Abstract
A previous study using a Nef-defective human immunodeficiency virus type 1 (HIV-1) mutant suggested that Nef-mediated down-regulation of HLA class I on the infected cell surface affects the cytolytic activity of HIV-1-specific cytotoxic T-lymphocyte (CTL) clones for HIV-1-infected primary CD4+ T cells. We confirmed this effect by using a nef-mutant HIV-1 strain (NL-M20A) that expresses a Nef protein which does not induce down-regulation of HLA class I molecules but is otherwise functional. HIV-1-specific CTL clones were not able to kill primary CD4+ T cells infected with a Nef-positive HIV-1 strain (NL-432) but efficiently lysed CD4+ T cells infected with NL-M20A. Interestingly, CTL clones stimulated with NL-432-infected CD4+ T cells were able to produce cytokines, albeit at a lower level than when stimulated with NL-M20A-infected CD4+ T cells. This indicates that Nef-mediated HLA class I down-regulation affects CTL cytokine production to a lesser extent than cytolytic activity. Replication of NL-432 was partially suppressed in a coculture of HIV-1-infected CD4+ T cells and HIV-1-specific CTL clones, while replication of NL-M20A was completely suppressed. These results suggest that HIV-1-specific CD8+ T cells are able to partially suppress the replication of HIV-1 through production of soluble HIV-1-suppressive factors such as chemokines and gamma interferon. These findings may account for the mechanism whereby HIV-1-specific CD8+ T cells are able to partially but not completely control HIV-1 replication in vivo.
Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells play a critical role in the control of HIV-1 infection (17, 23, 32, 34, 38). It is well known that CD8+ T cells inhibit the replication of HIV-1, not only by cytolytic mechanisms but also by the release of HIV-suppressive factors such as gamma interferon (IFN-γ) and chemokines (9, 25, 43, 45). β-Chemokines such as macrophage inflammatory protein 1α (MIP-1α) and RANTES suppress HIV-1 infection through competition for CCR5 (2, 8), while IFN-γ directly suppresses HIV-1 replication by inducing cellular antiviral proteins (30). In addition, several unknown factors produced by CD8+ T cells inhibit the transcription of HIV-1 (5, 26). However, although HIV-1-infected individuals exhibit a strong HIV-1-specific cytolytic response, these individuals usually develop AIDS if they are not treated with antiretrovirus therapy (22, 29). HIV-1 escape from HIV-1-specific CD8+ T cells may occur via different mechanisms. For example, mutations of immunodominant epitopes contribute to such escape in acute and chronic phases of HIV-1 infection (6, 15). In addition, the number of HIV-1-specific cytotoxic T lymphocytes (CTLs) is reduced by apoptosis of CD8+ T cells via Fas and tumor necrosis factor (TNF) (44). Furthermore, low perforin expression may result in impaired cytolytic function of HIV-1-specific CTLs (3, 4).
HLA class I down-regulation is a well-characterized event for HIV-1-infected cells (21, 31, 37). Several HIV-1 accessory genes are involved in this down-regulation, such as nef, vpu, and tat, with Nef protein having the strongest effect (20, 28, 39). Nef protein down-regulates HLA-A and HLA-B molecules but not HLA-C or HLA-E molecules (10, 24). The C terminus of HLA class I molecules and the N terminus of Nef protein are involved in Nef-mediated HLA class I down-regulation (10, 16, 27). Collins et al. showed that HIV-1-specific CTLs killed CD4+ T cells infected with nef-defective HIV-1 but failed to kill cells infected with Nef-positive HIV-1 (11, 12). Another study also demonstrated that HIV-1-specific CTL clones were able to kill CD4+ T-cell lines infected with a nef-defective HIV-1 strain (IIIB strain) (46). These studies suggest that Nef-mediated HLA class I down-regulation may be another mechanism that allows HIV-1 to evade CTLs.
The studies described above examined only the cytolytic activity of CTLs for HIV-1-infected cells; the effect of Nef-mediated HLA class I down-regulation on the production of soluble HIV-1-suppressive factors from HIV-1-specific CD8+ T cells was not examined. In the present study we investigated the effect of Nef-mediated HLA class I down-regulation on HIV-1-specific CTL cytotoxic activity, cytokine production, and ability to suppress HIV-1 replication, by using primary CD4+ T cells infected with a wild-type HIV-1 strain (NL-432), a Nef-defective HIV-1 strain (NL-Xh), or an HIV-1 strain that expresses a single-amino-acid-mutant Nef protein that fails to induce HLA class I down-regulation but retains other Nef functions (NL-M20A) (1).
MATERIALS AND METHODS
Purification of CD4+ T cells from PBMCs.
CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) of HIV-1-seronegative individuals (HLA-A2/24, B35/40). PBMCs were separated by using a Ficoll-Hypaque (Amersham Pharmacia, Little Chalfont, United Kingdom) density gradient and were then further purified with a nylon wool column. CD8+ T cells were depleted from nonadherent cells in the nylon wool column with anti-human CD8 monoclonal antibody (MAb)-coated magnetic beads (Dynal, Oslo, Norway). Remaining cells were cultured in an anti-human CD3 MAb-coated well and RPMI 1640 medium with 10% fetal calf serum (R10 medium) and supplemented with 100 U of recombinant human interleukin 2 (rhIL-2)/ml and 10 ng of rhIL-4/ml. After 8 days of culture, the percentage of CD4+ cells in the purified cells was determined by flow cytometry.
CTL clones.
Two HIV-1-specific, HLA-B*3501-restricted CTL clones (SF2-33-135 and SF2-4-2), an HLA-A*2402-restricted CTL clone (SF2-Env379-9-3), and an HLA-B*5101-restricted CTL clone (SF2-pol283-8-54) were previously generated (18, 41, 42). The HIV-1-specific, HLA-A*3303-restricted CTL clone SF2-pol594-9-1 was recently established (unpublished data). All CTL clones were cultured in R10 medium supplemented with 200 U of rhIL-2/ml and stimulated weekly with irradiated target cells prepulsed with the appropriate HIV-1-derived peptide.
Antibodies.
Hybridomas producing SFR8 B6 anti-HLA-Bw6 MAb and A11 1 M anti-HLA-A11/A24 MAb were purchased from the American Type Culture Collection. Phycoerythrin (PE)-labeled anti-HIV-1 p24 MAb KC-57 was purchased from Beckman Coulter (Miami, Fla.). Anti-human CD4, anti-human CD8, anti-human IFN-γ, anti-human MIP-1β, and anti-human TNF-α MAbs were purchased from Dako (Glostrup, Denmark).
HIV-1 clones.
An infectious proviral clone of HIV-1, pNL-432, and its mutants, pNL-Xh (containing a frameshift at a XhoI site within the nef gene) and pNL-M20A (containing a substitution of Ala for Met at residue 20 of Nef), were reported previously (1). HeLa or 293T cells were transfected with each proviral DNA clone by the calcium phosphate coprecipitation method. Supernatants from transfected HeLa or 293T cell cultures were stored at −80°C. The viral titer (50% tissue culture infectious doses) was determined with 174XCEM T1 cells.
Infection of target cells with HIV-1 for use in CTL assays.
Isolated primary CD4+ T cells (>95% CD4+ T cells) were incubated with HIV-1 clones for 7 h at 37°C with intermittent agitation. The cells were then washed once and cultured in R10 medium supplemented with 100 U of rIL-2/ml and 10 ng of rIL-4/ml. On the following 4 to 7 days, cells were harvested to determine the percentage of HIV-1-infected cells by intracellular HIV-1 p24 staining and flow cytometry.
CTL assay.
The cytotoxicity of CTL clones for target cells infected with HIV-1 (>40% p24 antigen [Ag]-positive cells) was determined by a standard 51Cr-release assay. Target cells were incubated for 120 min at 37°C with 100 μCi of Na251CrO4 in saline and then washed three times with R10 medium. Labeled target cells (2 × 103/well) were mixed with the indicated ratio of effector cells in a 96-well U-bottomed microtiter plate. After 6 h of incubation at 37°C, 100 μl of supernatant was harvested from each well and analyzed with a gamma counter. Spontaneous 51Cr release (cpm spn) was determined by measuring the counts per minute of the supernatant from the wells containing target cells alone. Maximum release (cpm max) was determined by measuring the counts per minute of supernatant from wells containing 2.5% Triton X-100. Percent specific lysis was calculated as follows: % specific lysis = (cpm exp − cpm spn)/(cpm max − cpm spn), where cpm exp is the counts per minute in supernatant from wells containing both target and effector cells.
Flow cytometry analysis.
For single-parameter analysis of HIV-1 p24 expression, HIV-1-infected CD4+ T cells were fixed in paraformaldehyde-lysolecithin, treated with cold absolute methanol, and permeabilized with 0.1% Nonidet P-40-phosphate-buffered saline. The cells were then incubated with PE-labeled anti-p24 MAb KC-57. In selected experiments, surface expression of HLA class I molecules on HIV-1-infected cells was examined. Cells were stained with anti-HLA class I MAb (SFR8 B6 or A 11 1 M) following by staining with fluorescein isothiocyanate-labeled anti-mouse immunoglobulin (Silenus Laboratories, Boronia, Victoria, Australia) and were then fixed and permeabilized for intracellular HIV-1 p24 staining. Cells were resuspended in 2% paraformaldehyde and analyzed on a FACSCalibur with CellQuest software (Becton Dickinson, San Jose, Calif.). Non-HIV-1-infected CD4+ T cells stained with anti-p24 MAb were gated out as a non-HIV-1-infected control.
For detection of intracellular cytokines, HIV-1-specific CTL clones were cocultured with peptide-prepulsed CD4+ T cells or HIV-1-infected CD4+ T cells for 6 h at a CTL clone/CD4+ T-cell ratio of 1:10. CTL clones cocultured with CD4+ T cells without peptide were used as a negative control. After 2 h of incubation, brefeldin A was added to each well (10 μg/ml). Cells were stained with a mixture of anti-CD4 MAb and anti-CD8 MAb. After washing, cells were fixed with paraformaldehyde and permeabilized with phosphate-buffered saline supplemented with 0.1% saponin containing 20% fetal calf serum (permeabilizing buffer) at 4°C for 10 min. Cells were resuspended in permeabilizing buffer and then stained with anti-IFN-γ MAb, anti-TNF-α MAb, or anti-MIP-1β MAb. Finally, cells were resuspended in 2% paraformaldehyde and the percentage of intracellular IFN-γ-, MIP-1β-, or TNF-α-positive cells was analyzed by flow cytometry.
Coculture of HIV-1-infected CD4+ T cells and HIV-1-specific CTL clones.
Primary CD4+ T cells were incubated with the indicated strain of HIV-1. After 7 h of incubation at 37°C with intermittent agitation, cells were washed three times with R10 medium. HIV-1-infected CD4+ T cells were cocultured with an HIV-1-specific CTL clone at a CD4+ T-cell/CTL clone ratio of 1:1. On days 3, 5, 9, and 15 postinfection, 10 μl of culture supernatant was collected and the mixtures were examined for p24 antigen by enzyme immunoassay (HIV-1 p24 Ag enzyme-linked immunosorbent assay kit; ZeptoMetrix Corporation, New York, N.Y.). For stimulation of HIV-1-specific CTL clones, HIV-1-infected CD4+ T cells were cocultured with an HIV-1-specific CTL clone at a CD4+ T-cell/CTL clone ratio of 1:1. On days 5, 10, and 15, cells in the coculture were stained with a mixture of PE-labeled anti-p24 MAb and PE-labeled anti-CD4 MAb or fluorescein isothiocyanate-labeled anti-CD8 MAb. The percentages of intracellular p24 Ag-positive cells as well as CD8+ and CD4− CD8− populations in the coculture were determined by flow cytometry.
RESULTS
Cytolytic activity of HIV-1-specific CTL clones for CD4+ T cells infected with HIV-1.
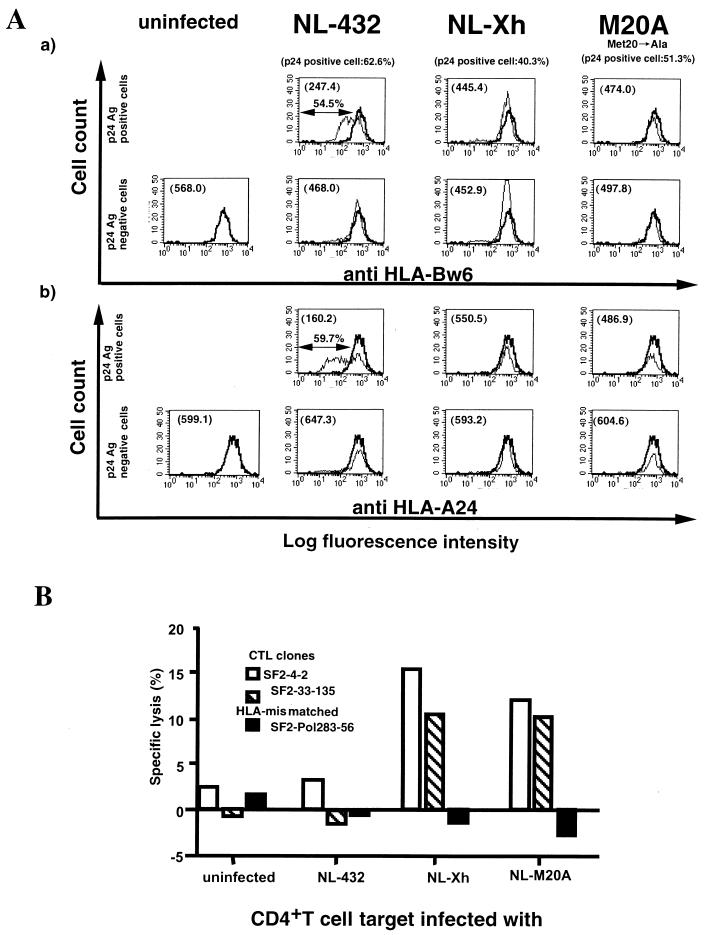
We analyzed the cytolytic activity of HIV-1-specific CTL clones for primary CD4+ T cells infected with Nef-positive or Nef-mutant HIV-1 strains by a standard 51Cr-release assay. CD4+ T cells purified from an HIV-1-seronegative donor (HLA-A2/24, B35/48) were infected with the HIV-1 strains NL-432 (Nef positive), NL-Xh (Nef negative), and NL-M20A (mutant Nef that fails to down-regulate HLA class I molecules but retains other Nef functions) (1). On day 6 after infection, intracellular p24 Ag-positive cells were measured by flow cytometry. The percentage of p24 Ag-positive cells in the NL-432-, NL-Xh-, and NL-M20A-infected populations was 62.6, 40.3, and 51.3%, respectively. Surface expression of HLA-A24 and HLA-B35 on the infected CD4+ T cells was analyzed by costaining with anti-HIV-1 p24 Ag MAb and A 11 1 M anti-HLA-A11/A24 MAb or SFR8 B6 anti-HLA-Bw6 MAb. The surface expression of both HLA class I molecules was much lower on CD4+ T cells infected with NL-432 than on uninfected cells. In contrast, the surface expression of these HLA class I molecules on CD4+ T cells infected with NL-Xh or NL-M20A was comparable to that on uninfected cells (Fig. 1A).
FIG. 1.
Cytolytic activity of HIV-1-specific CTL clones for primary CD4+ T cells infected with HIV-1 and Nef-mutant HIV-1 strains. (A) Expression of HLA class I molecules on primary CD4+ T cells infected with HIV-1 NL-432 or Nef-mutant HIV-1 strains (NL-Xh and NL-M20A). CD4+ T cells (HLA-A2/24, B35/48) were infected with NL-432, NL-Xh, or NL-M20A. On day 5 postinfection, the cells were costained with anti-HLA class I MAb (HLA-Bw6-specific MAb, SFR8 B6, or HLA-A24- and A11-specific MAb, A 11 1 M) and anti-HIV-1 p24 Ag. By using flow cytometry, p24 Ag-positive and p24 Ag-negative populations were gated out separately and the expression of HLA class I molecules on each population was analyzed. The expression level of HLA class I molecules on uninfected CD4+ T cells is shown by the heavy line, and that on infected cells is shown by the light line. The expression of HLA-B35 (a) and HLA-A24 (b) on p24 Ag-positive cells is given as mean fluorescence intensity in each upper left quadrant. The percentage of p24 Ag-positive cells in CD4+ T cells infected with NL-432, NL-Xh, and NL-M20A was 62.6, 40.3, and 51.3%, respectively. (B) Cytolytic activity of HIV-1-specific CTL clones for primary CD4+ T cells infected with HIV-1 or Nef-mutant strains. CD4+ T cells (HLA-A2/24, B35/48) were infected with NL-432, NL-Xh, or NL-M20A. On day 5 postinfection, the cells were harvested and used as target cells in standard 51Cr-release assays. The HLA-B*3501-restricted, HIV-1-specific CTL clones SF2-4-2 and SF2-33-135 were used at an effector/target ratio of 10:1. The HLA-B∗5101-restricted, HIV-1-specific CTL clone SF2-gag327-9-101 was used as an HLA-mismatched negative control. The results shown are the averages of duplicate assays. The specific lysis by CTL clones SF2-4-2 and SF2-33-135 for CD4+ T cells pulsed with the corresponding peptide (1 μM) was 67 and 77%, respectively.
The cytolytic activity of HIV-1-specific CTL clones for these HIV-1-infected targets was investigated by using the HLA-B*3501-restricted CTL clones SF2-4-2 and SF2-33-135. As shown in Fig. 1B, both CTL clones were able to kill CD4+ T cells infected with NL-Xh and NL-M20A but failed to kill cells infected with NL-432. Although the cytolytic activities of these CTL clones for CD4+ T cells infected with NL-M20A were relatively low (10.2 and 10.0% specific lysis, respectively), these activities were significantly higher than that of an HLA-mismatched CTL clone (HLA-B*5101-restricted HIV-1-specific CTL clone SF2-pol283-8-54) for CD4+ T cells infected with HIV-1 (−0.62% ± 1.62% specific lysis). In an independent experiment, an HIV-1-specific, HLA-A*2402-restricted CTL clone was similarly able to kill CD4+ T cells infected with NL-Xh and NL-M20A but failed to kill cells infected with NL-432 (data not shown). These results strongly suggest that the impaired cytolytic activity of HIV-1-specific CTL clones for primary CD4+ T cells infected with HIV-1 is due to Nef-mediated HLA class I down-regulation.
Cytokine production by HIV-1-specific CTL clones following stimulation with HIV-1-infected primary CD4+ T cells.
HIV-1-specific CD8+ T cells suppress the replication of HIV-1 both by cytotoxic activity against infected cells and through production of soluble HIV-1-suppressive factors such as IFN-γ and chemokines. While previous studies have shown that HIV-1-specific CD8+ T cells fail to kill HIV-1-infected primary CD4+ T cells, the ability of these CD8+ T cells to produce IFN-γ and chemokines following stimulation with HIV-1-infected primary CD4+ T cells has not been clarified. We therefore investigated the ability of these cells to produce cytokines following stimulation with CD4+ T cells infected with two different HIV-1 strains, NL-432 and NL-M20A.
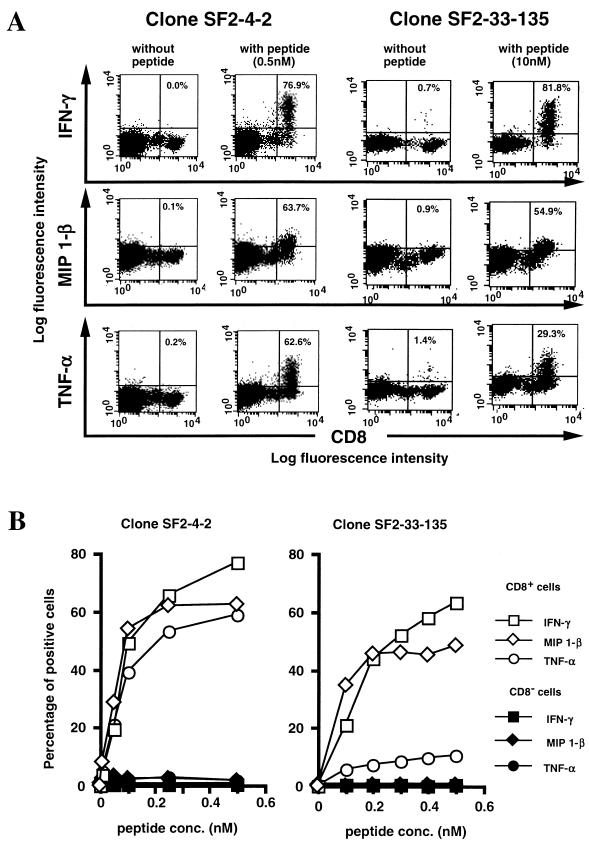
We first measured cytokine production by several HIV-1-specific CTL clones stimulated with CD4+ T cells prepulsed with HIV-1-derived peptides. The number of intracellular cytokine-positive cells was counted by flow cytometry. Results from two representative CTL clones (SF2-4-2 and SF2-33-135) are shown in Fig. 2. Both CTL clones produced significant levels of IFN-γ, MIP-1β, and TNF-α 6 h after stimulation with CD4+ T cells prepulsed with HIV-1-derived peptides (Fig. 2A), with cytokine production being dependent on peptide concentration (Fig. 2B).
FIG. 2.
Cytokine production of HIV-1-specific CTL clones stimulated with primary CD4+ T cells pulsed with HIV-1-specifc peptide. (A) Primary CD4+ T (HLA-A2/24, B35/48) cells prepulsed with HIV-1-specific peptides (SF2-4, 0.5 nM; SF2-33, 10 nM) were mixed with HIV-1-specific CTL clone SF2-4-2 or SF2-33-135 and then incubated for 6 h at 37°C with brefeldin A. Cells were costained with anti-CD8 MAb and anti-IFN-γ MAb, anti-MIP-1β MAb, or TNF-α MAb and analyzed by flow cytometry. The percentage of cytokine-positive CD8+ T cells is shown in each upper right quadrant. (B) Primary CD4+ T (HLA-A2/24, B35/48) cells prepulsed with serially diluted cognate peptide were mixed with HIV-1-specific CTL clone SF2-4-2 or SF2-33-135 and then incubated for 6 h at 37°C. Cells were costained with anti-CD8 MAb and anti-IFN-γ MAb, anti-MIP-1β MAb, or TNF-α MAb. The percentage of intracellular cytokine-positive CD8+ T cells and CD8− T cells was analyzed by flow cytometry. The results shown are the averages of duplicate assays.
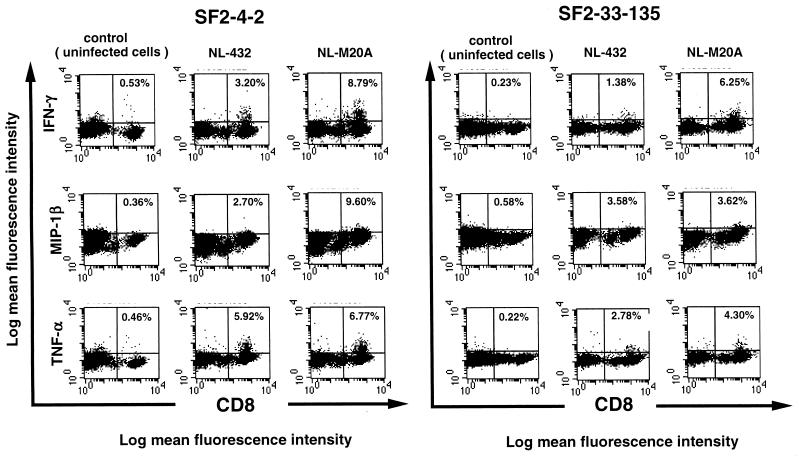
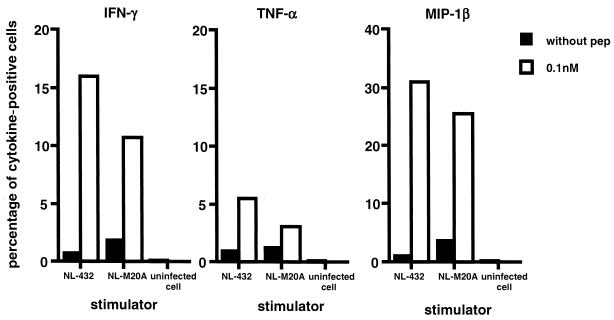
Cytokine production by HIV-1-specific CTL clones stimulated with HIV-1-infected primary CD4+ T cells was investigated by using the HLA-B*3501-restricted, HIV-1-specific CTL clones SF2-4-2 and SF2-33-135. Primary CD4+ T cells were infected with NL-432 or NL-M20A (the percentage of p24 Ag-positive cells in NL-432- and NL-M20A-infected populations was 49 and 47%, respectively). On day 7 postinfection, the CTL clones were stimulated with the HIV-1-infected CD4+ T cells and their intracellular cytokine expression was analyzed by flow cytometry. The number of cytokine-positive cells was lower in HIV-1-specific CTL clones stimulated with NL-M20A-infected or NL-432-infected CD4+ T cells than in those stimulated with CD4+ T cells prepulsed with 0.1 nM HIV-1-derived peptide (Fig. 2B and Fig. 3). This result suggests either that the number of epitope peptide-binding HLA-B*3501 molecules on NL-M20A- or NL-432-infected CD4+ T cells is lower than that on CD4+ T cells prepulsed with epitope peptide or that HIV-1-infected CD4+ T cells can suppress cytokine production. To exclude the second possibility, we investigated cytokine production by HIV-1-specific CTL clone SF2-33-135 stimulated with NL-M20A- or NL432-infected CD4+ T cells that were prepulsed with epitope peptide. The results showed that a peptide pulse restores cytokine production in this CTL clone (Fig. 4), indicating that HIV-1-infected CD4+ T cells do not suppress cytokine production.
FIG. 3.
Cytokine production by HIV-1-specific CTL clones stimulated with primary CD4+ T cells infected with HIV-1 and Nef-mutant HIV-1 strains. CD4+ T cells were infected with NL-432 or NL-M20A. On day 7 postinfection, HIV-1-infected or uninfected cells were cocultured with HIV-1-specific CTL clone SF2-4-2 or SF-2-33-135 for 6 h. Intracellular staining of IFN-γ, MIP-1β, and TNF-α was performed, and stained cells were analyzed by flow cytometry. The percentage of intracellular cytokine-positive CD8+ T cells is shown in each upper right quadrant.
FIG. 4.
Cytokine production by HIV-1-specific CTL clones stimulated with HIV-1-infected CD4+ T cells prepulsed with epitope peptide. CD4+ T cells were infected with NL-432 or NL-M20A. On day 9 postinfection, NL-432- or NL-M20A-infected CD4+ T cells prepulsed with or without peptide were mixed with HIV-1-specific CTL clone SF2-33-135 (effector/stimulator ratio of 1:5) and then incubated for 6 h. Intracellular staining of IFN-γ, TNF-α, or MIP-1β was performed, and cytokine-positive cells were analyzed by flow cytometry. The percentage of p24 Ag-positive cells in CD4+ T cells infected with NL-432 or NL-M20A was 41.2 and 38.5%, respectively.
Cytokine-positive cells were detected not only in CTL clones stimulated with NL-M20A-infected CD4+ T cells but also in those stimulated with NL-432-infected CD4+ T cells (Fig. 3). However, the number of cytokine-positive cells in CTL clones stimulated with NL-432-infected cells was significantly lower (1.4- to 5-fold) than that in clones stimulated with NL-M20A-infected cells. The surface expression of HLA-B35 molecules on NL-432-infected CD4+ T cells was twofold lower than that on NL-M20A-infected cells (mean fluorescence intensity: NL-432-infected cells, 53.2; M20A-infected cells, 103.1). These results suggest that Nef-mediated HLA class I down-regulation on HIV-1-infected cells influences, but does not completely eliminate, cytokine production by HIV-1-specific CD8+ T cells.
Suppression of HIV-1 replication by HIV-1-specific CTL clones.
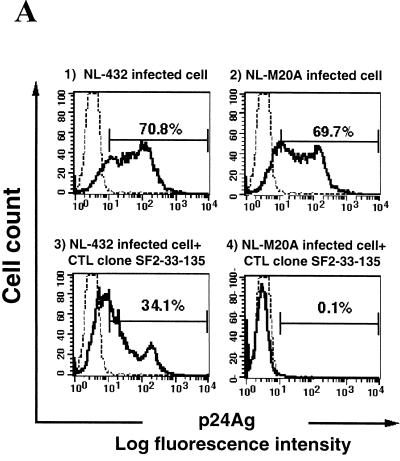
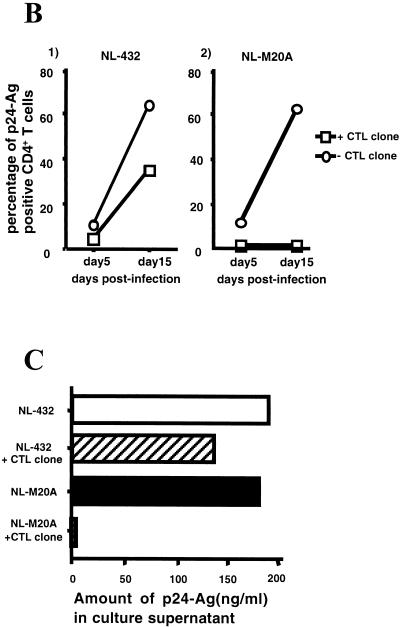
We investigated the ability of HIV-1-specific CTL clones to inhibit HIV-1 replication in vitro. Primary CD4+ T cells were mixed with NL-432 or NL-M20A for 6 h and then cocultured with HIV-1-specific CTL clones. The percentage of p24-positive cells in control CD4+ T-cell cultures infected with NL-432 or NL-M20A increased from approximately 10% on day 5 after infection to approximately 70% on day 15 (Fig. 5A -1, A-2, and B-2). In contrast, p24-positive cells were not detected following coculture of NL-M20A-infected CD4+ T cells and the SF2-33-135 CTL clone on days 5 and 15 postinfection (Fig. 5A-4 and B-2). In the coculture of NL-432-infected CD4+ T cells and the SF2-33-135 CTL clone, the percentage of p24-positive cells was significantly decreased on both days 5 and 15 postinfection compared to that in the control NL-432-infected CD4+ T-cell culture (Fig. 5A-3 and B-1). Similar results were observed for p24 Ag present in the culture medium of HIV-1-infected CD4+ T cells cocultured with or without the SF2-33-135 CTL clone (Fig. 5C). These results indicate that the SF2-33-135 CTL clone partially suppressed the replication of NL-432. This was also confirmed by an independent experiment using the SF2-33-135 CTL clone and the HLA-A∗3303-restricted CTL clone SF2-pol594-9-1. The SF2-33-135 CTL clone was able to suppress replication of NL-M20A (71% suppression) and could also partially suppress replication of NL-432 (43% suppression), while SF2-pol594-9-1 failed to suppress replication of either HIV-1 clone (16 and 13% suppression for NL-432 and NL-M20A, respectively) (data not shown).
FIG. 5.
Suppression of HIV-1 replication by an HIV-1-specific CTL clone. CD4+ T cells were infected with NL-432 or NL-M20A and then cocultured with or without the HIV-1-specific CTL clone SF2-33-135. On days 5 and 15 postinfection, cells and culture supernatant were harvested. (A) p24 Ag-positive, CD8-negative cells were analyzed on day 15 postinfection by flow cytometry. (B) The percentage of intracellular p24 Ag-positive cells in NL-432- or NL-M20A-infected CD4+ T cells cultured with or without the CTL clone SF2-33-135 was measured by flow cytometry on days 5 and 15 postinfection. (C) The culture supernatants (10 μl) from NL-432- or NL-M20A-infected CD4+ T cells cultured with or without CTL clone SF2-33-135 were taken on days 3, 5, 9, and 15 postinfection, and the amount of p24 Ag in the culture supernatants was measured by enzyme immunoassay. The percent suppression of NL-432 and NL-M20A replication by SF2-33-135 was 28.1 and 99.9%, respectively.
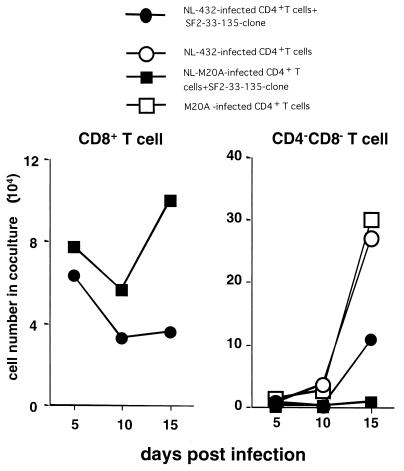
We next investigated the ability of HIV-1-infected primary CD4+ T cells to induce proliferation of HIV-1-specific CD8+ T cells by measuring the number of CD8+ T cells (SF2-33-135 CTL clone) and CD4− CD8− T cells (NL-M20A- or NL-432-infected primary CD4+ T cells) in cocultures of the two cell types. On day 15 postinfection, the number of CD8+ T cells present in the coculture with NL-M20A-infected CD4+ T cells was the same as the number added to the culture, while the number of CD8+ T cells present in the coculture with NL-432-infected CD4+ T cells decreased to one-third (Fig. 6). These results indicate that primary CD4+ T cells infected with NL-432 have very little ability to induce proliferation of HIV-1-specific CD8+ T cells. On days 10 and 15 postinfection, the number of CD4− CD8− T cells was significantly lower in the coculture of the SF2-33-135 CTL clone and NL-432-infected CD4+ T cells than in the control culture of NL-432-infected CD4+ T cells (Fig. 6). These results also support the idea that the SF2-33-135 CTL clone can partially suppress NL-432 replication.
FIG. 6.
Proliferation of HIV-1-specific CTL clones following stimulation with HIV-1-infected CD4+ T cells. CD4+ T cells infected with NL-432 or NL-M20A were cocultured with or without CTL clone SF2-33-135. Infected CD4+ T cells (105) were mixed with the same number of CTLs. On days 5, 10, and 15 postinfection, the number of total cells in each coculture was counted and the percentage of each type of cells in the coculture was determined by flow cytometry. The number of each type of cell in the coculture was calculated as follows: total cell number × percentage of each population.
DISCUSSION
Previous studies demonstrated that HIV-1-specific CTLs killed Nef-defective HIV-1-infected CD4+ T cells but not Nef-positive HIV-1-infected CD4+ T cells (11, 12). These studies indicated that the cytolytic activity of HIV-1-specific, HLA-A2-restricted CTL clones for HIV-1-infected CD4+ T cells is eliminated by Nef-mediated HLA class I down-regulation. However, as Nef has many other functions, such as CD4 down-regulation, enhancement of viral infectivity, blocking of IP3-induced calcium release, and blocking of CD3 signaling (35), it remained possible that unknown functions of Nef affect recognition of HIV-1-infected cells by HIV-1-specific CTLs. To clarify the role of Nef-mediated HLA class I down-regulation in CTL function, we used an HIV-1 strain that encodes a single-amino-acid mutant of Nef, the NL-M20A clone. NL-M20A has a single-amino-acid substitution at position 20 in Nef and has previously been reported to fail to induce HLA class I down-regulation on infected cells but to retain other Nef functions such as CD4 down-regulation and enhancement of viral infectivity (1). We confirmed that the surface expression of HLA-A*2402 and HLA-B*3501 was not suppressed on NL-M20A-infected CD4+ T cells. Moreover, both HLA-A*2402-restricted and HLA-B*3501-restricted CTL clones failed to kill HIV-1-infected CD4+ T cells but effectively killed NL-M20A-infected CD4+ T cells. Thus, we confirmed that the impaired cytolytic activity of HIV-1-specific CTLs for HIV-1-infected CD4+ T cells was due to Nef-mediated HLA class I down-regulation. We also provided evidence that Nef-mediated HLA class I down-regulation affects the cytolytic activity of HLA-B-restricted CTL clones in addition to HLA-A-restricted CTL clones. However, since we used CTL clones in the present study, analysis using polyclonal CTLs will be required to exclude the possibility that this effect of Nef is not restricted with CTL clones used in the present study.
HLA-B35 molecules were previously reported to be associated with rapid progression of AIDS (35). A recent study showed that HLA-B*3502 and HLA-B*3503 molecules are strongly associated with disease progression to AIDS while HLA-B*3501 is not (14). It will be very interesting to analyze the recognition of HIV-1-specific CTLs restricted by these HLA-B*35 molecules and by HLA-B27 or HLA-B57 molecules, which are associated with delayed progression to AIDS (21).
In the experiment shown in Fig. 1, approximately 40% of NL-432-infected CD4+ T cells (approximately 25% of target cells) showed high expression of HLA-A24 and -B35 (Fig. 1A), but no cytolytic activity for these cells by two CTL clones was detected. While 25% of target cells might not be enough to detect specific lysis in a standard 51Cr-release assay, it remains possible that presentation of HIV-1 epitopes is abolished by an unknown mechanism due to HLA class I down-regulation in HIV-1-infected CD4+ T cells.
The failure of HIV-1-specific CTL clones to kill HIV-1-infected CD4+ T cells indicates that the T-cell-receptor-mediated signal induced by recognizing epitopes presented by down-regulated HLA class I molecules is too weak to induce cytolytic activity in CTL clones. However, we showed that CTL clones stimulated with HIV-1-infected CD4+ T cells were still able to produce the HIV-1-suppressive factors IFN-γ, MIP-1β, and TNF-α. This indicates that HIV-1-specific CTLs can produce cytokines by recognizing a small number of HIV-1 epitopes. These findings suggest that HIV-1 replication may be partially inhibited by HIV-1-suppressive factors secreted from HIV-1-specific CD8+ T cells in HIV-1-infected individuals. Indeed, we demonstrated that HIV-1-specific CTL clones partially suppressed the replication of NL-432 in a coculture of NL-432-infected CD4+ T cells and HIV-1-specific CTL clones. Similar results were reported in a previous study, which showed that an HIV-1-specific CTL clone could suppress replication of HIV-1 strain JR-CSF in primary CD4+ T cells infected with this HIV-1 clone (40). The strong suppression of NL-M20A replication in a coculture of NL-M20A-infected CD4+ T cells with an HIV-1-specific CTL clone observed in the present study was due to both CTL cytolytic activity and cytokine production. It is likely that the partial suppression of NL-432 replication in coculture was due to cytokine production alone, as the cytolytic activity of CTL clones for NL-432-infected CD4+ T cells was impaired. However, it is very difficult to rule out the possibility that CTL clones partially lysed NL-432-infected CD4+ T cells in long-term cultures.
Highly active antiretroviral therapy dramatically diminished HIV-1 viral load and the number of HIV-1-specific CD8+ T cells in HIV-1-infected individuals (33). This finding suggests that HIV-1-infected cells can effectively present HIV-1 epitopes to CD8+ T cells and that the stimulated HIV-1-specific CD8+ T cells can proliferate. In the present study, we investigated proliferation of HIV-1-specific CTL clones in cocultures with HIV-1-infected CD4+ T cells. The number of HIV-1-specific CD8+ T cells decreased when they were cocultured with NL-432-infected CD4+ T cells but increased during days 10 to 15 when they were cocultured with NL-M20A-infected CD4+ T cells. Thus, since the ability of HIV-1-infected CD4+ T cells to induce proliferation of HIV-1-specific CD8+ T cells is very weak, other HIV-1-infected cells such as macrophages and dendritic cells may be able to effectively induce proliferation of HIV-1-specific CD8+ T cells. A recent study showed that Nef does not down-regulate HLA class I on dendritic cells infected with an adenovirus clone expressing Nef (13). Nef-mediated HLA class I down-regulation might therefore have a minimal effect on Ag presentation by HIV-1-infected dendritic cells.
In the present study, HLA class I down-regulation on HIV-1-infected CD4+ T cells and CTL recognition of HIV-1-infected CD4+ T cells were analyzed by using laboratory-isolated HIV-1 clones. Therefore, it is not clear whether Nef-mediated HLA class I down-regulation is an active event in vivo. A previous study demonstrated that HLA class I expression on PBMCs and CD4+ T cells is much lower in AIDS patients than in HIV-1-seronegative individuals (36). In contrast, a recent study showed that HIV-1 isolates from asymptomatic individuals were able to down-regulate HLA class I while those from AIDS patients were not (7). Thus, events related to Nef-mediated HLA class I down-regulation are still unclear in HIV-1-infected individuals.
In the present study, we investigated production of three cytokines, IFN-γ, MIP-1β, and TNF-α, by HIV-1-specific CTL clones. Since IL-2, IL-4, and IL-12 influence Th1 immune responses, it is also important to analyze the production of these cytokines by the CTL clones. Further studies are expected to clarify the effect of Nef-mediated HLA class I down-regulation on the total immune responses of HIV-1-infected individuals.
In summary, we show here that Nef-mediated down-regulation of HLA class I molecules on HIV-1-infected CD4+ T cells affects CTL cytokine production to a lesser extent than CTL cytolytic activity. In a coculture of HIV-1-infected CD4+ T cells with HIV-1-specific CTL clones, replication of NL-432 was partially suppressed while that of NL-M20A was completely suppressed. These findings suggest that HIV-1-specific CD8+ T cells are able to partially suppress the replication of HIV-1 by production of soluble HIV-1-suppressive factors such as chemokines and interferon.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sport and Culture and the Ministry of Health Science, the government of Japan, and a grant from the Japan Health Science Foundation.
We thank Hideaki Tada (Minase Research Institute Ono Pharmaceutical Co. Ltd.) for providing rhIL-4 and Sachiko Sakai and Kumiko Yamashita for secretarial assistance.
REFERENCES
- 1.Akari, H., S. Arold, T. Fukumori, T. Okazaki, K. Strebel, and A. Adachi. 2000. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J. Virol. 74:2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, J., H. Behbahani, J. Lieberman, E. Connick, A. Landay, B. Patterson, A. Sonnerborg, K. Lore, S. Uccini, and T. E. Fehniger. 1999. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS 13:1295-1303. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. A. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. L. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgne, L. S., M. Fevrier, C. Callebaut, S. P. Lee, and Y. Riviere. 2000. CD8+-cell antiviral factor activity is not restricted to human immunodeficiency virus (HIV)-specific T cells and can block HIV replication after initiation of reverse transcription. J. Virol. 74:4456-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. A. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 7.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choppin, J., F. Martinon, F. Connan, M. Pauchard, E. Gomard, and J. P. Levy. 1991. HLA-binding regions of HIV-1 proteins. II. A systematic study of viral proteins. J. Immunol. 147:575-583. [PubMed] [Google Scholar]
- 9.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 11.Collins, K. L., and D. Baltimore. 1999. HIV's evasion of the cellular immune response. Immunol. Rev. 168:65-74. [DOI] [PubMed] [Google Scholar]
- 12.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 13.Cramer, L. A., and J. A. Frelinger. 2001. Dendritic cells transduced with HIV Nef express normal levels of HLA-A and HLA-B class I molecules. J. Acquir. Immune Defic. Syndr. 27:417-425. [DOI] [PubMed] [Google Scholar]
- 14.Gao, X., G. W. Nelson, P. Karacki, B. A. Maureen, P. Martin, J. Phair, R. Kaslow, J. Goedert, S. Buchinder, K. Hoots, D. Vlahov, S. J. O'Brien, and M. Carrington. 2001. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N. Engl. J. Med. 344:1668-1675. [DOI] [PubMed] [Google Scholar]
- 15.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrer, T., E. Harrer, S. A. Kalams, T. Elbeik, S. I. Staprans, M. B. Feinberg, Y. Cao, D. D. Ho, T. Yilma, A. M. Caliendo, R. P. Johnson, S. P. Buchbinder, and B. D. Walker. 1996. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS. Res. Hum. Retrovir. 12:585-592. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda-Moore, Y., H. Tomiyama, K. Miwa, S. Oka, A. Iwamoto, Y. Kaneko, and M. Takiguchi. 1997. Identification and characterization of multiple HLA-A24-restricted HIV-1 CTL epitopes: strong epitopes are derived from V regions of HIV-1. J. Immunol. 159:6242-6252. [PubMed] [Google Scholar]
- 19.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 20.Kerkau, T., I. Bacik, J. R. Bennink, J. W. Yewdell, T. Hunig, A. Schimpl, and U. Schubers. 1997. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 185:1295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerkau, T., R. Schmitt-Landgraf, A. Schimpl, and E. Wecker. 1989. Downregulation of HLA class I antigens in HIV-1-infected cells. AIDS Res. Hum. Retrovir. 5:613-620. [DOI] [PubMed] [Google Scholar]
- 22.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. K. Kerkhof Garde, R. J. Bende, I. P. M. Keet, J. K. M. Eeftinck-Schattenkerk, A. D. M. E. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the M subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 25.Levy, J. A., C. E. Mackewicz, and E. Barker. 1996. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today. 17:217-224. [DOI] [PubMed] [Google Scholar]
- 26.Mackewicz, C. E., D. J. Blackbourn, and J. A. Levy. 1995. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl. Acad. Sci. USA 92:2308-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangasarian, A., V. Piguet, J. K. Wang, Y. L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui, M., R. J. Warburton, P. C. Cogswell, A. S. Baldwin, Jr., and J. A. Frelinger. 1996. Effects of HIV-1 Tat on expression of HLA class I molecules. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:233-240. [DOI] [PubMed] [Google Scholar]
- 29.McMichael, A. J., and R. E. Phillips. 1997. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 15:271-296. [DOI] [PubMed] [Google Scholar]
- 30.Meylan, R. P., J. C. Guatelli, J. R. Munis, D. D. Richman, and R. S. Kornbluth. 1993. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology 193:138-148. [DOI] [PubMed] [Google Scholar]
- 31.Noraz, N., B. Verrier, C. Fraisier, and C. Desgranges. 1995. Cell surface phenotypic changes induced in H9 T cells chronically infected with HTLV type I or HIV type 1 or coinfected with the two viruses. AIDS Res. Hum. Retrovir. 11:145-154. [DOI] [PubMed] [Google Scholar]
- 32.Norris, P. J., and E. S. Rosenberg. 2001. Cellular immune response to human immunodeficiency virus. AIDS 15(Suppl. 2):S16-S21. [DOI] [PubMed] [Google Scholar]
- 33.Ogg, G. S., X. Jin, S. Bonhoeffer, P. Moss, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, A. Hurley, M. Markowitz, D. D. Ho, A. J. McMichael, and D. F. Nixon. 1999. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 73:797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 35.Peter, F. 1998. HIV nef: the mother of all evil? Immunity 9:433-437. [DOI] [PubMed] [Google Scholar]
- 36.Puppo, F., S. Brenci, O. Bosco, L. Lanza, S. Barocci, A. Nocera, M. Ghio, P. Contin, M. Setti, M. Scudeletti, and F. Indiveri. 1997. Downregulation of HLA class I antigen expression in CD4+ T lymphocytes from HIV type 1-infected individuals. AIDS Res. Hum. Retrovir. 13:1509-1516. [DOI] [PubMed] [Google Scholar]
- 37.Scheppler, J. A., J. K. Nicholson, D. C. Swan, A. Ahmed-Ansari, and J. S. McDougal. 1989. Down-modulation of MHC-I in a CD4+ T cell line, CEM-E5, after HIV-1 infection. J. Immunol. 143:2858-2866. [PubMed] [Google Scholar]
- 38.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 40.Severino, M. E., N. V. Sipsas, P. T. Nguyen, S. A. Kalams, B. D. Walker, R. P. Johnson, and O. O. Yang. 2000. Inhibition of human immunodeficiency virus type 1 replication in primary CD4+ T lymphocytes, monocytes, and dendritic cells by cytotoxic T lymphocytes. J. Virol. 74:6695-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomiyama, H., K. Miwa, H. Shiga, Y. I. Moore, S. Oka, A. Iwamoto, Y. Kaneko, and M. Takiguchi. 1997. Evidence of presentation of multiple HIV-1 cytotoxic T lymphocyte epitopes by HLA-B∗3501 molecules that are associated with the accelerated progression of AIDS. J. Immunol. 158:5026-5034. [PubMed] [Google Scholar]
- 42.Tomiyama, H., T. Sakaguchi, K. Miwa, S. Oka, A. Iwamoto, Y. Kaneko, and M. Takiguchi. 1999. Identification of multiple HIV-1 CTL epitopes presented by HLA-B∗5101 molecules. Hum. Immunol. 60:177-186. [DOI] [PubMed] [Google Scholar]
- 43.Truong, M. J., E. C. Darcissac, E. Hermann, J. Dewulf, A. Capron, and G. M. Bahr. 1999. Interleukin-16 inhibits human immunodeficiency virus type 1 entry and replication in macrophages and in dendritic cells. J. Virol. 73:7008-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 9:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, O. O., S. A. Kalams, M. Rosenzweig, A. Trocha, N. Jones, M. Koziel, B. D. Walker, and R. P. Johnson. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 70:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]