;336: 291-6. [DOI] [PubMed] [Google Scholar]
- 10.Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935-1984. Epilepsia 1995;36: 327-33. [DOI] [PubMed] [Google Scholar]
- 11.Hirtz D, Ashwal S, Berg A, Bettis D, Camfield C, Camfield P, et al. Practice parameter: evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, the Child Neurology Society and the American Epilepsy Society. Neurology 2000;55: 616-23. [DOI] [PubMed] [Google Scholar]
- 12.American College of Emergency Physicians, American Academy of Neurology, American Association of Neurological Surgeons, American Society of Neuroradiology. Practice parameter: neuroimaging in the emergency patient presenting with seizure (summary statement). Ann Emerg Med 1996;28: 114-8. [PubMed] [Google Scholar]
- 13.Schreiner A, Pohlmann-Eden B. Value of the early electroencephalogram after a first unprovoked seizure. Clin Electroencephalogr 2003;34: 140-6. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Riviello JJ, Harper MB, Baskin MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics 2003;111: 1-5. [DOI] [PubMed] [Google Scholar]
- 15.Pohlmann-Eden B, Schreiner A. Epileptology of the first-seizure presentation [letter]. Lancet 1998;352: 1855-6. [DOI] [PubMed] [Google Scholar]
- 16.Schreiner A, Pohlmann-Eden B, Schwartz A, Hennerici M. Epileptic seizures in subcortical vascular encephalopathy. J Neurol Sci 1995;130: 171-7. [DOI] [PubMed] [Google Scholar]
- 17.Berg A, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 1991;41: 965-72. [DOI] [PubMed] [Google Scholar]
- 18.Camfield P, Camfield C. Epilepsy can be diagnosed when the first two seizures occur on the same day. Epilepsia 2000;41: 1230-3. [DOI] [PubMed] [Google Scholar]
- 19.ILAE Commission report. Restrictions for children with epilepsy. Epilepsia 1997;38: 1054-6. [DOI] [PubMed] [Google Scholar]
- 20.Schmedding E, for the Belgian Working Group on Epilepsy and Driving. Epilepsy and driving in Belgium: proposals and justification. Acta Neurol Belg 2004;104: 68-79. [PubMed] [Google Scholar]
- 21.Musicco M, Beghi E, Solai A, Viani F. Treatment of first tonic-clonic seizure does not improve the diagnosis of epilepsy. First Seizure Trial Group (FIRST). Neurology 1997;49: 991-8. [DOI] [PubMed] [Google Scholar]
- 22.Marson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 2005;365: 2007-13. [DOI] [PubMed] [Google Scholar]
- 23.Hirtz D, Berg A, Bettis D, Camfield C, Camfield P, Crumrine P, et al. Practice parameter: treatment of the child with a first unprovoked seizure. Neurology 2003;60: 166-75. [DOI] [PubMed] [Google Scholar]
- 24.Camfield CS, Camfield PR, Gordon KG, Dooley JM. Does the number of seizures before treatment influence ease of control or remission of childhood epilepsy? Not if the number is 10 or less. Neurology 1996;46: 41-4. [DOI] [PubMed] [Google Scholar]
- 25.Berg AT, Shinnar S. Relapse following discontinuation of antiepileptic drugs: a meta-analysis. Neurology 1994;44: 601-8. [DOI] [PubMed] [Google Scholar]


