Abstract
Virion uncoating is a critical step in the life cycle of mammalian orthoreoviruses. In cell culture, and probably in extraintestinal tissues in vivo, reovirus virions undergo partial proteolysis within endosomal or/or lysosomal compartments. This process converts the virion into a form referred to as an intermediate subvirion particle (ISVP). In natural enteric reovirus infections, proteolytic uncoating takes place extracellularly within the intestinal lumen. The resultant proteolyzed particles, unlike intact virions, have the capacity to penetrate cell membranes and thereby gain access to cytoplasmic components required for viral gene expression. We hypothesized that the capacity of reovirus outer capsid proteins to be proteolyzed is a determinant of cellular host range. To investigate this hypothesis, we asked if the addition of protease to cell culture medium would expand the range of cultured mammalian cell lines that can be productively infected by reoviruses. We identified many transformed and nontransformed cell lines, as well as primary cells, that restrict viral infection. In several of these restrictive cells, virion uncoating is inefficient or blocked. Addition of proteases to the cell culture medium generates ISVP-like particles and promotes viral growth in nearly all cell lines tested. Interestingly, we found that some cell lines that restrict reovirus uncoating still express mature cathepsin L, a lysosomal protease required for virion disassembly in murine L929 cells. This finding suggests that factors in addition to cathepsin L are required for efficient intracellular proteolysis of reovirus virions. Our results demonstrate that virion uncoating is a critical determinant of reovirus cellular host range and that many cells which otherwise support productive reovirus infection cannot efficiently mediate this essential early step in the virus life cycle.
Mammalian reoviruses (reoviruses) are prototypic members of the Reoviridae family, which includes the pathogenic rotaviruses, coltiviruses, and orbiviruses. Whereas reovirus causes infections that are generally asymptomatic in humans, it can induce respiratory, enteric, and nervous system diseases in animal models (reviewed in reference 67). Reovirus virions comprise a multilayered protein capsid that surrounds a segmented, double-stranded RNA genome (reviewed in reference 51). The outermost capsid layer consists of protein σ3. The presence of σ3 imparts environmental stability to the virion (54) but also appears to negatively regulate critical virion functions such as membrane penetration. The ability of reovirus to establish a productive infection requires proteolysis of the outer capsid (13, 62, 65). More recent data point toward σ3 as the critical target for degradation (19, 20).
The first step in reovirus infection is attachment to cellular receptors through interactions with the viral receptor protein σ1 (46, 75). Following attachment, virions are internalized by receptor-mediated endocytosis and delivered to endosomal compartments (12, 13, 65). In cell culture and probably in extraintestinal tissues in vivo, reovirus virions undergo partial proteolysis within cellular endosomal and/or lysosomal compartments (13, 21, 62, 65). It has been suggested that the lysosomal cysteine protease cathepsin L is sufficient to mediate reovirus disassembly in murine L929 cells (4). During virion disassembly, the outer capsid protein σ3 is proteolytically degraded and the underlying protein μ1 is cleaved, generating particles referred to as intermediate (or infectious) subvirion particles (ISVPs) (13, 21, 62, 65). ISVPs, unlike intact virions, have the capacity to penetrate cell membranes, thereby gaining access to cytoplasmic components required for viral gene expression (12, 38, 39, 48, 66). Studies using the mouse model have revealed that in enteric reovirus infections, proteolytic uncoating takes place within the intestinal lumen, where it is likely mediated by pancreatic serine proteases such as trypsin or chymotrypsin (CHT) (7, 11). There is debate as to whether ISVPs generated extracellularly are internalized by receptor-mediated endocytosis or enter cells by directly penetrating the plasma membrane (12, 65).
Other members of the Reoviridae family have a markedly more restricted tropism than reovirus. Whereas reovirus can infect a variety of cells in vivo, including those of the respiratory and intestinal tracts, heart, muscle, and brain (50, 58, 61, 68, 74), rotavirus replication is generally restricted to villus tip enterocytes of the small intestine (15, 27, 34). In vitro, rotavirus is typically cultured in the presence of exogenous trypsin. The addition of trypsin causes cleavage of the outer capsid protein VP4. This enables rotavirus to permeabilize membranes via an activity of VP5, one of the cleavage products (28, 33). In the absence of exogenous trypsin, rotavirus can be internalized by receptor-mediated endocytosis, but the virus fails to uncoat (6).
We hypothesized that the efficiency of reovirus outer capsid proteolysis is one determinant of cellular host range, the types of cells that can be productively infected by reovirus. To test this, we asked if addition of protease to cell culture medium would expand the range of cell types that would support reovirus infection. We identified a number of transformed and nontransformed cell lines, as well as primary cells, that are restrictive for viral infection. We demonstrated that addition of intestinal proteases to the cell culture medium promotes viral growth in these cells, in some cases dramatically. Our results indicate that virion uncoating is a critical determinant of cellular host range and that many cells which otherwise support productive reovirus infection restrict this early step in the virus life cycle.
MATERIALS AND METHODS
Cells and viruses.
Murine L929 cells were maintained as suspension cultures as described previously (41). A549, U937, RAW 264.7 (RAW), BHK, primary human prostate stromal, primary human prostate epithelial, and primary human tonsil cells were maintained as monolayer cultures in RPMI medium (GIBCO-BRL, Gaithersburg, Md.). Vero cells, primary mouse embryo fibroblasts (MEFs), and 293, CRL-1492, HL-60, and 3T6 cells were maintained as monolayers in Dulbecco's modified Eagle medium (GIBCO-BRL). Both RPMI medium and Dulbecco's modified Eagle medium were supplemented to contain 10% fetal calf serum (HyClone, Logan, Utah), 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 2 mM glutamine. RPMI medium also contained 25 mM HEPES. Katherine Staskus (University of Minnesota) provided primary cultures from prostatic tissues, Peter Southern (University of Minnesota) provided primary tonsillar epithelial cells, and Bryan Williams (Cleveland Clinic) provided primary MEFs.
Reovirus serotype 1 Lang, serotype 2 Jones, and serotype 3 Dearing and c87 (Abney) are prototypic laboratory strains. Mutants 3-1 (29) and L/C (1) are variants of strain Dearing that were isolated from persistently infected L929 cells. Purified virions were prepared by CsCl density gradient centrifugation of extracts from cells infected with third-passage lysate stocks (35). Purified virions containing [35S]methionine-labeled proteins were prepared by adding 5 mCi of [35S]methionine in the form of EasyTag express protein labeling mix (NEN Life Science Products Inc., Boston, Mass.) to cell suspensions (2.0 × 108 cells at 5 × 105 cells/ml) at 17 h postinfection (p.i.). ISVPs and detergent-plus-protease subvirion particles (dpSVPs) were prepared by treating purified virions with CHT according to published methods (19, 53). To assess the efficiency of ISVP and dpSVP generation, we determined the extent to which μ1C was cleaved in various particles. Particles were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Dried gels were scanned, protein band intensity was determined by using NIH Image software, and the mean density ratio of σ2 to μ1C or δ was calculated for virions, ISVPs, and dpSVPs.
Analysis of viral protein expression in infected cells.
Cell suspensions were prepared by using 0.05% trypsin-0.53 mM EDTA to remove adherent cells from culture dishes. Cells were plated at a density of 106/ml in 6-well plates 18 to 24 h prior to infection. Cells were washed with phosphate-buffered saline (PBS) containing 2 mM MgCl2 and then infected at the specified multiplicity of infection (MOI) with virus diluted in PBS-2 mM MgCl2. Virus was allowed to adsorb to cells for 1.5 h at 4°C. At this temperature, virus binds to cells but is not internalized (63). After adsorption, cultures were fed with serum-free medium and incubated at 37°C. As indicated, some samples included 10 μg of CHT (Sigma, St. Louis, Mo.)/ml or 10 μg of trypsin (Sigma)/ml in the postadsorption medium. At the indicated times p.i., cells were removed from culture dishes (with scraping of adherent cells), collected by low-speed centrifugation (at 179 × g), and lysed in Tris lysis buffer (TLB) (10 mM Tris [pH 7.5], 2.5 mM MgCl2, 100 mM NaCl, 0.5% Triton X-100, 5 μg of leupeptin [Sigma]/ml, 1 mM phenylmethylsulfonyl fluoride [PMSF]). After 30 min on ice, samples were pelleted by low-speed centrifugation for 10 min to remove cellular debris. Protein sample buffer (1.2 M sucrose, 0.5 M Tris [pH 8.0], 20% SDS, 0.01% bromphenol blue, 50 μl of β-mercaptoethanol/ml) was added to cell lysate samples.
Protein samples (representing 105 cells) were resolved by electrophoresis on SDS-12% polyacrylamide gels and transferred to nitrocellulose membranes for 2 h at 100 V in 25 mM Tris-192 mM glycine-20% methanol. Nitrocellulose membranes (Bio-Rad Laboratories, Hercules, Calif.) were blocked overnight at 4°C in Tris-buffered saline (10 mM Tris [pH 8.0], 150 mM NaCl)-0.05% Tween (TBST) containing 5% nonfat dry milk, rinsed with TBST, and incubated with a rabbit anti-μNS polyclonal antiserum (14) (1:12,500 in TBST) for 1 h. Membranes were subsequently washed with TBST and incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) (1:7,500 in TBST) (Amersham, Arlington Heights, Ill.). Bound antibody was detected by treating the nitrocellulose filters with enhanced chemiluminescence (ECL) detection reagents (Amersham) and exposing the filters to Full Speed Blue X-ray film (Eastman Kodak, Rochester, N.Y.).
To determine if CHT-facilitated infection is influenced by agents which inhibit intracellular proteolysis of reovirus virions (3, 65), L929 cell monolayers were, in some cases, pretreated for 1 h with a medium which contained either 300 μM trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane (E-64) or 20 mM NH4Cl (both from Sigma). Cells were infected as described above. The postadsorption medium included either 300 μM E-64, 20 mM NH4Cl, or no inhibitor; some samples also included 10 μg of CHT/ml.
Analysis of intracellular proteolysis of reovirus virions.
Cells were plated at a density of 106 per 35-mm culture dish and incubated for 18 to 24 h. Prior to infection, cell monolayers were washed twice with PBS. [35S]methionine-labeled Lang virions (5 × 105 cpm/sample, which reflected an MOI of 25) were allowed to adsorb to cells for 1 h at 4°C. After adsorption, cells were washed twice with ice-cold PBS to remove unbound virions, and time zero samples were collected. Fresh serum-free medium was added to the monolayers, and they were transferred to a 37°C incubator. Some samples included CHT (10 μg/ml). At the indicated times p.i., cells were removed from culture dishes and concentrated by low-speed centrifugation for 10 min. Cell pellets were resuspended in immunoprecipitation buffer (0.1 M NaCl, 10 mM Tris [pH 7.5], 1 mM EDTA, 0.5% NP-40) and incubated for 10 min on ice. Lysates were centrifuged at 716 × g to pellet nuclei. Proteins were precipitated from the supernatants by using acetone (51), collected by centrifugation at 12,000 × g for 10 min, solubilized in protein sample buffer, and resolved on SDS-15% polyacrylamide gels. Following electrophoresis, gels were fixed, treated with Amplify (Amersham), dried under a vacuum, and placed on PhosphorImager screens. Labeled viral protein bands were analyzed by using a Storm 840 PhosphorImager and quantified by using ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.).
Analysis of viral growth.
Cells were infected at an MOI of 3 (in batch suspensions), and attachment was allowed to proceed for 1.5 h on ice at 4°C. After adsorption, virus and cells were added to 3-dram vials (4 × 105 cells/vial) containing 1 ml of chilled serum-free medium. Some samples included CHT (10 μg/ml). Triplicate samples were prepared for each time point. For each cell type and condition (with or without CHT), one set of samples (time zero) was frozen immediately at −70°C. The remaining samples were placed at 37°C. Long-term exposure of cells to CHT negatively affected cell viability. To avoid this problem, at 9 h p.i. fetal calf serum was added to each 3-dram vial so that the medium was completed with the concentration of serum normally used to culture the cells. The addition of serum effectively inhibited the activity of CHT in the CHT-containing cultures. Samples were harvested at the times indicated and subjected to three cycles of freezing and thawing, and virus was quantified by a modified CHT plaque assay on L929 cells (see below). Viral yields were calculated as (log10 PFU/ml)t = x h − (log10 PFU/ml)t = 0 h ± standard deviation (SD).
CHT plaque assay.
Protease-facilitated plaque assays have been described elsewhere (40, 73). Briefly, L929 cells were plated in 6-well plates at 106 cells per well. After 18 to 24 h of incubation, the medium was removed and monolayers were washed with 1 ml of PBS containing 2 mM MgCl2. Samples, diluted in gelatin-saline (136.9 mM NaCl, 0.27 mM CaCl2-2H2O, 0.84 mM MgCl2, 19.4 mM H3BO3, 0.13 mM Na2B3O7, 0.3% gelatin [pH 7.4]), were added and allowed to adsorb to cells for 1 h at 37°C. Following adsorption, the inoculum was removed. Cell monolayers were covered with 1% agar and serum-free 199 medium (Irving Scientific, Santa Ana, Calif.), supplemented to contain 2 mM glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 250 ng of amphotericin/ml, and 10 μg of CHT/ml. Plaques were counted 3 days after infection.
Neutralization experiments.
Purified Lang virions (2.3 × 105 PFU, corresponding to 7.2 × 107 particles) were diluted into either 1 ml of PBS, rabbit anti-reovirus core serum (20) (diluted 1:1,000 in PBS), or the anti-σ1 monoclonal antibody 5C6 (diluted to 1 μg/ml in PBS) (71) and were incubated for 1 h at 37°C. The virus-antibody mixture was then added to L929 cells at an MOI of 3 and allowed to adsorb for 1.5 h at 4°C. After adsorption, infected cells were transferred into vials (4 × 105 infected cells/vial) containing serum-free medium. Some samples included CHT (10 μg/ml). Triplicate samples were prepared for each time point and antibody treatment. Time zero samples were frozen immediately at −70°C, and the remaining samples were incubated at 37°C for 24 h. Cell-associated virus was released by three cycles of freezing and thawing. Viral growth was analyzed by plaque assay on L929 cell monolayers as described elsewhere (72).
Immunoblot analysis for cathepsin L.
Cell lysates were prepared from L929, MEF, RAW, and 3T6 cells using TLB as described above for the analysis of viral protein expression. The concentration of protein within each sample was determined by using the Bio-Rad DC protein assay kit. Secreted proteins from culture supernatants were precipitated with 20% trichloroacetic acid containing 25 μg of salmon sperm DNA/ml, centrifuged at 12,000 × g for 10 min, and resuspended in sample buffer.
Protein samples, normalized for either protein content (cellular proteins) or cell number (secreted proteins), were resolved by electrophoresis on SDS-15% polyacrylamide gels and transferred to nitrocellulose membranes. Nitrocellulose membranes were blocked overnight in TBST containing 10% nonfat dry milk, washed with TBST, and incubated with a rabbit antiserum raised against murine cathepsin L (1:5,000 in TBST) (56). Membranes were subsequently washed with TBST and then incubated with a horseradish peroxidase-conjugated anti-rabbit IgG (1:7,500 in TBST). Protein bands were detected by using ECL reagents as described above.
RESULTS
Addition of exogenous protease facilitates viral infection in many restrictive cell lines.
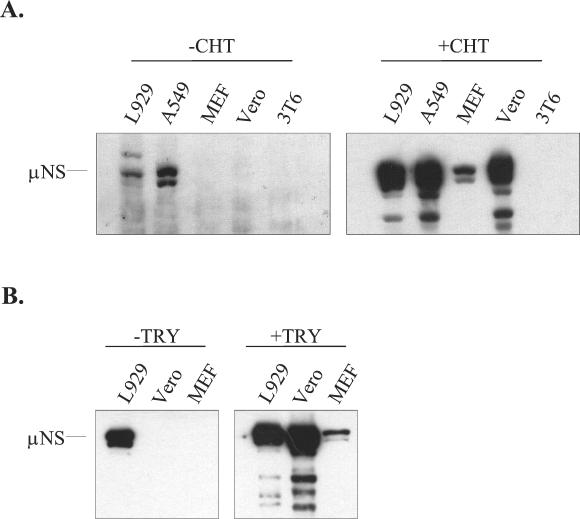
To investigate whether the efficiency of reovirus outer capsid proteolysis is an important determinant of cellular host range, we asked if addition of protease to the culture medium could expand the range of cell types that could be infected in vitro. Replicate cultures of L929, A549, MEF, Vero, and 3T6 cells were infected with reovirus type 1 Lang. After adsorption, half of the samples were incubated in a culture medium that contained CHT. CHT was chosen because it has been widely used to generate reovirus subvirion particles in vitro (for example, see reference 52) and because of its probable role in proteolysis of reovirus particles in the intestinal tract of the host (7, 11). At 9 h p.i., cell lysates were harvested and viral protein expression was analyzed by immunoblotting. At this time point, viral protein synthesis is readily detectable in permissive mouse L929 fibroblasts. We used an antiserum directed against the reovirus nonstructural protein μNS to ensure that only new viral protein synthesis was detected. Results are shown in Fig. 1A. Synthesis of μNS was observed in L929 fibroblasts and human A549 respiratory epithelial cells in the absence of protease. The presence of CHT enhanced the level of μNS detected at 9 h p.i. in these cell lines. In contrast, μNS was not detected at this time point in lysates of primary MEFs or Vero cells unless CHT was included in the culture medium. We did not detect μNS synthesis in mouse 3T6 fibroblasts infected with strain Lang even when CHT was included in the culture medium. Increasing the MOI from 3 to 50 did not overcome the apparent block to viral protein synthesis in infected Vero, MEF, or 3T6 cells (data not shown).
FIG. 1.
Effects of exogenous protease on viral protein expression in L929, A549, MEF, Vero, and 3T6 cells. (A) L929, A549, MEF, Vero, and 3T6 cells were infected with reovirus serotype 1 Lang at an MOI of 3. CHT (10 μg/ml) either was (+ CHT) or was not (− CHT) included in the postadsorption medium. Cell extracts were prepared from samples at 9 h p.i., electrophoresed on SDS-12% polyacrylamide gels, transferred to nitrocellulose membranes (Bio-Rad), and probed with a rabbit anti-μNS antiserum (diluted 1:12,500). Bound antibody was detected by using reagents that generate a chemiluminescent signal. (B) Cultures of the indicated cell types were infected as described for panel A, except that an MOI of 20 was used. Trypsin (10 μg/ml) either was (+ TRY) or was not (− TRY) included. Cell extracts were harvested and analyzed for μNS expression as described for panel A.
In natural enteric infections, reovirus is exposed to multiple intestinal proteases (7, 11). In vitro, the intestinal protease trypsin has been used to generate ISVPs that are structurally similar to those generated by CHT treatment (53). To determine if trypsin, like CHT, could enable reovirus to infect restrictive cells, we used the protocol described above, except that trypsin was substituted for CHT. The results of this experiment, shown in Fig. 1B, demonstrate that viral infection of MEFs and Vero cells is also facilitated by trypsin.
Addition of exogenous CHT generates ISVP-like particles capable of bypassing normal requirements for viral uncoating.
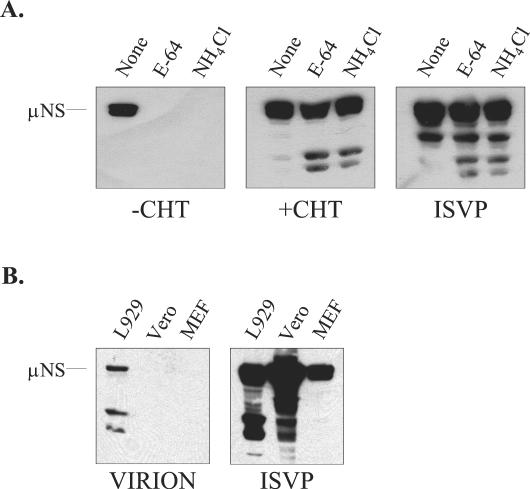
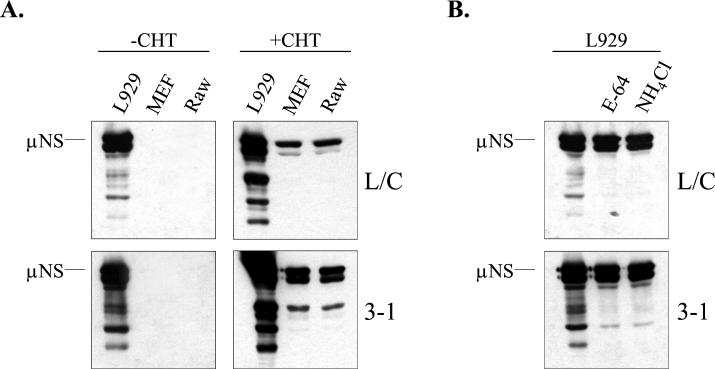
There are several mechanisms by which inclusion of CHT in the postadsorption culture medium might have facilitated infection of restrictive cells. One possibility was that the addition of CHT generated ISVP-like particles capable of bypassing the normal cellular requirements for acidic endosomal or lysosomal pH and cysteine protease activity. However it was also possible that CHT facilitated infection by altering cell surface components involved in infection. To gain insight into the mechanism by which CHT facilitated infection of restrictive cells, we first asked whether this treatment generated ISVP-like particles in the cultures. We examined the effects of the cysteine protease inhibitors E-64 and NH4Cl on infection of L929 cells by reovirus strain Lang in the presence or absence of exogenous CHT. In permissive cells, these agents have been shown to inhibit reovirus uncoating and to block infection by virions; infection with in vitro-generated ISVPs is not inhibited by these agents (3, 65). Infection was monitored by assaying expression of the viral nonstructural protein μNS (Fig. 2A). As expected, both E-64 and NH4Cl inhibited infection by viral stocks when CHT was not included in the postadsorption medium. In contrast, μNS was synthesized in infected cultures treated with 10 μg of CHT/ml, even if the postadsorption culture medium also contained E-64 or NH4Cl. μNS expression was also seen in the positive-control samples that were infected with in vitro-generated ISVPs prepared from purified Lang virions. These findings indicate that addition of CHT to the postadsorption medium generates particles that, like ISVPs, bypass the normal requirements for pH-dependent proteolysis in endocytic vesicles.
FIG. 2.
Effects of E-64 and NH4Cl on CHT-facilitated infection. (A) L929 cells were infected at an MOI of 20 either with a crude virus stock, with or without CHT at 10 μg/ml (+ CHT or − CHT, respectively), or with ISVPs generated from purified Lang virions. After adsorption, some of the samples received medium containing E-64 (300 μM) or NH4Cl (20 mM). At 9 h p.i., samples were harvested and μNS expression was detected by immunoblotting as described in the legend to Fig. 1. (B) Purified Lang virions or ISVPs were used to infect L929, Vero, and MEF cells at an MOI of 20. At 9 h p.i., μNS expression was analyzed by immunoblotting as described in the legend to Fig. 1.
To explore the possibility that CHT modification of cell surface components contributed to the mechanism by which CHT facilitated infection, we asked if in vitro-generated ISVPs had the capacity to infect restrictive cells. To prepare ISVPs, we treated purified Lang virions with CHT for 30 min and terminated the reaction with PMSF (53). Infectivity was determined by plaque assay, and the particles were used to infect permissive L929 cells and restrictive Vero and MEF cells. At 9 h p.i., viral protein expression was analyzed by immunoblotting. The results are shown in Fig. 2B. μNS expression was evident at 9 h p.i. in each of the three cell types when ISVPs were used to initiate infection. In contrast, when intact purified virions were used, μNS was detected only in L929 cells. These results argue strongly that CHT facilitates infection of restrictive cells by generating ISVP-like particles rather than by affecting the host cell.
Virion uncoating is impaired in some restrictive cell lines.
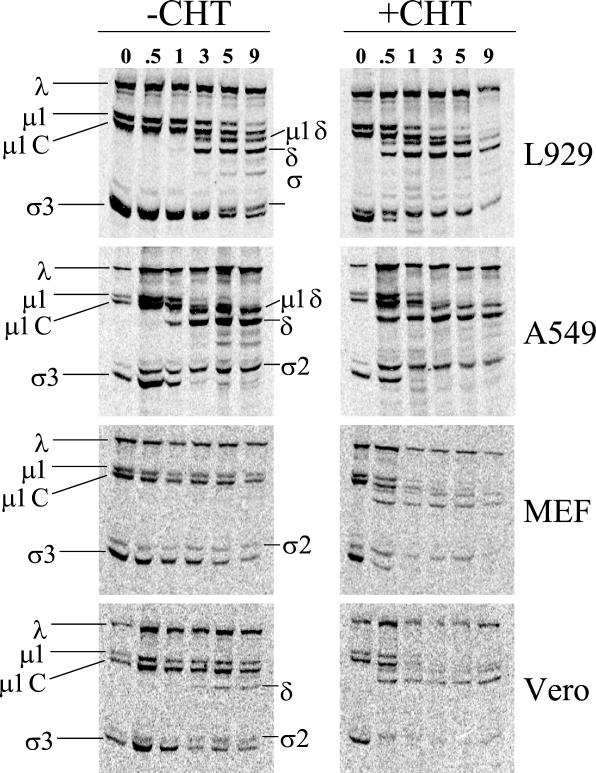
The results shown in Fig. 2 strongly suggest that the block to infection in some restrictive cells is at the level of virion uncoating, since restrictive cells can be infected either by ISVPs or by including CHT in the postadsorption medium. To examine uncoating directly, we infected L929, A549, MEF, or Vero cells with [35S]methionine-labeled Lang virions, prepared cell extracts at various times p.i., and subjected samples to SDS-PAGE and phosphorimager analysis (Fig. 3). The characteristic pattern of proteolytic uncoating (4, 65) can be observed in infected L929 and A549 cells in the absence of CHT. Early in infection, the outer capsid protein σ3 is degraded and the underlying protein μ1/μ1C undergoes endoproteolytic cleavage, yielding the detectable N-terminal fragments δ/μ1δ and the smaller C-terminal fragment φ (not retained on this gel). Addition of exogenous protease potentiates uncoating in infected L929 and A549 cell cultures, as evidenced by the increased extents of σ3 and μ1/μ1C cleavage at early times (0.5 and 1 h) in the CHT-exposed samples. This is consistent with previous findings that uncoating occurs within 3 h p.i. in the permissive L929 cell line (65). In contrast, there was little detectable σ3 or μ1/μ1C cleavage in infected MEF cells in the absence of exogenous enzyme, even at 9 h p.i. We observed a similar block to uncoating in infected 3T6 cells (data not shown). In contrast, the block to uncoating did not appear to be as severe in infected Vero cells. In the absence of CHT, we detected a small degree of outer capsid proteolysis in infected Vero cells at the later time points analyzed. In the presence of CHT, infected MEF and Vero cells showed the characteristic pattern of σ3 and μ1/μ1C cleavage. The finding that postadsorption CHT-treatment rapidly generates ISVP-like particles is consistent with our observation that infections in the presence of CHT and E-64 or NH4Cl progress to the stage of viral protein expression (Fig. 2).
FIG. 3.
Analysis of virion uncoating in the absence or presence of exogenous CHT. Monolayers of L929, A549, MEF, or Vero cells were infected with 5 × 105 cpm of purified [35S]methionine-labeled Lang virions (equivalent to an MOI of 25). After 1 h of adsorption at 4°C, the inoculum was removed, and fresh medium was added (with [+] or without [−] CHT). Samples were incubated at 37°C for the times (in hours) indicated at the top. Cells were then lysed, and extracts were prepared as described by Sturzenbecker and colleagues (65). Samples were subjected to electrophoresis on SDS-12% polyacrylamide gels. Gels were dried under a vacuum, and radioactivity was detected by PhosphorImager analysis (Molecular Dynamics). The positions of capsid proteins are indicated. Virion uncoating is characterized by the removal of σ3, cleavage of μ1 and μ1C, and the presence of the μ1/μ1C cleavage fragments μ1δ and δ.
CHT-facilitated infection requires receptor interactions but is not a consequence of increased cell binding.
In the conversion of virions to ISVPs, σ3 is removed, the underlying protein μ1/μ1C is cleaved, and the vertex-associated σ1 molecules are believed to assume an extended conformation (reviewed in reference 30). To elucidate the role of the cell attachment protein σ1 in CHT-facilitated infection, we first asked if infection could be blocked with 5C6, a neutralizing σ1-specific monoclonal antibody (71). We incubated purified Lang virions with either 5C6 or a control anti-core antiserum and then used the particles to infect L929 cell monolayers in the presence or absence of protease. Viral yields were measured at 24 h p.i. The results of this experiment are shown in Table 1. We found that the σ1-specific antibody neutralized infection even if CHT was included in the postadsorption medium. In the absence of antibody or in the presence of a core-specific antiserum, we observed a ∼2-log-unit increase in viral yield at 24 h p.i. This result indicates that CHT-facilitated infection is dependent on σ1-cell receptor interactions, as is infection by purified ISVPs (19).
TABLE 1.
Capacities of anti-σ1 and core-specific antibodies to neutralize infectivitya
| Sample | Log10 PFU/ml
|
|
|---|---|---|
| Without CHT | With CHT | |
| No antibody | 1.88 ± 0.12 | 2.23 ± 0.07 |
| Anti-core | 1.92 ± 0.12 | 2.12 ± 0.09 |
| Anti-σ1 (5C6) | 0.14 ± 0.12 | 0.20 ± 0.08 |
Purified Lang virions were incubated with either PBS, a rabbit antiserum raised against reovirus cores, or the anti-σ1 monoclonal antibody 5C6 for 1 h at 37°C. Virus-antibody mixtures were used to infect L929 cells in the absence or presence of CHT. Yields at 24 h p.i. were measured by standard plaque assay on L929 cells. Each value represents the yield from three independent samples, calculated as described in Materials and Methods.
It has been suggested that the extended conformation of σ1 may enhance the capacity of ISVPs to interact with cell receptors (2). To determine if the mechanism by which CHT facilitates infection involves enhanced cell binding, we quantitated the levels of cell-associated virus at time zero in the experiment for which results are shown in Fig. 3. As described earlier, equal amounts of radiolabeled virus were allowed to attach to cells for 1 h at 4°C, and unbound virus was removed by washing with ice-cold PBS. At 4°C, virus binds to cells but is not internalized; by 30 min p.i. at 37°C, a majority of bound virus has been reported to be internalized (63). As shown in Table 2, CHT treatment does not enhance viral binding, since the level of cell-associated radioactivity in the λ core proteins is not higher in infections with CHT. The fact that there were fewer cell-associated counts in most of the samples that contained CHT is consistent with the observation that ISVPs may have fewer σ1 molecules than do virions (25). We obtained similar results in experiments in which scintillation counting was used to compare the capacities of purified radiolabeled virions and ISVPs to bind to L929 cells and a macrophage cell line (J. Linke and L. Schiff, unpublished data).
TABLE 2.
Quantitation of cell-associated inoculuma
| Cell line | Amt of cell-associated virus (cpm)
|
Ratio (amt with CHT/amt without CHT) | |
|---|---|---|---|
| Without CHT | With CHT | ||
| L929 | 37,864 | 24,800 | 0.65 |
| A549 | 8,321 | 6,659 | 0.88 |
| Vero | 5,207 | 4,864 | 0.93 |
| MEF | 7,879 | 6,706 | 0.85 |
Equal amounts of purified [35S]methionine-labeled virus (strain Lang) were added to cells at 4°C. After adsorption, unbound virus was removed by a wash with ice-cold PBS. Medium was added (with or without CHT at 10 μg/ml). Cells were collected immediately (t = 0 h); cell-associated counts were recovered by acetone precipitation. Equivalent amounts of the cell extracts from samples with and without CHT were analyzed by SDS-PAGE. Cell-associated radioactivity in the viral λ proteins was quantitated after phosphorimaging using ImageQuant software (Molecular Dynamics).
Protease-facilitated infection requires cleavage of σ3 and not the endoproteolytic cleavage of μ1C that generates δ and φ.
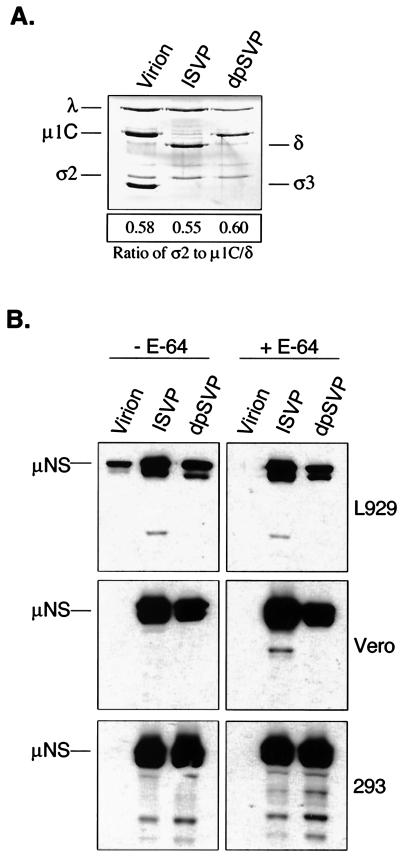
As mentioned above, proteolytic conversion of virions to ISVPs results in the removal of σ3 and endoproteolyic cleavage of the underlying protein μ1/μ1C. To determine whether one or both of these events is critical to the capacity of proteolyzed particles to infect restrictive cells, we investigated the infectivity of dpSVPs. dpSVPs are ISVPs that are prepared in the presence of alkyl sulfate detergents (19). They differ from ISVPs in that μ1/μ1C is uncleaved (as it is in virions); similar levels of μ1-related proteins are present in the three particle types (Fig. 4A). We infected permissive L929 and restrictive Vero and 293 cells with strain Lang virions, ISVPs, and dpSVPs. In one set of samples, E-64 was added to inhibit intracellular cleavage of σ3 (in the virion-infected cells) and μ1/μ1C (in the virion- and dpSVP-infected cells) (3, 19). At 9 h p.i., cell lysates were prepared and μNS expression was analyzed by immunoblotting. Results are shown in Fig. 4B. As expected, after infection with virions, μNS was detected only in the L929 cell extracts that had not been treated with E-64. ISVPs were capable of establishing infection in L929, Vero, and 293 cells, even when the medium included E-64. The phenotype of dpSVP infections was identical to that of ISVP infections, indicating that the endoproteolytic cleavage of μ1/μ1C that generates δ and φ is not required for infection of restrictive cells. This result strongly suggests that protease treatment facilitates infection in restrictive cells by effecting σ3 cleavage.
FIG. 4.
Analysis of the capacities of different subvirion particles to infect restrictive cells. (A) dpSVPs were prepared by treating purified Lang virions for 10 min with CHT in the presence of sodium tetradecyl sulfate (19). Digestions were terminated with PMSF, and 1010 particles were analyzed by SDS-PAGE. Viral proteins (indicated on the left) were stained with Coomassie brilliant blue. The mean density ratio of σ2 to μ1C/δ was calculated as described in Materials and Methods. (B) Purified Lang virions, ISVPs, and dpSVPs were used to infect L929, Vero, and 293 cells at an MOI of 20. At 9 h p.i., μNS expression was analyzed by immunoblotting as described in the legend to Fig. 1.
Cells differ in the extent to which CHT facilitates viral growth.
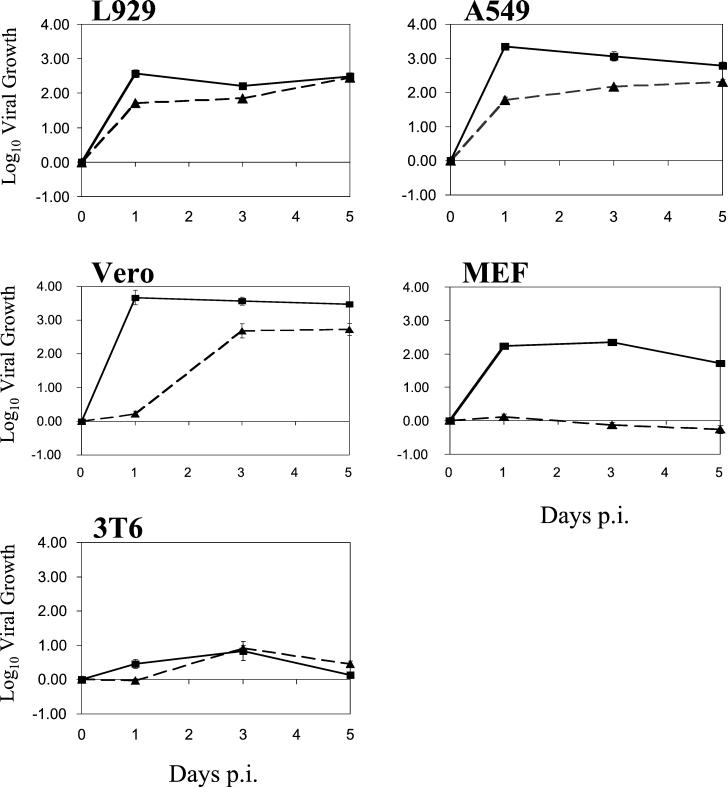
Our analysis of viral uncoating and protein expression suggests that in some restrictive cells, such as Vero cells, inefficient viral disassembly might delay replication. In other cells, such as MEFs, infection by reovirus virions might be inhibited due to a more complete block to uncoating. It is also conceivable that reovirus might initiate an abortive infection in some cell types, such that viral proteins would be expressed but infectious progeny would not be produced due to a block at a later stage, such as viral assembly. To investigate the extent to which replication is inhibited within restrictive cells and to ask whether CHT can rescue restrictive infections, we performed single-cycle growth analyses in the presence or absence of CHT. Cells were infected with reovirus strain Lang at an MOI of 3, and viral yields were measured at 1, 3, and 5 days p.i. Results of representative experiments are shown in Fig. 5. The extent of reovirus replication and the effect of CHT differed among the various cell lines, but results were consistent with those of protein expression and uncoating experiments. In both L929 and A549 cells, Lang replicated efficiently, even in the absence of protease. Viral yields were increased at early times p.i. in the presence of CHT, in agreement with reports that infection by ISVPs has a shorter eclipse phase than does infection by virions (12, 26). In MEFs, in the absence of CHT, we did not detect growth at 5 days p.i. This result is consistent with the inability of reovirus to uncoat (Fig. 3) and express protein (Fig. 1 and 6; also data not shown) efficiently in these cells. However, addition of CHT enabled Lang virions to productively infect MEFs. In the presence of protease, yields in MEFs were comparable to those in permissive L929 cells. The growth phenotype in Vero cells was distinct. In the absence of CHT, there was little replication at 24 h p.i., consistent with our protein expression results (Fig. 1). At later time points, however, viral yields were similar to those in permissive L929 and A549 cells. This long eclipse phase is consistent with the delay in uncoating observed after infection of Vero cells with radiolabeled virions (Fig. 3) and the fact that some viral protein expression could be detected at 24 h p.i. (data not shown). In the presence of CHT, reovirus strain Lang replicated very efficiently in Vero cells, with a 3.5-log-unit increase in growth detectable at 24 h p.i. Lastly, viral yields were poor in 3T6 cells, even in the presence of CHT. This suggests that 3T6 cells restrict replication at a step other than, or in addition to, virion uncoating.
FIG. 5.
Analysis of viral growth in the presence or absence of CHT. L929, A549, MEF, Vero, and 3T6 cells were infected with virus at an MOI of 3. After adsorption, samples were incubated in medium with (solid lines) or without (dashed lines) the addition of CHT at 10 μg/ml. The amounts of virus present at time zero and at 1, 3, and 5 days p.i. were determined by a modified CHT plaque assay on L929 cell monolayers, and yields were determined as described in Materials and Methods. Each time point represents the mean (± SD) derived from three independent samples.
FIG. 6.
Capacity of PI virus isolates L/C and 3-1 to replicate in L929, MEF, and RAW cells. (A) L929, MEF, and RAW cells were infected at an MOI of 5 with PI virus isolates 3-1 and L/C. After adsorption, samples were incubated in media that either did or did not contain CHT at 10 μg/ml (+ CHT and − CHT, respectively). At 15 h p.i., cell lysates were prepared and μNS synthesis was analyzed by immunoblotting as described in the legend to Fig. 1. (B) Control L929 cell infections were performed with the PI viruses at an MOI of 5 in the presence of NH4Cl or E-64, as described in the legend to Fig. 3. Analysis of viral protein expression was performed at 15 h p.i., as described in the legend to Fig. 1.
Reovirus infection of many cell lines and primary cell cultures can be facilitated by CHT treatment.
To investigate how widespread the restriction to virion uncoating is, we assayed a variety of transformed and nontransformed cell lines, as well as primary cell cultures, for their abilities to support reovirus growth in the absence or presence of CHT. We infected cells with strain Lang in the presence or absence of CHT and incubated them for 24 h. Triplicate samples were harvested, and yields were determined by plaque assay on L929 cells. The amount of viral growth was calculated, as was the enhancement afforded by CHT treatment. The results of this analysis are shown in Table 3. Some established cell lines, including L929, A549, and U937 cells, supported reovirus infection in the absence of exogenous protease but demonstrated increased yields (1 to 1.5 log units) at 24 h p.i. in the presence of CHT. Vero, CRL-1492, and 293 cells were relatively restrictive to reovirus infection in the absence of exogenous protease but demonstrated more than a 2-log-unit increase in yield when the postadsorption medium included CHT. Other restrictive cell lines, such as RAW cells and HL-60 cells, showed more-modest enhancement of viral growth in the presence of CHT. CHT-mediated enhancement of viral growth was not strictly a phenomenon of continuous cell lines. In addition to MEFs, two other primary cell cultures (from a human tonsil and from human prostatic epithelium) could be infected by reovirus when CHT was included in the culture medium. In summary, addition of CHT increased 24-h viral yields to some extent in all cells tested, although replication remained severely restricted in a few cell lines. The results of this survey suggest that the early, essential step of viral uncoating is blocked or extremely inefficient in many cell types that are otherwise permissive for reovirus infection.
TABLE 3.
Effects of CHT on the replication of reovirus strain Lang in a panel of primary cell cultures and continuous cell linesa
| Cell type | Description | Viral yield (log10) at 24 h
|
Log10 increase in viral growth with CHT | |
|---|---|---|---|---|
| Without CHT | With CHT | |||
| L929 | Mouse fibroblast cell line | 1.70 ± 0.08 | 2.77 ± 0.16 | 1.07 |
| A549 | Human respiratory epithelial cell line | 1.59 ± 0.09 | 2.54 ± 0.04 | 0.95 |
| U937 | Human monocyte cell line | 1.19 ± 0.03 | 2.76 ± 0.06 | 1.58 |
| BHK | Baby hamster kidney cell line | 0.67 ± 0.06 | 1.07 ± 0.19 | 0.40 |
| PrEp | Primary human prostatic epithelial cells | 0.51 ± 0.03 | 1.61 ± 0.09 | 1.10 |
| Vero | African green monkey kidney cell line | 0.39 ± 0.33 | 2.64 ± 0.16 | 2.25 |
| Primary tonsil | Primary human tonsillar cells | 0.21 ± 0.13 | 1.25 ± 0.07 | 1.04 |
| CRL-1492 | Rat pancreatic tumor cell line | 0.16 ± 0.08 | 2.47 ± 0.04 | 2.30 |
| 293 | Human kidney epithelial cell line | 0.13 ± 0.07 | 2.17 ± 0.11 | 2.26 |
| MEF | Primary mouse embryo fibroblast cells | 0.03 ± 0.09 | 1.67 ± 0.04 | 1.63 |
| PrSc | Primary human prostate stromal cells | 0.11 ± 0.05 | 0.57 ± 0.11 | 0.46 |
| HL-60 | Human promyelocytic leukemia cell line | −0.03 ± 0.08 | 1.21 ± 0.14 | 1.07 |
| RAW | Mouse macrophage cell line | −0.04 ± 0.19 | 0.82 ± 0.25 | 0.86 |
| 3T6 | Mouse embryo fibroblast cell line | −0.09 ± 0.08 | 0.27 ± 0.04 | 0.35 |
Cells were infected at an MOI of 3 with reovirus strain Lang. After adsorption, cells were incubated at 37°C in media that either did or did not contain CHT at 10 μg/ml. Samples were harvested at 24 h p.i., and yields were determined by a modified CHT plaque assay on L929 cells. Each value represents the yield from three independent samples, calculated as described in Materials and Methods. Cells are ranked relative to the amount of viral growth in the absence of CHT; the least permissive cells are found at the bottom of the table.
Protease-facilitated infection of restrictive cells is not strain specific.
Different reovirus strains infect distinct cell types and cause disease in different tissues in vivo (reviewed in reference 69). For example, it has been known for some time that reovirus strain type 3 Dearing does not cause disease in the mouse model when inoculated perorally, whereas some other strains efficiently cause disease by this route (10, 57). The molecular basis for this strain difference has recently been shown to involve the sensitivity of the Dearing cell attachment protein σ1 to proteolytic cleavage (22, 52). To determine if protease-facilitated infection of cultured cells is specific to reovirus strain Lang, we infected a small but representative panel of the cell lines analyzed in Table 3 with reovirus type 2 Jones and two serotype 3 strains, Dearing and c87 (Abney), and measured 24-h viral yields in the presence and absence of CHT. We included c87 in our analysis because, in vitro, the response of host cells to different serotype 3 strains can vary substantially (for example, see reference 70). Results are shown in Table 4. With respect to infection by strain Dearing, in the absence of CHT, Vero cells were the most permissive and RAW cells were the least permissive of the restrictive cells analyzed. The pattern was similar when we analyzed infection by other strains. After L929 cells, Vero cells were the most permissive cell type in the absence of added protease, supporting 1.3 log units of replication after infection with c87 and approximately one-half log unit after infection with strain Jones. CHT treatment substantially increased the viral yields of serotype 2 and serotype 3 strains in Vero, 293, RAW, and HL-60 cells. Together with the results of experiments using strain Lang, these data indicate that there is no serotype specificity to the ability of exogenous CHT to facilitate infection. It is notable that even strain Dearing, whose cell attachment protein is susceptible to proteolysis by CHT, is capable of infecting cells under these conditions.
TABLE 4.
Effects of CHT on the replication of reovirus serotypes 2 and 3a
| Virus | Cell type | Viral yield (log10) at 24 h
|
Log10 increase in viral growth with CHT | |
|---|---|---|---|---|
| Without CHT | With CHT | |||
| Dearing | L929 | 2.11 ± 0.02 | 2.48 ± 0.06 | 0.37 |
| Vero | 0.23 ± 0.12 | 1.45 ± 0.10 | 1.22 | |
| 293 | −0.04 ± 0.03 | 2.26 ± 0.06 | 2.29 | |
| HL-60 | 0.01 ± 0.09 | 1.25 ± 0.04 | 1.24 | |
| Raw | −0.08 ± 0.04 | 1.32 ± 0.00 | 1.40 | |
| Jones | L929 | 2.19 ± 0.10 | 2.00 ± 0.06 | −0.19 |
| Vero | 0.52 ± 0.04 | 2.35 ± 0.04 | 1.81 | |
| 293 | 0.07 ± 0.07 | 1.66 ± 0.07 | 1.59 | |
| HL-60 | −0.03 ± 0.06 | 1.17 ± 0.04 | 1.20 | |
| Raw | 0.21 ± 0.05 | 1.02 ± 0.09 | 0.81 | |
| c87 (Abney) | L929 | 2.50 ± 0.04 | 2.61 ± 0.09 | 0.11 |
| Vero | 1.31 ± 0.09 | 2.39 ± 0.06 | 1.08 | |
| 293 | 0.05 ± 0.07 | 2.66 ± 0.05 | 2.61 | |
| HL-60 | 0.04 ± 0.04 | 1.42 ± 0.03 | 1.38 | |
| Raw | 0.10 ± 0.09 | 1.29 ± 0.06 | 1.19 | |
Cells were infected at an MOI of 3 with reovirus serotype 2 Jones, serotype 3 Dearing, or the serotype 3 strain c87 (Abney). After adsorption, cells were incubated at 37°C in media that did or did not contain CHT at 10 μg/ml. Samples were harvested at 24 h p.i.; yields were determined by a modified CHT plaque assay on L929 cells. Each value represents the yield from three independent samples, calculated as described in Materials and Methods.
The block to uncoating in restrictive cells appears to be distinct from that which develops during persistent infection of L929 cells.
Our results suggest that many cells restrict reovirus infection because they are unable to efficiently mediate cleavage of the outer capsid protein σ3. To begin to elucidate the nature of the block(s) to uncoating within restrictive cell lines, we took advantage of reagents developed during the study of persistent reovirus infection. During persistent reovirus infections of murine L929 cells, mutant cells are selected that restrict viral disassembly (3, 29, 77) because they do not express mature cathepsin L, a lysosomal cysteine protease (4). These mutant cells fully support reovirus growth when infection is initiated with ISVPs or with the viral mutants that coevolve with the cells (1, 29). The mutant viruses (PI viruses) that are coselected during persistent infection of L929 cells have an enhanced disassembly phenotype. These viruses uncoat more rapidly than wild-type virus in vitro and replicate in E-64- and NH4Cl-treated cells (3, 29, 77). Genetic studies reveal that pertinent viral mutations are contained within the gene which encodes σ3 (3, 77).
To determine if the block to uncoating in restrictive cells is similar to that which develops during persistent infection of L929 cells, we asked if viruses isolated from persistently infected L929 cell cultures could infect restrictive MEFs and RAW cells. RAW is a murine macrophage cell line that is restrictive to reovirus infection in the absence of exogenous protease (Table 3). We used two independently isolated L929 cell-derived PI viruses, L/C (1) and 3-1 (29), to infect L929, MEF, and RAW cells in the presence or absence of CHT. At 15 h. p.i., cell lysates were prepared and viral protein expression was analyzed by immunoblotting. The results of a representative experiment are shown in Fig. 6. Figure 6A shows that neither MEF nor RAW cells synthesize viral proteins when infected with 3-1 or L/C in the absence of CHT; however, addition of CHT to the postadsorption medium facilitates infection by these viruses. Figure 6B shows that, as previously reported, both PI viruses can infect L929 cells in the presence of E-64 and NH4Cl, like ISVPs (3, 29). In another experiment (data not shown), we examined the capacity of the PI virus 3-1 to infect restrictive Vero cells and found no evidence of viral protein production when cells were infected in the absence of exogenous protease.
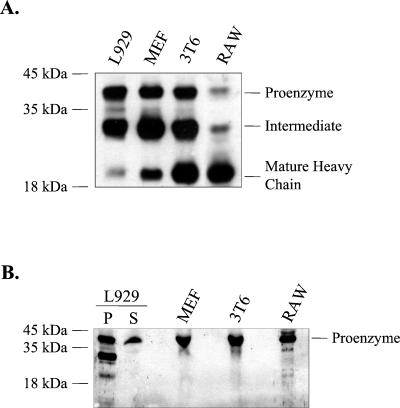
The finding that MEF, RAW, and Vero cells could not be infected by PI viruses suggests that the restriction to virion uncoating in these cells is distinct from that which develops during persistent infection of L929 cells. This conclusion would be further supported if the naturally restrictive cells were found to express mature cathepsin L. To examine this, we probed immunoblots containing extracts and supernatants from murine L929, MEF, 3T6, and RAW cells with a rabbit antiserum directed against murine cathepsin L (56). Results of a representative experiment are shown in Fig. 7. Figure 7A shows the immunoblot results for cell-associated cathepsin L. Cathepsin L is synthesized as a 38-kDa proenzyme that is either secreted or cleaved into the mature form, consisting of a 23-kDa heavy chain and a 5-kDa light chain (36, 60). We detected the mature form of cathepsin L in the restrictive MEF, 3T6, and RAW cells as well as in the permissive L929 cells. Figure 7B shows the immunoblotting results for protein secreted into the media of the various cell cultures. Each cell type secreted the precursor form of cathepsin L. These findings indicate that the restriction to uncoating in MEFs and RAW cells, which can be overcome by exogenous treatment with CHT, is not a consequence of the lack of expression of mature cathepsin L.
FIG. 7.
Immunoblot analysis of cathepsin L expression in permissive and restrictive cells. (A) Cell lysates, normalized for protein content, were resolved by SDS-15% PAGE and electrophoretically transferred onto nitrocellulose filters. Filters were incubated with a polyclonal rabbit antiserum directed against murine cathepsin L (1:5,000). Bound antibody was detected by using reagents that generate a chemiluminescent signal. Bands representing the three forms of cathepsin L are indicated. The 38-kDa proenzyme is either secreted from cells or transported to the endosyme or lysosome, where it is converted into a transient 30-kDa intermediate form. The intermediate form is further processed into a 23-kDa mature cathepsin L heavy chain and a 5-kDa light chain (not retained on the 15% polyacrylamide gel). (B) Proteins were precipitated from cell culture supernatants (normalized for cell number). Protein samples were resolved on an SDS-15% polyacrylamide gel and analyzed for the presence of the cathepsin L proenzyme, as described for panel A. P, pellet; S, supernatant.
DISCUSSION
Many cells restrict reovirus uncoating.
Virion uncoating is an essential early event in the reovirus life cycle (12, 13, 65). This pH-sensitive and protease-dependent (3, 65) step results in the degradation of the outermost capsid protein, σ3, resulting in exposure of the underlying capsid protein μ1. Protein μ1 plays a critical role in the capacity of reovirus particles to disrupt membranes (12, 38, 39, 48, 66). In this report, we have presented evidence that the requirement for capsid proteolysis profoundly restricts the reovirus cellular host range. Although this definition is somewhat arbitrary, we have defined restrictive growth in this study as less than 1/2 log unit of growth at 24 h p.i. By this criterion, we identified a number of cell lines and primary cell cultures that restrict reovirus infection. Remarkably, the majority of restrictive cells (8 out of 10) support efficient viral replication (between 0.9 and 2.3 log units of growth at 24 h p.i.) when protease is included in the postadsorption culture medium. Direct analysis of virion binding and uncoating in some of these restrictive cell lines revealed that virus efficiently bound to the cell surface but that virion disassembly was affected. We found that restrictions to viral uncoating vary in degree. Whereas some cell lines, such as MEFs, showed no evidence of significant uncoating even at 9 h p.i., the process appeared delayed in others, such as Vero cells. The range of phenotypes suggests that alternative proteases may function in different cells at varying degrees of efficiency. Alternatively, endocytic transport may be inefficient in some cells, resulting in the slow delivery of infecting virions to a vesicle containing one or more proteases that can initiate disassembly.
In our uncoating experiments, the outer capsid protein σ3 was rapidly and efficiently degraded when exogenous protease was included in the postadsorption medium. Under these conditions, infections were productive even when the medium also contained E-64 or NH4Cl. Thus, addition of exogenous protease to the infected cultures rapidly generates ISVP-like particles that are capable of bypassing the essential acid- and cysteine protease-dependent step in reovirus infection (3, 16, 65). In the presence of CHT, we observed a significant increase in the 24-h viral yield in almost all cell types analyzed, including cell lines, such as A549 and L929, that efficiently support infection in the absence of protease. This observation suggests that the requirement for virion uncoating limits reovirus infection even in permissive cell lines. Since reoviruses naturally infect the enteric tracts of their mammalian hosts, they may have evolved to be most efficiently uncoated by intestinal proteases.
What is the mechanism by which CHT facilitates infection?
Protease treatment profoundly alters reovirus outer capsid structure (30). Given that each of the three outer capsid proteins is affected by protease treatment, what is (are) the relevant viral target(s) in CHT-facilitated infection? Our data are most consistent with a role for σ3. Protease treatment of reovirus virions can result in several types of changes in the cell attachment protein σ1. In some serotype 3 strains, proteases cleave the molecule such that the globular head is separated from the fibrous tail (31, 52). The fact that we see expanded host range by protease treatment of all three serotypes indicates that cleavage of σ1 is not key to the expanded host range, since, of the viruses we examined, only strain Dearing exhibits this phenotype (45). Protease treatment can also cause a striking change in the morphology of σ1, converting it from a poorly visualized, more compact form to an extended, flexible fiber (30). This conformational change in σ1 may play a role in the interaction of reovirus with receptors on intestinal M cells (2). Our data are not consistent with a specific role for σ1 extension in the mechanism of CHT-facilitated infection. Protease treatment did not enhance viral binding in either the restrictive cells or the nonrestrictive cells that we analyzed. Neither did we see infection of restrictive cells by serotype 2 Jones virions, which are reported to have constitutively extended σ1 molecules (35).
Proteolytic degradation and removal of the outermost capsid protein, σ3, renders the underlying protein μ1/μ1C susceptible to proteolysis. This protein undergoes endoproteolytic cleavage in the conversion from virions to ISVPs. Our experiments with dpSVPs, which lack σ3 but have uncleaved μ1/μ1C, strongly suggest that σ3 removal is the critical target for CHT. Our results are consistent with findings by Nibert and colleagues that the μ1/μ1C cleavage that generates μ1δ, δ, and φ is not required for penetration in either L929 cells or MDCK cells (19, 20). Thus, the block to infection in restrictive cells likely involves the requirement for σ3 degradation, a requirement that is mitigated when CHT is included in the culture medium or when ISVPs or dpSVPs are used to initiate infection.
What is the nature of the block to uncoating in restrictive cells?
We have begun to characterize the molecular basis for the restriction(s) to virion uncoating within cells that support efficient replication by ISVPs or ISVP-like particles. Experiments described in this paper have investigated the hypothesis that the restriction is similar to that which develops during a persistent reovirus infection. During the establishment of persistent reovirus infections in L929 cells, mutant cells which resist infection by wild-type virus are selected. Recent studies reveal that these mutant cells fail to express the mature form of the lysosomal cysteine protease cathepsin L (4). These cells can be infected by mutant viruses that coevolve in the cultures or by ISVPs generated in vitro from wild-type virus (1, 29). The fact that neither of two PI viruses could infect restrictive cells in the absence of protease suggests that the block to uncoating in these cells is distinct from that which develops during persistence in L929 cells. This conclusion is further supported by our observation that many restrictive cells express and process cathepsin L.
Our own preliminary data suggest that a persistent infection can be established in MEF cells by 8 days after infection with virions (data not shown). This is characterized by minimal cell culture crisis and continuous viral output. Others have demonstrated that cells with blocks to reovirus entry, such as murine erythroleukemia cells, become persistently infected rapidly without significant culture crisis (76). Persistently infected carrier cultures have also been established by blocking uncoating in permissive L929 cells with ammonium chloride (16). We expect that insight into the nature of the block(s) to infection in MEF cells will come from future studies of the relationship between virus and cells isolated from persistently infected carrier cultures.
Cellular processes that may restrict uncoating include vesicle acidification and trafficking. It has been shown that reovirus infection is dependent on an acidic pH within the vesicular compartment (16, 49, 65). It is not clear, however, if low pH is required only to activate an essential protease or if it is also required for another early step in reovirus infection, such as an essential conformational change in a capsid protein. Some genetic evidence suggests that the pH- and protease-dependent steps in infection are separable (3). Interestingly, HL-60 cells, which we have shown fail to support reovirus replication in the absence of exogenous protease, have a more alkaline lysosomal pH than do L929 cells (6.4 versus 5.2) (3, 9). This finding is consistent with a model in which lysosomal pH plays a pivotal role in determining the susceptibility of some cells to infection by intact reovirus virions, but ISVPs or proteolyzed particles establish infection by a mechanism independent of vesicular pH.
This study also identified cells, such as 3T6 cells, that were restrictive to infection even after addition of exogenous protease. There was very limited viral growth in 3T6 cells, even at 5 days p.i. in the presence of protease (Fig. 5.). Viral binding to these cells was relatively inefficient, and bound virus did not appear to uncoat in the absence of exogenous protease (data not shown). Thus, 3T6 cells may restrict infection by virions at several different steps in the life cycle. One component of the restrictive phenotype in 3T6 cells could be similar to that described for R1.1 thymoma cells. Reovirus has been reported to bind to these cells but not to be internalized, instead becoming localized to cell membrane elaborations (59). Indeed, more work will be required to elucidate the nature of the block(s) to viral infection in cells where protease treatment does not overcome the restriction.
Importance of proteolysis in viral tropism and disease.
Mounting evidence suggests that viral pathogenesis in animal hosts is influenced by protease expression. Influenza virus is predominantly pneumotropic, despite use of a broadly expressed receptor. This restricted tropism may be the consequence of Clara cell-specific expression of a protease that can activate the influenza virus hemagglutinin for membrane penetration (42, 43). Exposure to extracellular protease is critical for infections by rotaviruses and astroviruses (8, 23, 33, 47). In vivo, these viruses replicate almost exclusively in the enteric tract and require the addition of exogenous protease, usually trypsin, for propagation in cell culture. Clearly, the extent to which cells express and secrete various types of proteases can have a significant effect on viral susceptibility for those cells and surrounding cells.
Unlike many other viruses whose tissue tropism is restricted at the level of receptor expression, reoviruses appear to utilize one or more common cellular molecules as receptors (5; reviewed in reference 67). This is supported by our finding that a variety of cell types can be infected by reovirus when CHT is included in the postadsorption medium. The fact that reovirus receptors are widely expressed, together with the observation that reovirus does not cause serious disease in its host (reviewed in reference 69), suggests that other factors must limit systemic spread of the virus within a host. We suggest that at least one of those factors is the requirement for proteolytic uncoating of the infecting virion and the resultant activation of its capacity to penetrate cell membranes. Future studies will determine whether protease-treated particles (ISVPs) have increased pathogenic potential in the mouse model because they can infect cells that normally restrict uncoating and thus serve as natural barriers to infection by virions.
Practical implications.
Our findings have important practical applications. Addition of exogenous protease, either CHT or trypsin, to cell culture medium can be used to readily generate ISVP-like particles from crude viral stocks. This simple method may be used to study reovirus biology within a wide range of cells that do not naturally support virion uncoating. We are currently using this approach to study reovirus replication in Vero cells and in MEFs from various knockout mice. Although most of our experiments utilized crude viral stocks, restrictive cells were also productively infected with ISVPs prepared by in vitro digestion of purified virions with CHT. The latter approach, while significantly more time-consuming and expensive, may be preferable in animal studies when the size of the inoculum must be small and in studies of phenotypes that would be influenced by cytokines present within crude viral stocks. Also, CHT-facilitated infection should allow preparation of higher-titer virus stocks. We have shown that inclusion of CHT in the postadsorption medium increases viral yields in permissive cells, including L929, A549, and Vero cells. Comparison of viral yields in Vero and L929 cells suggests that CHT-facilitated infection of Vero cells, which do not express alpha/beta interferons (32), may generate stocks with particularly high titers.
Finally, reovirus has been shown to preferentially infect cells with an activated Ras pathway (24, 64). This finding has led to the study of reovirus as an anticancer therapy (24, 37, 55). The mechanism by which Ras activation increases reovirus growth has been suggested to involve inactivation of the interferon-induced eIF2α kinase PKR (64). Interestingly, it has been demonstrated that Ras activation can result in increased expression of various proteases, including cathepsin L (17, 18, 44). Since reovirus infection can be mediated by cathepsin L, higher levels of this protease could make Ras-activated cells more permissive to infection. Studies in our laboratory are currently under way to determine if increased expression of cellular proteases in Ras-activated cells is a requirement for reovirus oncolysis.
Acknowledgments
We are grateful to all those who provided cells for our studies, including Stephen Rice, Katherine Staskus, Ron Jemmerson, Peter Southern, Bryan Williams, Vivian Bardwell, and Christian Mohr. We also thank those who graciously provided antibodies or virus, including Max Nibert, Simon Noble, and Theresa Broering (anti-μNS and anti-core antisera), Kenneth Tyler (5C6), Terence Dermody (virus 3-1), and Daniel Portnoy (anti-cathepsin L). We thank Stephen Rice, Jennifer Smith, and Max Nibert for critical reviews of preliminary versions of the manuscript. Finally, we thank the members of the Schiff, Rice, and Conklin laboratories for constructive input throughout these studies.
This work was supported by NIH grant AI45990 to L.A.S. J.W.G. received support from NIH training grants T32 CA09138 and 2T32 AI07421.
REFERENCES
- 1.Ahmed, R., W. M. Canning, R. S. Kauffman, A. H. Sharpe, J. V. Hallum, and B. N. Fields. 1981. Role of the host cell in persistent viral infection: co-evolution of L cells and reovirus during persistent infection. Cell 25:325-332. [DOI] [PubMed] [Google Scholar]
- 2.Amerongen, H. M., G. A. Wilson, B. N. Fields, and M. R. Neutra. 1994. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J. Virol. 68:8428-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer, G. S., and T. S. Dermody. 1997. Mutations in reovirus outer-capsid protein σ3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J. Virol. 71:4921-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baer, G. S., D. H. Ebert, C. J. Chung, A. H. Erickson, and T. S. Dermody. 1999. Mutant cells selected during persistent reovirus infection do not express mature cathepsin L and do not support reovirus disassembly. J. Virol. 73:9532-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 6.Bass, D. M., M. Baylor, C. Chen, and U. Upadhyayula. 1995. Dansylcadaverine and cytochalasin D enhance rotavirus infection of murine L cells. Virology 212:429-437. [DOI] [PubMed] [Google Scholar]
- 7.Bass, D. M., D. Bodkin, R. Dambrauskas, J. S. Trier, B. N. Fields, and J. L. Wolf. 1990. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J. Virol. 64:1830-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass, D. M., and S. Qiu. 2000. Proteolytic processing of the astrovirus capsid. J. Virol. 74:1810-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belhoussine, R., H. Morjani, R. Gillet, V. Palissot, and M. Manfait. 1999. Two distinct modes of oncoprotein expression during apoptosis resistance in vincristine and daunorubicin multidrug-resistant HL-60 cells. Adv. Exp. Med. Biol. 457:365-381. [DOI] [PubMed] [Google Scholar]
- 10.Bodkin, D. K., and B. N. Fields. 1989. Growth and survival of reovirus in intestinal tissue: role of the L2 and S1 genes. J. Virol. 63:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodkin, D. K., M. L. Nibert, and B. N. Fields. 1989. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J. Virol. 63:4676-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borsa, J., B. D. Morash, M. D. Sargent, T. P. Copps, P. A. Lievaart, and J. G. Szekely. 1979. Two modes of entry of reovirus particles into L cells. J. Gen. Virol. 45:161-170. [DOI] [PubMed] [Google Scholar]
- 13.Borsa, J., M. D. Sargent, P. A. Lievaart, and T. P. Copps. 1981. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology 111:191-200. [DOI] [PubMed] [Google Scholar]
- 14.Broering, T. J., A. M. McCutcheon, V. E. Centonze, and M. L. Nibert. 2000. Reovirus nonstructural protein μNS binds to core particles but does not inhibit their transcription and capping activities. J. Virol. 74:5516-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet, J. P., N. Jourdan, J. Cotte-Laffitte, C. Linxe, M. Geniteau-Legendre, A. Servin, and A. M. Quero. 2000. Rotavirus infection induces cytoskeleton disorganization in human intestinal epithelial cells: implication of an increase in intracellular calcium concentration. J. Virol. 74:10801-10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canning, W. M., and B. N. Fields. 1983. Ammonium chloride prevents lytic growth of reovirus and helps to establish persistent infection in mouse L cells. Science 219:987-988. [DOI] [PubMed] [Google Scholar]
- 17.Casson, A. G., S. M. Wilson, J. A. McCart, F. P. O'Malley, H. Ozcelik, M. S. Tsao, and A. F. Chambers. 1997. Ras mutation and expression of the ras-regulated genes osteopontin and cathepsin L in human esophageal cancer. Int. J. Cancer 72:739-745. [DOI] [PubMed] [Google Scholar]
- 18.Chambers, A. F., R. Colella, D. T. Denhardt, and S. M. Wilson. 1992. Increased expression of cathepsins L and B and decreased activity of their inhibitors in metastatic, ras-transformed NIH 3T3 cells. Mol. Carcinog. 5:238-245. [DOI] [PubMed] [Google Scholar]
- 19.Chandran, K., and M. L. Nibert. 1998. Protease cleavage of reovirus capsid protein μ1/μ1C is blocked by alkyl sulfate detergents, yielding a new type of infectious subvirion particle. J. Virol. 72:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandran, K., S. B. Walker, Y. Chen, C. M. Contreras, L. A. Schiff, T. S. Baker, and M. L. Nibert. 1999. In vitro recoating of reovirus cores with baculovirus-expressed outer-capsid proteins μ1 and σ3. J. Virol. 73:3941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang, C.-T., and H. J. Zweerink. 1971. Fate of parental reovirus in infected cell. Virology 46:544-555. [DOI] [PubMed] [Google Scholar]
- 22.Chappell, J. D., E. S. Barton, T. H. Smith, G. S. Baer, D. T. Duong, M. L. Nibert, and T. S. Dermody. 1998. Cleavage susceptibility of reovirus attachment protein σ1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the σ1 neck. J. Virol. 72:8205-8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark, S. M., J. R. Roth, M. L. Clark, B. B. Barnett, and R. S. Spendlove. 1981. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J. Virol. 39:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffey, M. C., J. E. Strong, P. A. Forsyth, and P. W. Lee. 1998. Reovirus therapy of tumors with activated Ras pathway. Science 282:1332-1334. [DOI] [PubMed] [Google Scholar]
- 25.Coombs, K. M. 1998. Stoichiometry of reovirus structural proteins in virus, ISVP, and core particles. Virology 243:218-228. [DOI] [PubMed] [Google Scholar]
- 26.Cox, D. C., and W. Clinkscales. 1976. Infectious reovirus subviral particles: virus replication, cellular cytopathology, and DNA synthesis. Virology 74:259-261. [DOI] [PubMed] [Google Scholar]
- 27.Cuadras, M. A., C. F. Arias, and S. Lopez. 1997. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065-9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denisova, E., W. Dowling, R. LaMonica, R. Shaw, S. Scarlata, F. Ruggeri, and E. R. Mackow. 1999. Rotavirus capsid protein VP5* permeabilizes membranes. J. Virol. 73:3147-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dermody, T. S., M. L. Nibert, J. D. Wetzel, X. Tong, and B. N. Fields. 1993. Cells and viruses with mutations affecting viral entry are selected during persistent infections of L cells with mammalian reoviruses. J. Virol. 67:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dryden, K. A., G. Wang, M. Yeager, M. L. Nibert, K. M. Coombs, D. B. Furlong, B. N. Fields, and T. S. Baker. 1993. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 122:1023-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan, R., and P. W. Lee. 1994. Localization of two protease-sensitive regions separating distinct domains in the reovirus cell-attachment protein σ1. Virology 203:149-152. [DOI] [PubMed] [Google Scholar]
- 32.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43:247-252. [DOI] [PubMed] [Google Scholar]
- 33.Estes, M. K., D. Y. Graham, and B. B. Mason. 1981. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J. Virol. 39:879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flewett, T. H., and G. N. Woode. 1978. The rotaviruses. Arch. Virol. 57:1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlong, D. B., M. L. Nibert, and B. N. Fields. 1988. σ1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62:246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gal, S., M. C. Willingham, and M. M. Gottesman. 1985. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J. Cell Biol. 100:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirasawa, K., S. G. Nishikawa, K. L. Norman, T. Alain, A. Kossakowska, and P. W. Lee. 2002. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 62:1696-1701. [PubMed] [Google Scholar]
- 38.Hooper, J. W., and B. N. Fields. 1996. Monoclonal antibodies to reovirus σ1 and μ1 proteins inhibit chromium release from mouse L cells. J. Virol. 70:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooper, J. W., and B. N. Fields. 1996. Role of the μ1 protein in reovirus stability and capacity to cause chromium release from host cells. J. Virol. 70:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jane-Valbuena, J., M. L. Nibert, S. M. Spencer, S. B. Walker, T. S. Baker, Y. Chen, V. E. Centonze, and L. A. Schiff. 1999. Reovirus virion-like particles obtained by recoating infectious subvirion particles with baculovirus-expressed σ3 protein: an approach for analyzing σ3 functions during virus entry. J. Virol. 73:2963-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kedl, R., S. Schmechel, and L. Schiff. 1995. Comparative sequence analysis of the reovirus S4 genes from 13 serotype 1 and serotype 3 field isolates. J. Virol. 69:552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kido, H., M. Murakami, K. Oba, Y. Chen, and T. Towatari. 1999. Cellular proteinases trigger the infectivity of the influenza A and Sendai viruses. Mol. Cells 9:235-244. [PubMed] [Google Scholar]
- 43.Kido, H., T. Towatari, Y. Niwa, Y. Okumura, and Y. Beppu. 1996. Cellular proteases involved in the pathogenicity of human immunodeficiency and influenza viruses. Adv. Exp. Med. Biol. 389:233-240. [DOI] [PubMed] [Google Scholar]
- 44.Kim, K., J. Cai, S. Shuja, T. Kuo, and M. J. Murnane. 1998. Presence of activated Ras correlates with increased cysteine proteinase activities in human colorectal carcinomas. Int. J. Cancer 79:324-333. [DOI] [PubMed] [Google Scholar]
- 45.Lee, P. W. K. 1989. The cell attachment proteins of type 1 and type 3 reovirus are differentially susceptible to trypsin and chymotrypsin. Virology 170:62-70. [DOI] [PubMed] [Google Scholar]
- 46.Lee, P. W. K., E. C. Hayes, and W. K. Joklik. 1981. Protein σ1 is the reovirus cell attachment protein. Virology 108:156-163. [DOI] [PubMed] [Google Scholar]
- 47.Lee, T. W., and J. B. Kurtz. 1981. Serial propagation of astrovirus in tissue culture with the aid of trypsin. J. Gen. Virol. 56:421-424. [DOI] [PubMed] [Google Scholar]
- 48.Lucia-Jandris, P., J. W. Hooper, and B. N. Fields. 1993. Reovirus M2 gene is associated with chromium release from mouse L cells. J. Virol. 67:5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez, C. G., R. Guinea, J. Benavente, and L. Carrasco. 1996. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J. Virol. 70:576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin, M. J., A. Warner, and B. N. Fields. 1996. Reovirus infection in rat lungs as a model to study the pathogenesis of viral pneumonia. J. Virol. 70:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nibert, M. L. 1998. Structure of mammalian orthoreovirus particles. Curr. Top. Microbiol. Immunol. 233:1-30. [DOI] [PubMed] [Google Scholar]
- 52.Nibert, M. L., J. D. Chappell, and T. S. Dermody. 1995. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved σ1 protein. J. Virol. 69:5057-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nibert, M. L., and B. N. Fields. 1992. A carboxy-terminal fragment of protein μ1/μ1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J. Virol. 66:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nibert, M. L., D. B. Furlong, and B. N. Fields. 1991. Mechanisms of viral pathogenesis. Distinct forms of reoviruses and their roles during replication in cells and host. J. Clin. Investig. 88:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norman, K. L., M. C. Coffey, K. Hirasawa, D. J. Demetrick, S. G. Nishikawa, L. M. DiFrancesco, J. E. Strong, and P. W. Lee. 2002. Reovirus oncolysis of human breast cancer. Hum. Gene Ther. 13:641-652. [DOI] [PubMed] [Google Scholar]
- 56.Portnoy, D. A., A. H. Erickson, J. Kochan, J. V. Ravetch, and J. C. Unkeless. 1986. Cloning and characterization of a mouse cysteine proteinase. J. Biol. Chem. 261:14697-14703. [PubMed] [Google Scholar]
- 57.Rubin, D. H., and B. N. Fields. 1980. Molecular basis of reovirus virulence. Role of the M2 gene. J. Exp. Med. 152:853-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin, D. H., M. J. Kornstein, and A. O. Anderson. 1985. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J. Virol. 53:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubin, D. H., D. B. Weiner, C. Dworkin, M. I. Greene, G. G. Maul, and W. V. Williams. 1992. Receptor utilization by reovirus type 3: distinct binding sites on thymoma and fibroblast cell lines result in differential compartmentalization of virions. Microb. Pathog. 12:351-365. [DOI] [PubMed] [Google Scholar]
- 60.Salminen, A., and M. M. Gottesman. 1990. Inhibitor studies indicate that active cathepsin L is probably essential to its own processing in cultured fibroblasts. Biochem. J. 272:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherry, B., F. J. Schoen, E. Wenske, and B. N. Fields. 1989. Derivation and characterization of an efficiently myocarditic reovirus variant. J. Virol. 63:4840-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silverstein, S. C., C. Astell, D. H. Levin, M. Schonberg, and G. Acs. 1972. The mechanisms of reovirus uncoating and gene activation in vivo. Virology 47:797-806. [DOI] [PubMed] [Google Scholar]
- 63.Silverstein, S. C., and S. Dales. 1968. The penetration of reovirus RNA and initiation of its genetic function in L-strain fibroblasts. J. Cell Biol. 36:197-230. [PubMed] [Google Scholar]
- 64.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sturzenbecker, L. J., M. Nibert, D. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61:2351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tosteson, M. T., M. L. Nibert, and B. N. Fields. 1993. Ion channels induced in lipid bilayers by subvirion particles of the nonenveloped mammalian reoviruses. Proc. Natl. Acad. Sci. USA 90:10549-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyler, K. L. 2001. Reoviruses, p. 1729-1746. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 68.Tyler, K. L., R. T. Bronson, K. B. Byers, and B. Fields. 1985. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology 35:88-92. [DOI] [PubMed] [Google Scholar]
- 69.Tyler, K. L., and B. N. Fields. 1996. Reoviruses, p. 1597-1623. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 70.Tyler, K. L., M. K. Squier, S. E. Rodgers, B. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein σ1. J. Virol. 69:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Virgin, H. W., IV, M. A. Mann, B. N. Fields, and K. L. Tyler. 1991. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J. Virol. 65:6772-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virgin, H. W., IV, R. Bassel-Duby, B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallis, C., J. L. Melnick, and F. Rapp. 1966. Effects of pancreatin on the growth of reovirus. J. Bacteriol. 92:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiner, H. L., D. Drayna, D. R. Averill, Jr., and B. N. Fields. 1977. Molecular basis of reovirus virulence: role of the S1 gene. Proc. Natl. Acad. Sci. USA 74:5744-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiner, H. L., M. L. Powers, and B. N. Fields. 1980. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J. Infect. Dis. 141:609-616. [DOI] [PubMed] [Google Scholar]
- 76.Wetzel, J. D., J. D. Chappell, A. B. Fogo, and T. S. Dermody. 1997. Efficiency of viral entry determines the capacity of murine erythroleukemia cells to support persistent infections by mammalian reoviruses. J. Virol. 71:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wetzel, J. D., G. J. Wilson, G. S. Baer, L. R. Dunnigan, J. P. Wright, D. S. Tang, and T. S. Dermody. 1997. Reovirus variants selected during persistent infections of L cells contain mutations in the viral S1 and S4 genes and are altered in viral disassembly. J. Virol. 71:1362-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]