Abstract
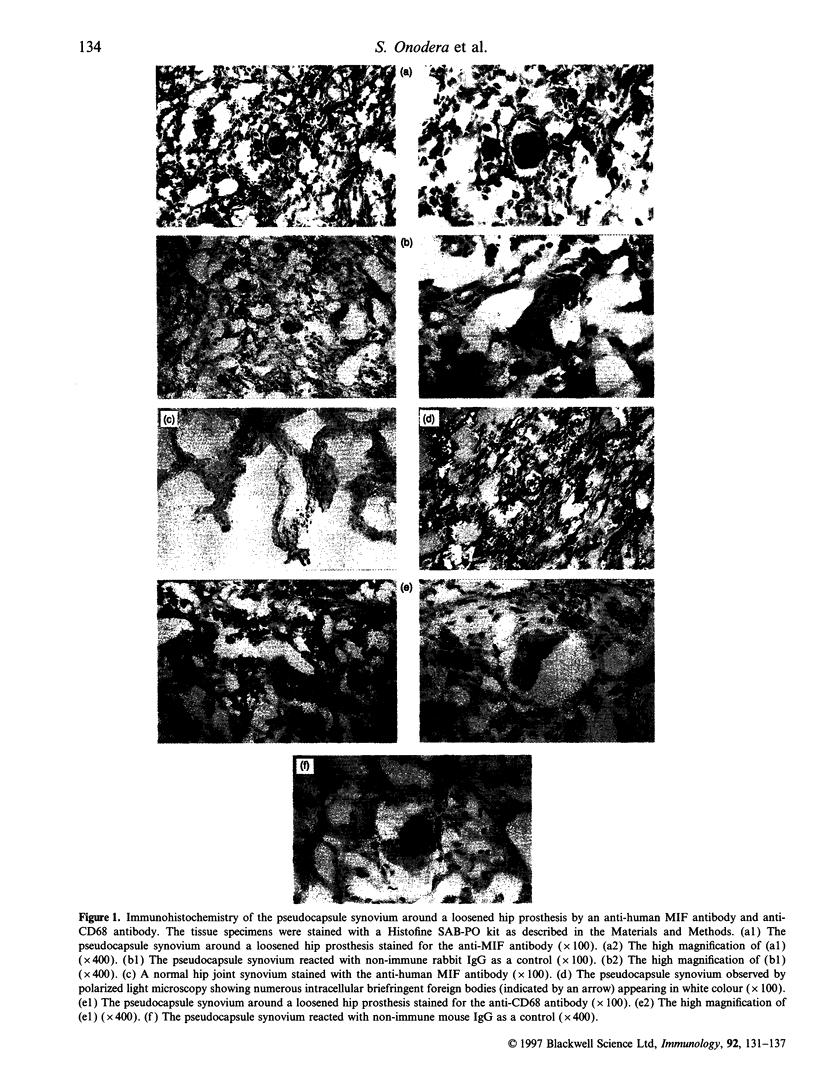
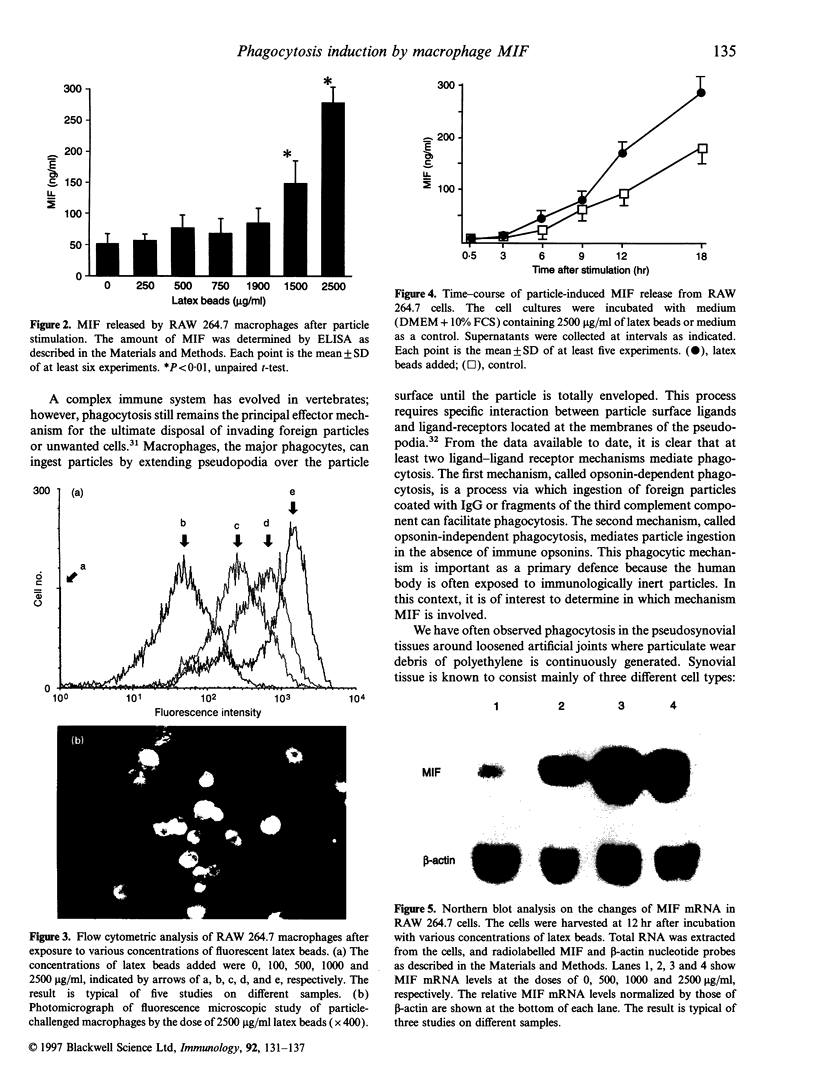
Exposure to foreign particles sometimes causes inflammatory reactions through production of cytokines and chemoattractants by phagocytic cells. In this study, we focused on macrophage migration inhibitory factor (MIF) to evaluate its pathophysiological role in the phagocytic process. Immunohistochemical analysis of human pseudosynovial tissues retrieved at revision of total hip arthroplasty showed that infiltrating mononuclear and multinuclear cells were positively stained by both an anti-CD68 antibody and anti-human MIF antibody. For in vitro study, MIF was released from murine macrophage-like cells (RAW 264.7) in response to phagocytosis of fluorescent-latex beads in a particle dose-dependent manner. Northern blot analysis showed marked elevation of the MIF mRNA level in the phagocytic macrophage-like cells. Moreover, pretreatment of RAW 264.7 cells with rat recombinant MIF increased the extent of phagocytosis by 1.6-fold compared with the control. Taken together, these results suggest that MIF plays an important role by activating macrophages in autocrine and paracrine fashion to phagocytose foreign particles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995 Sep 7;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Mitchell R. A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994 Jun 1;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Weiser W. Y., David J. R., Moss D. W., Moncada S., Liew F. Y. Recombinant migration inhibitory factor induces nitric oxide synthase in murine macrophages. J Immunol. 1993 Mar 1;150(5):1908–1912. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels A. O., Cohn Z. A. The alveolar macrophage. J Appl Physiol (1985) 1986 Feb;60(2):353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Jacobs J. J., Molnár G., Shanbhag A. S., Valyon M., Galante J. O. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993 Sep;8(9):1071–1079. doi: 10.1002/jbmr.5650080907. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Jacobs J. J. Response of three murine macrophage populations to particulate debris: bone resorption in organ cultures. J Orthop Res. 1994 Sep;12(5):720–731. doi: 10.1002/jor.1100120515. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Schiller A. L., Roelke M., Rourke C. M., O'Neil D. A., Harris W. H. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983 Jun;65(5):575–584. [PubMed] [Google Scholar]
- Goto M., Sasano M., Yamanaka H., Miyasaka N., Kamatani N., Inoue K., Nishioka K., Miyamoto T. Spontaneous production of an interleukin 1-like factor by cloned rheumatoid synovial cells in long-term culture. J Clin Invest. 1987 Sep;80(3):786–796. doi: 10.1172/JCI113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. M., Doty S. B., Lane J. M., Burstein A. H. Studies of the mechanism by which the mechanical failure of polymethylmethacrylate leads to bone resorption. J Bone Joint Surg Am. 1993 Jun;75(6):802–813. doi: 10.2106/00004623-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Jiranek W. A., Machado M., Jasty M., Jevsevar D., Wolfe H. J., Goldring S. R., Goldberg M. J., Harris W. H. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am. 1993 Jun;75(6):863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Chiba J., Rubash H. E. In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. J Bone Joint Surg Am. 1994 Feb;76(2):172–180. doi: 10.2106/00004623-199402000-00002. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Rubash H. E., Wilson S. C., D'Antonio J. A., McClain E. J. A histologic and biochemical comparison of the interface tissues in cementless and cemented hip prostheses. Clin Orthop Relat Res. 1993 Feb;(287):142–152. [PubMed] [Google Scholar]
- Kobzik L., Huang S., Paulauskis J. D., Godleski J. J. Particle opsonization and lung macrophage cytokine response. In vitro and in vivo analysis. J Immunol. 1993 Sep 1;151(5):2753–2759. [PubMed] [Google Scholar]
- Maguire J. K., Jr, Coscia M. F., Lynch M. H. Foreign body reaction to polymeric debris following total hip arthroplasty. Clin Orthop Relat Res. 1987 Mar;(216):213–223. [PubMed] [Google Scholar]
- Matsuda A., Tagawa Y., Matsuda H., Nishihira J. Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett. 1996 May 6;385(3):225–228. doi: 10.1016/0014-5793(96)00386-9. [DOI] [PubMed] [Google Scholar]
- Mirra J. M., Marder R. A., Amstutz H. C. The pathology of failed total joint arthroplasty. Clin Orthop Relat Res. 1982 Oct;(170):175–183. [PubMed] [Google Scholar]
- Nishihira J., Koyama Y., Mizue Y. Identification of macrophage migration inhibitory factor in human leukemia HL-60 cells and its induction by lipopolysaccharide. Biochem Mol Biol Int. 1996 Nov;40(5):861–869. doi: 10.1080/15216549600201473. [DOI] [PubMed] [Google Scholar]
- Nishihira J., Kuriyama T., Nishino H., Ishibashi T., Sakai M., Nishi S. Purification and characterization of human macrophage migration inhibitory factor: evidence for specific binding to glutathione and formation of subunit structure. Biochem Mol Biol Int. 1993 Dec;31(5):841–850. [PubMed] [Google Scholar]
- Nishihira J., Kuriyama T., Sakai M., Nishi S., Ohki S., Hikichi K. The structure and physicochemical properties of rat liver macrophage migration inhibitory factor. Biochim Biophys Acta. 1995 Feb 22;1247(1):159–162. doi: 10.1016/0167-4838(94)00215-3. [DOI] [PubMed] [Google Scholar]
- Onodera S., Suzuki K., Matsuno T., Kaneda K., Kuriyama T., Nishihira J. Identification of macrophage migration inhibitory factor in murine neonatal calvariae and osteoblasts. Immunology. 1996 Nov;89(3):430–435. doi: 10.1046/j.1365-2567.1996.d01-751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L. A., Weiser W. Y. Human recombinant migration inhibitory factor activates human macrophages to kill tumor cells. Cell Immunol. 1992 Dec;145(2):372–379. doi: 10.1016/0008-8749(92)90339-q. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. R., Griffin F. M., Jr Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981 Jan 29;289(5796):409–411. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ohkawara A., Nishihira J., Sakamoto W. Identification of macrophage migration inhibitory factor (MIF) in human skin and its immmunohistochemical localization. FEBS Lett. 1996 Mar 4;381(3):199–202. doi: 10.1016/0014-5793(96)00120-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Murata E., Tanaka I., Nishihira J., Sakai M. Crystallization and a preliminary X-ray diffraction study of macrophage migration inhibitory factor from human lymphocytes. J Mol Biol. 1994 Jan 21;235(3):1141–1143. doi: 10.1006/jmbi.1994.1063. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sugimoto H., Nakagawa A., Tanaka I., Nishihira J., Sakai M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996 Mar;3(3):259–266. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- Weiser W. Y., Pozzi L. M., David J. R. Human recombinant migration inhibitory factor activates human macrophages to kill Leishmania donovani. J Immunol. 1991 Sep 15;147(6):2006–2011. [PubMed] [Google Scholar]
- Weiser W. Y., Temple P. A., Witek-Giannotti J. S., Remold H. G., Clark S. C., David J. R. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert H. G., Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977 Mar;11(2):157–164. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis: an overview. Methods Enzymol. 1986;132:3–15. doi: 10.1016/s0076-6879(86)32003-2. [DOI] [PubMed] [Google Scholar]