Abstract
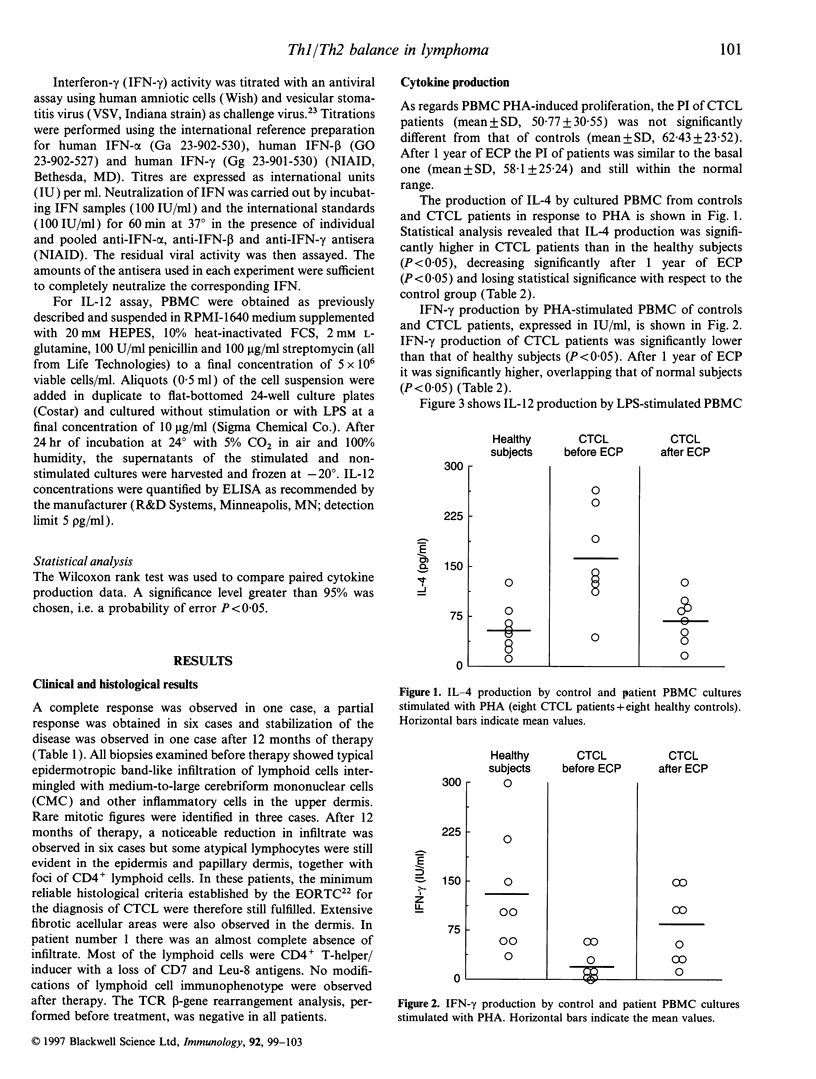
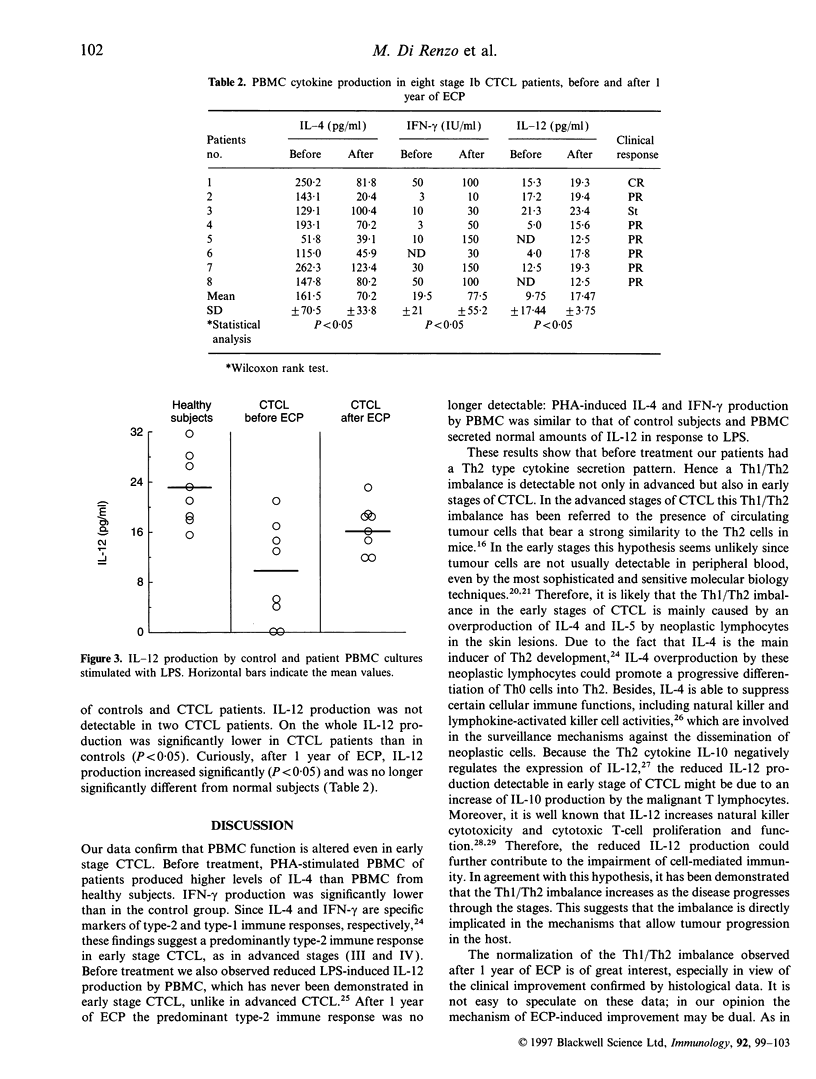
Extracorporeal photochemotherapy (ECP) has been shown to be a potent activator of peripheral blood macrophages because it causes a marked release of macrophage-dependent proinflammatory cytokines, and it is therefore currently considered to be a safe and non-toxic immunomodulatory treatment. On this basis we studied the function of peripheral blood mononuclear cells (PBMC) in eight patients with early stage (Ib) cutaneous T-cell lymphoma (CTCL), before and 1 year after ECP, together with their clinical and histological responses. In particular we evaluated in vitro phytohaemagglutinin (PHA)-stimulated proliferation and production of interleukin-4 (IL-4) and interferon-gamma (IFN-gamma) as well as lipopolysaccharide (LPS)-induced production of IL-12. Before treatment we observed that PBMC of patients produced significantly higher levels of IL-4 and lower levels of IFN-gamma and IL-12 than those of healthy control subjects. After 1 year of ECP, IL-4, IFN-gamma and IL-12 production no longer differed from that of control subjects. Moreover, we observed a good clinical result matched by histological response. Our data confirm that early-stage CTCL patients show a predominantly type-2 immune response that might be responsible for several immunological abnormalities found in this disease. We have demonstrated that ECP reverses the T-helper type 1/T-helper type 2 (Th1/Th2) imbalance and may therefore be considered an efficient biological response modifier.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakels V., van Oostveen J. W., Gordijn R. L., Walboomers J. M., Meijer C. J., Willemze R. Frequency and prognostic significance of clonal T-cell receptor beta-gene rearrangements in the peripheral blood of patients with mycosis fungoides. Arch Dermatol. 1992 Dec;128(12):1602–1607. [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993 Sep 1;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer R., Kohl O., Gillessen J., Kägi M., Burg G. Peripheral blood mononuclear cells in patients with nonleukemic cutaneous T-cell lymphoma. Reduced proliferation and preferential secretion of a T helper-2-like cytokine pattern on stimulation. Arch Dermatol. 1993 Apr;129(4):433–436. [PubMed] [Google Scholar]
- Edelson R. L. Photopheresis. J Clin Apher. 1990;5(2):77–79. [PubMed] [Google Scholar]
- Edelson R. L. Photopheresis: a clinically relevant immunobiologic response modifier. Ann N Y Acad Sci. 1991 Dec 30;636:154–164. doi: 10.1111/j.1749-6632.1991.tb33446.x. [DOI] [PubMed] [Google Scholar]
- Edelson R., Berger C., Gasparro F., Jegasothy B., Heald P., Wintroub B., Vonderheid E., Knobler R., Wolff K., Plewig G. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987 Feb 5;316(6):297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- Fimiani M., Rubegni P., D'Ascenzo G., Andreassi L. Extracorporeal photochemotherapy in the early treatment of cutaneous T-cell lymphoma. J Am Acad Dermatol. 1994 Nov;31(5 Pt 1):828–830. doi: 10.1016/s0190-9622(09)80066-2. [DOI] [PubMed] [Google Scholar]
- Fimiani M., Rubegni P., Pimpinelli N., Mori M., De Aloe G., Andreassi L. Extracorporeal photochemotherapy induces a significant increase in CD36+ circulating monocytes in patients with mycosis fungoides. Dermatology. 1997;194(2):107–110. doi: 10.1159/000246076. [DOI] [PubMed] [Google Scholar]
- Heald P., Rook A., Perez M., Wintroub B., Knobler R., Jegasothy B., Gasparro F., Berger C., Edelson R. Treatment of erythrodermic cutaneous T-cell lymphoma with extracorporeal photochemotherapy. J Am Acad Dermatol. 1992 Sep;27(3):427–433. doi: 10.1016/0190-9622(92)70212-x. [DOI] [PubMed] [Google Scholar]
- Holloway K. B., Flowers F. P., Ramos-Caro F. A. Therapeutic alternatives in cutaneous T-cell lymphoma. J Am Acad Dermatol. 1992 Sep;27(3):367–378. doi: 10.1016/0190-9622(92)70202-q. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Custer M. C., Rosenberg S. A., Lotze M. T. IL-4 regulates IL-2 induction of lymphokine-activated killer activity from human lymphocytes. J Immunol. 1989 May 15;142(10):3452–3461. [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Stanton G. J., Baron S. Virus plaque-reduction assay for interferon: microplaque and regular macroplaque reduction assays. Methods Enzymol. 1981;78(Pt A):339–346. doi: 10.1016/0076-6879(81)78139-4. [DOI] [PubMed] [Google Scholar]
- Laroche L., Kaiserlian D. Decreased natural-killer-cell activity in cutaneous T-cell lymphomas. N Engl J Med. 1983 Jan 13;308(2):101–102. doi: 10.1056/NEJM198301133080213. [DOI] [PubMed] [Google Scholar]
- Lessin S. R., Vowels B. R., Rook A. H. Th2 cytokine profile in cutaneous T-cell lymphoma. J Invest Dermatol. 1995 Dec;105(6):855–856. doi: 10.1111/1523-1747.ep12326693. [DOI] [PubMed] [Google Scholar]
- Levy S., Tempé J. L., Caussade P., Aleksijevic A., Grosshans E., Mayer S., Lang J. M. Stage-related decrease in natural killer cell activity in untreated patients with mycosis fungoides. Cancer Immunol Immunother. 1984;18(2):138–140. doi: 10.1007/BF00205749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra P. T., Wu D., Crim J. A., Mostowski H. S., Siegel J. P. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993 Sep 1;151(5):2444–2452. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Perez M., Edelson R., Laroche L., Berger C. Inhibition of antiskin allograft immunity by infusions with syngeneic photoinactivated effector lymphocytes. J Invest Dermatol. 1989 May;92(5):669–676. doi: 10.1111/1523-1747.ep12696853. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Kubin M., Cassin M., Vonderheid E. C., Vowels B. R., Wolfe J. T., Wolf S. F., Singh A., Trinchieri G., Lessin S. R. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol. 1995 Feb 1;154(3):1491–1498. [PubMed] [Google Scholar]
- Rook A. H., Vowels B. R., Jaworsky C., Singh A., Lessin S. R. The immunopathogenesis of cutaneous T-cell lymphoma. Abnormal cytokine production by Sézary T cells. Arch Dermatol. 1993 Apr;129(4):486–489. [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels B. R., Cassin M., Boufal M. H., Walsh L. J., Rook A. H. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol. 1992 May;98(5):686–692. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]
- Vowels B. R., Cassin M., Vonderheid E. C., Rook A. H. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992 Jul;99(1):90–94. doi: 10.1111/1523-1747.ep12611877. [DOI] [PubMed] [Google Scholar]
- Vowels B. R., Lessin S. R., Cassin M., Jaworsky C., Benoit B., Wolfe J. T., Rook A. H. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994 Nov;103(5):669–673. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Tung R. M., Haeffner A. C., Crooks C. F., Liao S., Orozco R., Veelken H., Kadin M. E., Koh H., Heald P. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol. 1994 Jul;103(1):34–41. doi: 10.1111/1523-1747.ep12389114. [DOI] [PubMed] [Google Scholar]
- du Vivier A., Harper R. A., Vonderheid E., Van Scott E. J. Lymphocyte transformation in patients with staged mycosis fungoides and Sézary syndrome. Cancer. 1978 Jul;42(1):209–213. doi: 10.1002/1097-0142(197807)42:1<209::aid-cncr2820420134>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]



