Abstract
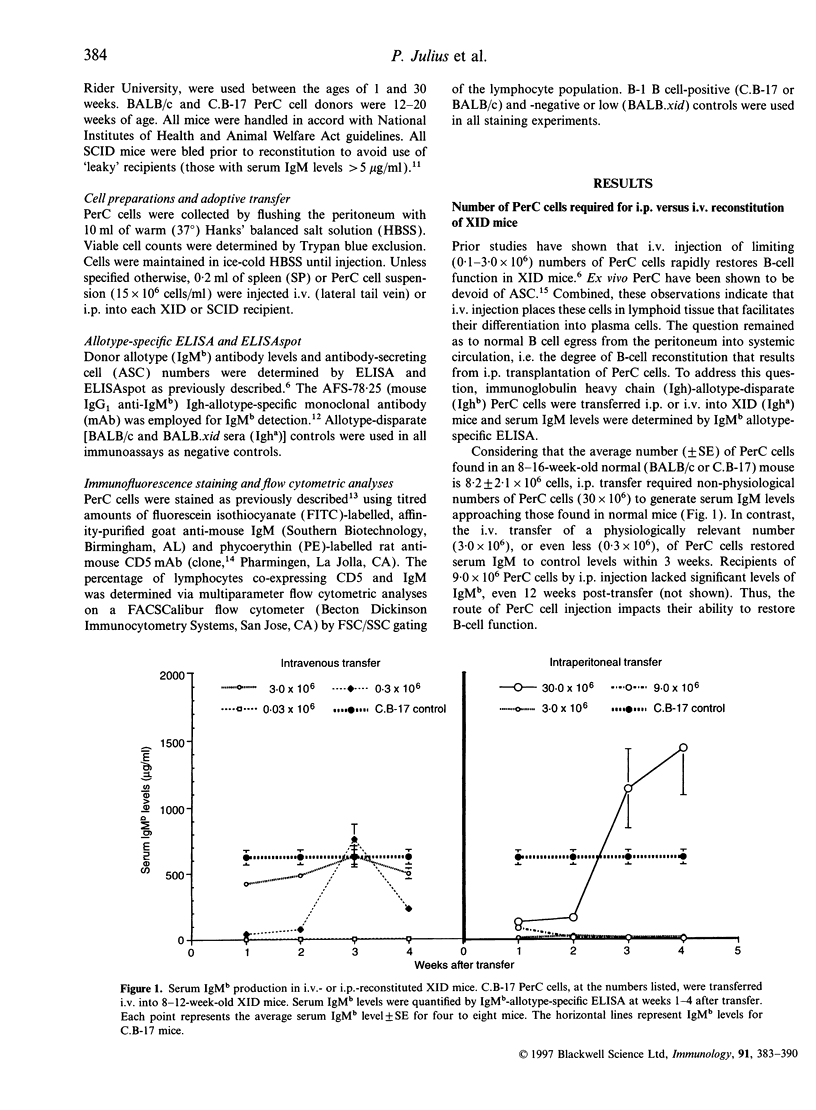
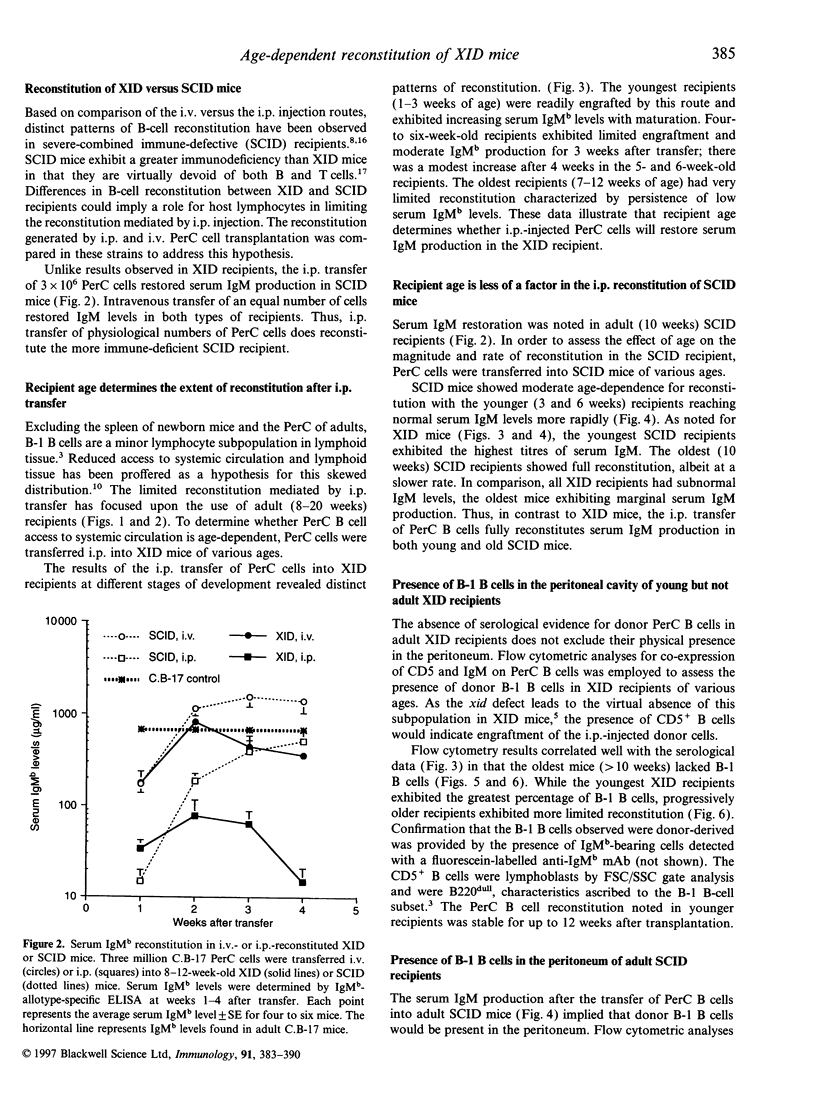
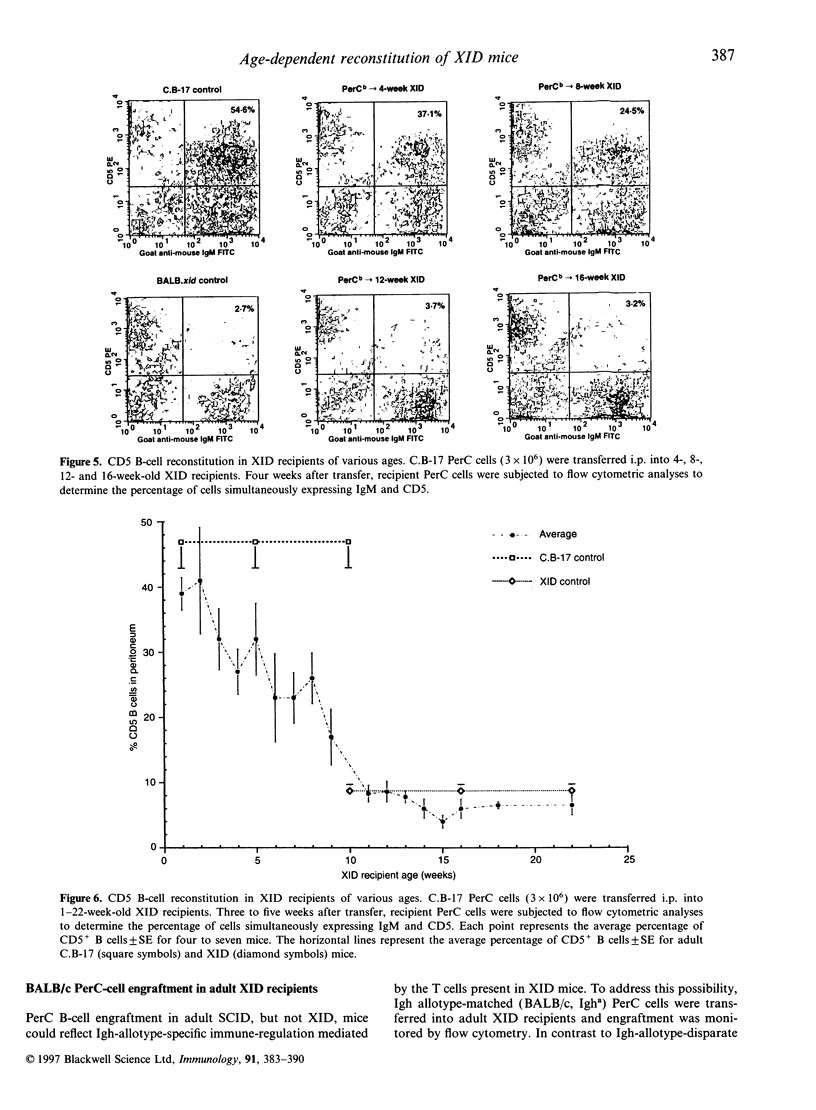
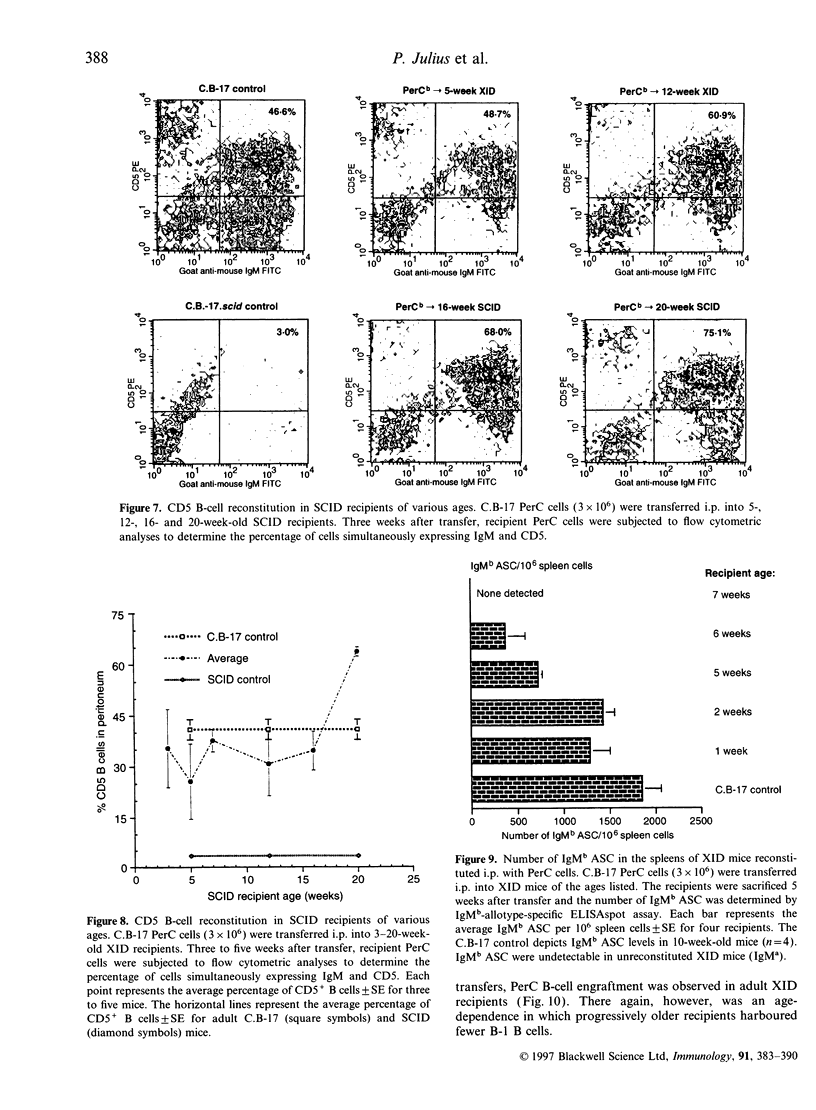
In vivo studies of lymphocyte biology have used intravenous (i.v.) injection as the primary mode of cell transfer, a protocol consistent with the anatomic distribution of most lymphocytes. However, for study of peritoneal cavity B cells, i.v. injection does not correlate with anatomical localization. This report describes the restoration of B-cell function in B lymphocyte-defective X-chromosome-linked immune-defective (XID) mice after intraperitoneal transfer of immunoglobulin heavy chain (Igh)-disparate peritoneal cavity (PerC) cells. In contrast to i.v. transfer, intraperitoneal (i.p.) transfer restored B-cell function in young, but not adult (> 8 weeks), XID mice. When host and donor Igh allotype matched, PerC B-cell engraftment was noted in older recipients; this reconstitution however, was also age-dependent. Migration from the peritoneum to systemic circulation was necessary for serum IgM production as shown by the presence of donor antibody-secreting cells in the host spleen. Host lymphocytes also influenced the success of i.p. transplantation as severe combined immune-deficient mice, regardless of age, exhibited donor serum IgM production. Recipient age, Igh allotype, and immune-deficiency were found to have an impact on the ability of i.p.-transferred PerC B cells to restore B-cell function in XID mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Gibson D. M., Custer R. P., Bosma M. J. Reconstitution of scid mice by injection of varying numbers of normal fetal liver cells into scid neonates. Curr Top Microbiol Immunol. 1989;152:151–159. doi: 10.1007/978-3-642-74974-2_18. [DOI] [PubMed] [Google Scholar]
- Bösing-Schneider R., Selinka H. C., Lehle G. B cell dependence of the congeneic barrier towards idiotype-carrying cells. Eur J Immunol. 1986 Nov;16(11):1401–1405. doi: 10.1002/eji.1830161114. [DOI] [PubMed] [Google Scholar]
- Fidler J. M., Morgan E. L., Weigle W. O. B lymphocyte differentiation in the CBA/N mouse: a delay in maturation rather than a total arrest. J Immunol. 1980 Jan;124(1):13–19. [PubMed] [Google Scholar]
- Förster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987 Apr;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kantor A. B. The development and repertoire of B-1 cells (CD5 B cells). Immunol Today. 1991 Nov;12(11):389–391. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- Kolb C., Weiler E. Regulation of B-cell expression as revealed in the congenic barrier: exerted by B cells, augmented by T cells, and directed toward memory as well as naive donor B lymphocytes. Cell Immunol. 1988 Feb;111(2):433–442. doi: 10.1016/0008-8749(88)90106-2. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Herzenberg L. A., Adams S., Stall A. M. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989 Mar;19(3):507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- Marcos M. A., Sundblad A., Malenchère E., Coutinho A. Peritoneal B cells regulate the numbers of allotype-matched pre-B and B cells in bone marrow. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9944–9948. doi: 10.1073/pnas.88.22.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Holmes K. L., Steinberg A. D., Kastner D. L. CD5+ peritoneal B cells express high levels of membrane, but not secretory, C mu mRNA. J Immunol. 1991 May 15;146(10):3639–3645. [PubMed] [Google Scholar]
- Murakami M., Tsubata T., Okamoto M., Shimizu A., Kumagai S., Imura H., Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992 May 7;357(6373):77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Pecquet S. S., Ehrat C., Ernst P. B. Enhancement of mucosal antibody responses to Salmonella typhimurium and the microbial hapten phosphorylcholine in mice with X-linked immunodeficiency by B-cell precursors from the peritoneal cavity. Infect Immun. 1992 Feb;60(2):503–509. doi: 10.1128/iai.60.2.503-509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior L., Pierson S., Woodland R. T., Riggs J. Rapid restoration of B-cell function in XID mice by intravenous transfer of peritoneal cavity B cells. Immunology. 1994 Oct;83(2):180–183. [PMC free article] [PubMed] [Google Scholar]
- Ridge J. P., Fuchs E. J., Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996 Mar 22;271(5256):1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Hobbs M. V., Mosier D. E. CD4+CD8- thymocytes from neonatal mice induce IgM production in SCID mice. J Immunol. 1992 Mar 1;148(5):1389–1395. [PubMed] [Google Scholar]
- Riggs J. E., Stowers R. S., Mosier D. E. Adoptive transfer of neonatal T lymphocytes rescues immunoglobulin production in mice with severe combined immune deficiency. J Exp Med. 1991 Jan 1;173(1):265–268. doi: 10.1084/jem.173.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs J. E., Stowers R. S., Mosier D. E. The immunoglobulin allotype contributed by peritoneal cavity B cells dominates in SCID mice reconstituted with allotype-disparate mixtures of splenic and peritoneal cavity B cells. J Exp Med. 1990 Aug 1;172(2):475–485. doi: 10.1084/jem.172.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs J., Stowers R. Ability of spleen, peritoneal cavity, and lymph node B cells to reconstitute serum immunoglobulin in SCID mice. Immunology. 1996 May;88(1):20–27. doi: 10.1046/j.1365-2567.1996.d01-636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I. CBA/N immune defective mice; evidence for the failure of a B cell subpopulation to be expressed. Immunol Rev. 1982;64:117–136. doi: 10.1111/j.1600-065x.1982.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]


