Abstract
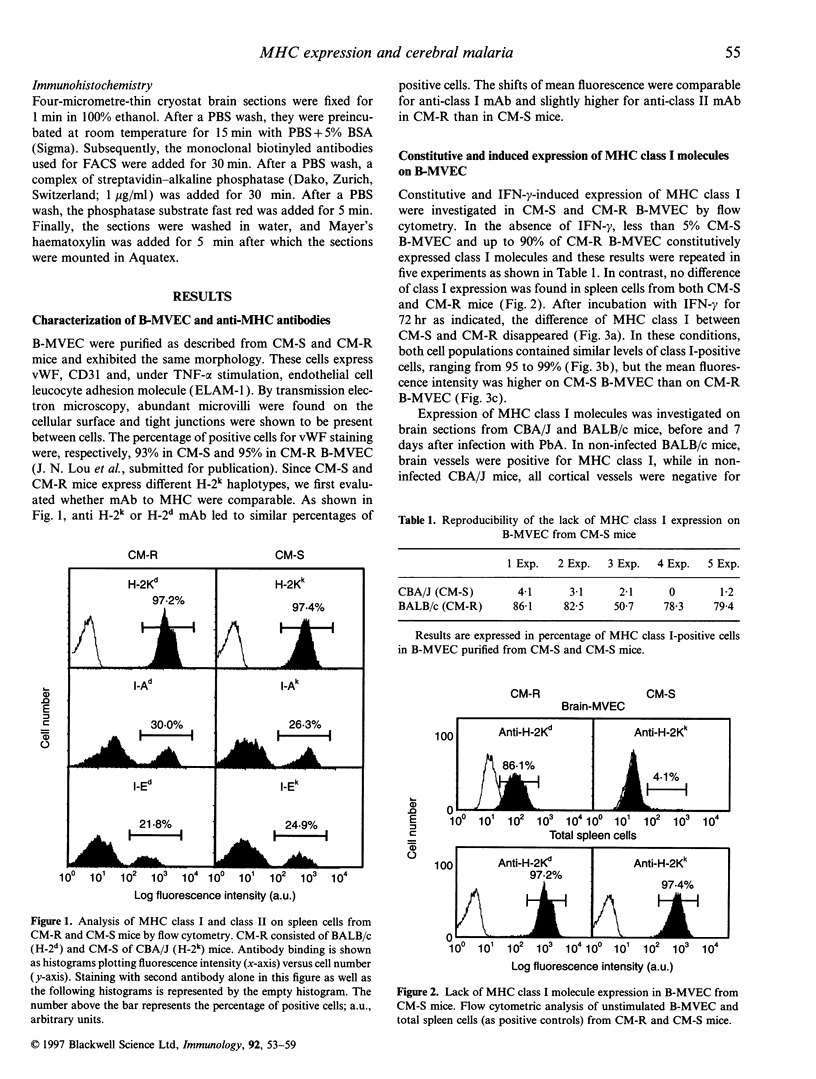
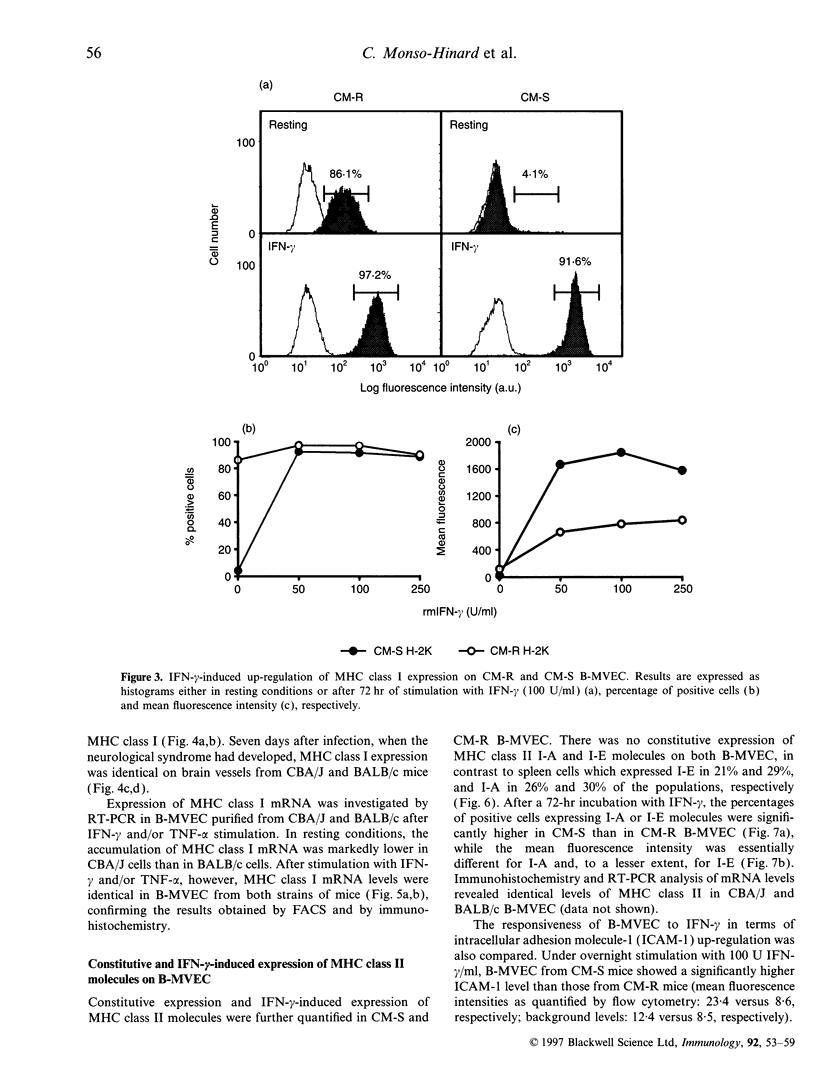
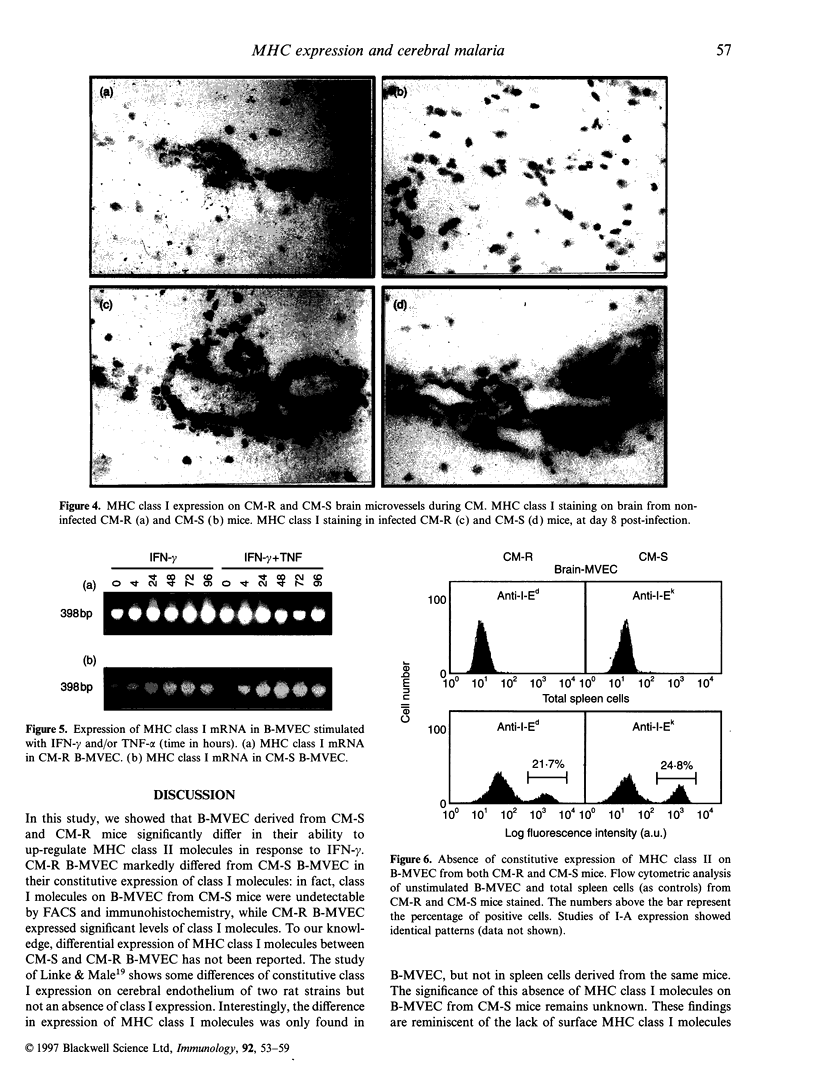
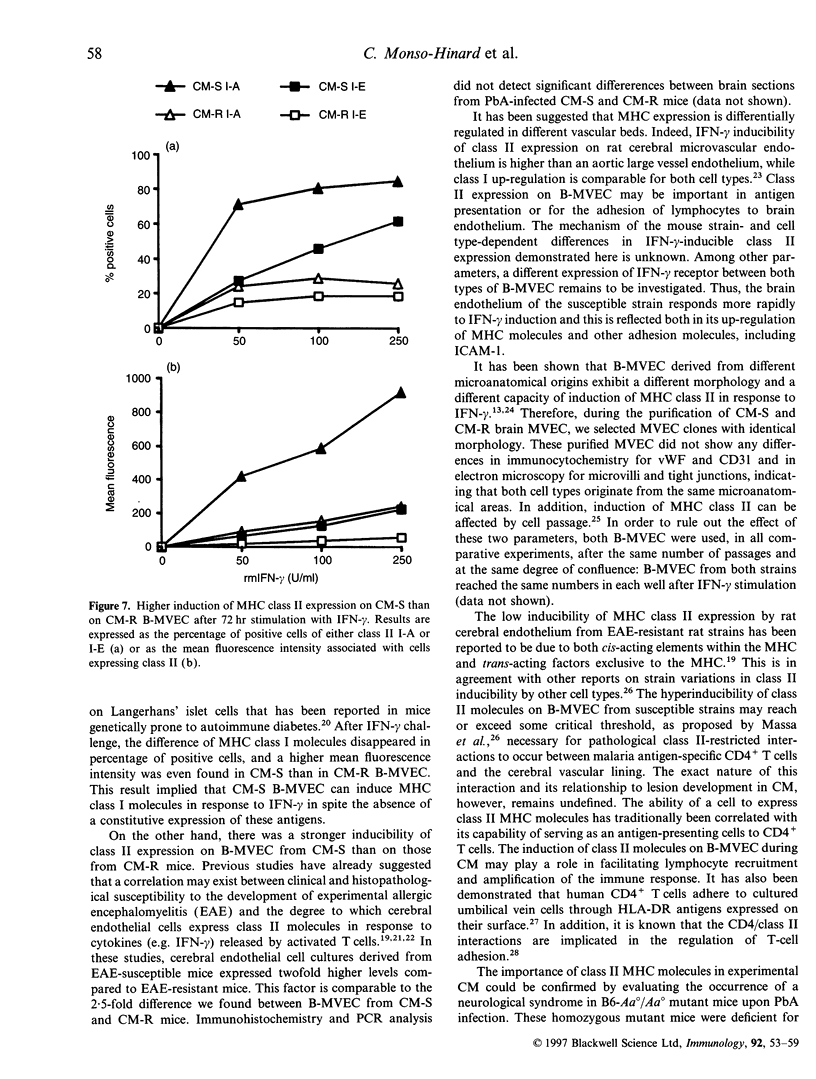
The physiopathology of experimental cerebral malaria (CM), an acute neurological complication of Plasmodium berghei ANKA (PbA) infection, involves interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha), two cytokines that are known to modulate major histocompatibility complex (MHC) molecule expression. The aim of this study was to evaluate whether the genetic susceptibility to CM is related to the constitutive or IFN-gamma-induced expression of MHC molecules on brain microvessels. To this end, brain microvascular endothelial cells (B-MVEC) were isolated from CM-susceptible (CM-S, CBA/J) and resistant (CM-R, BALB/c) mice. By flow cytometry, we found that less than 5% of CM-S B-MVEC constitutively expressed MHC class I molecules, in contrast to up to 90% of CM-R B-MVEC. Upon stimulation with IFN-gamma, the percentage of positive cells for MHC class I molecules in CM-S B-MVEC became comparable to CM-R B-MVEC, but a higher fluorescence intensity existed on CM-S B-MVEC compared with CM-R B-MVEC. MHC class II molecules were not constitutively expressed on B-MVEC from either strain. IFN-gamma-induced expression of MHC class II (I-A, I-E) molecules was significantly higher in CM-S than CM-R B-MVEC both in percentage of positive cells and fluorescence intensity. These data demonstrate that absent or low MHC class I and higher inducibility of MHC class II expression on B-MVEC are associated with the genetic susceptibility to CM.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Faustman D., Li X. P., Lin H. Y., Fu Y. E., Eisenbarth G., Avruch J., Guo J. Linkage of faulty major histocompatibility complex class I to autoimmune diabetes. Science. 1991 Dec 20;254(5039):1756–1761. doi: 10.1126/science.1763324. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985 Dec;135(6):3750–3762. [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A., Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Greenwood B. M., Bradley A. K., Greenwood A. M., Byass P., Jammeh K., Marsh K., Tulloch S., Oldfield F. S., Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81(3):478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Savage C. O., Pober J. S. The endothelial cell as a regulator of T-cell function. Immunol Rev. 1990 Oct;117:85–102. doi: 10.1111/j.1600-065x.1990.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Huynh H. K., Dorovini-Zis K. Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. Am J Pathol. 1993 Apr;142(4):1265–1278. [PMC free article] [PubMed] [Google Scholar]
- Jemison L. M., Williams S. K., Lublin F. D., Knobler R. L., Korngold R. Interferon-gamma-inducible endothelial cell class II major histocompatibility complex expression correlates with strain- and site-specific susceptibility to experimental allergic encephalomyelitis. J Neuroimmunol. 1993 Aug;47(1):15–22. doi: 10.1016/0165-5728(93)90280-c. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch M. S., Strominger J. L., Doyle C. Cell adhesion mediated by CD4 and MHC class II proteins requires active cellular processes. J Immunol. 1993 Nov 1;151(9):4552–4561. [PubMed] [Google Scholar]
- Köntgen F., Süss G., Stewart C., Steinmetz M., Bluethmann H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int Immunol. 1993 Aug;5(8):957–964. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- Linke A. T., Male D. K. Strain-specific variation in constitutive and inducible expression of MHC class II, class I and ICAM-1 on rat cerebral endothelium. Immunology. 1994 May;82(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- Male D., Pryce G., Rahman J. Comparison of the immunological properties of rat cerebral and aortic endothelium. J Neuroimmunol. 1990 Dec;30(2-3):161–168. doi: 10.1016/0165-5728(90)90100-2. [DOI] [PubMed] [Google Scholar]
- Malissen B., Shastri N., Pierres M., Hood L. Cotransfer of the Ed alpha and Ad beta genes into L cells results in the surface expression of a functional mixed-isotype Ia molecule. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3958–3962. doi: 10.1073/pnas.83.11.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., ter Meulen V., Fontana A. Hyperinducibility of Ia antigen on astrocytes correlates with strain-specific susceptibility to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4219–4223. doi: 10.1073/pnas.84.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama J., Minato N., Kano S. Mechanisms of lymphocyte adhesion to human vascular endothelial cells in culture. T lymphocyte adhesion to endothelial cells through endothelial HLA-DR antigens induced by gamma interferon. J Clin Invest. 1986 May;77(5):1596–1605. doi: 10.1172/JCI112475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Collins T., Cotran R. S., Ault K. A., Fiers W., Krensky A. M., Clayberger C., Reiss C. S., Burakoff S. J. Interactions of T lymphocytes with human vascular endothelial cells: role of endothelial cells surface antigens. Immunobiology. 1984 Dec;168(3-5):483–494. doi: 10.1016/s0171-2985(84)80132-1. [DOI] [PubMed] [Google Scholar]
- Rupnick M. A., Carey A., Williams S. K. Phenotypic diversity in cultured cerebral microvascular endothelial cells. In Vitro Cell Dev Biol. 1988 May;24(5):435–444. doi: 10.1007/BF02628495. [DOI] [PubMed] [Google Scholar]
- Spanel-Borowski K., Bein G. Different microvascular endothelial cell phenotypes exhibit different class I and II antigens under interferon-gamma. In Vitro Cell Dev Biol Anim. 1993 Aug;29A(8):601–602. doi: 10.1007/BF02634540. [DOI] [PubMed] [Google Scholar]
- Turner G. D., Morrison H., Jones M., Davis T. M., Looareesuwan S., Buley I. D., Gatter K. C., Newbold C. I., Pukritayakamee S., Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994 Nov;145(5):1057–1069. [PMC free article] [PubMed] [Google Scholar]
- Waldman W. J., Knight D. A., Adams P. W., Orosz C. G., Sedmak D. D. In vitro induction of endothelial HLA class II antigen expression by cytomegalovirus-activated CD4+ T cells. Transplantation. 1993 Dec;56(6):1504–1512. doi: 10.1097/00007890-199312000-00043. [DOI] [PubMed] [Google Scholar]
- Welsh J., Sapatino B., Rosenbaum B., Smith R., Linthicum S. Correlation between susceptibility to demyelination and interferon-gamma induction of major histocompatibility complex class II antigens on murine cerebrovascular endothelial cells. J Neuroimmunol. 1993 Oct;48(1):91–97. doi: 10.1016/0165-5728(93)90062-4. [DOI] [PubMed] [Google Scholar]
- White N. J., Pukrittayakamee S. Clinical malaria in the tropics. Med J Aust. 1993 Aug 2;159(3):197–203. [PubMed] [Google Scholar]





