Abstract
Human cytomegalovirus (HCMV) possesses low pathogenic potential in an immunocompetent host. In the immunosuppressed host, however, a wide spectrum of infection outcomes, ranging from asymptomatic to life threatening, can follow either primary or nonprimary infection. The variability in the manifestations of HCMV infection in immunosuppressed individuals implies that there is a threshold of host antiviral immunity that can effectively limit disease potential. We used a nonhuman primate model of CMV infection to assess the relationship between CMV disease and the levels of developing anti-CMV immunity. Naive rhesus macaques were inoculated with rhesus cytomegalovirus (RhCMV) followed 2 or 11 weeks later by inoculation with pathogenic simian immunodeficiency virus SIVmac239. Two of four monkeys inoculated with SIV at 2 weeks after inoculation with RhCMV died within 11 weeks with simian AIDS (SAIDS), including activated RhCMV infection. Neither animal had detectable anti-SIV antibodies. The other two animals died 17 and 27 weeks after SIV inoculation with either SAIDS or early lymphoid depletion, although no histological evidence of activated RhCMV was observed. Both had weak anti-SIV antibody titers. RhCMV antibody responses for this group of monkeys were significantly below those of control animals inoculated with only RhCMV. In addition, all animals of this group had persistent RhCMV DNA in plasma and high copy numbers of RhCMV in tissues. In contrast, animals that were inoculated with SIV at 11 weeks after RhCMV infection rarely exhibited RhCMV DNA in plasma, had low copy numbers of RhCMV DNA in most tissues, and did not develop early onset of SAIDS or activated RhCMV. SIV antibody titers were mostly robust and sustained in these monkeys. SIV inoculation blunted further development of RhCMV humoral responses, unlike the normal pattern of development in control monkeys following RhCMV inoculation. Anti-RhCMV immunoglobulin G levels and avidity were slightly below control values, but levels maintained were higher than those observed following SIV infection at 2 weeks after RhCMV inoculation. These findings demonstrate that SIV produces long-lasting insults to the humoral immune system beginning very early after SIV infection. The results also indicate that anti-RhCMV immune development at 11 weeks after infection was sufficient to protect the host from acute RhCMV sequelae following SIV infection, in contrast to the lack of protection afforded by only 2 weeks of immune response to RhCMV. As previously observed, monkeys that were not able to mount a significant immune response to SIV were the most susceptible to SAIDS, including activated RhCMV infection. Rapid development of SAIDS in animals inoculated with SIV 2 weeks after RhCMV inoculation suggests that RhCMV can augment SIV pathogenesis, particularly during primary infection by both viruses.
The pathogenic potential of human cytomegalovirus (HCMV) is dependent on the immune status of the infected individual. In immunocompetent hosts, antiviral immune responses are protective (1, 18, 26). Primary infections are usually asymptomatic despite active replication and systemic dissemination. In addition, periodic reactivation of latent HCMV genomes and production of infectious virus are rarely associated with sequelae. HCMV infection can be dramatically different in those lacking a competent immune system, such as in congenitally infected fetuses (2-4, 6, 17), AIDS patients (5), and immunosuppressed transplant recipients (19). In these individuals, HCMV can produce a wide spectrum of outcomes ranging from subclinical infection to a disseminated fulminant disease that often results in death.
Currently, it is not known what distinguishes at-risk individuals who develop HCMV end organ disease from those who do not. The wide disparity in outcomes implies that variations in the specificity and/or magnitude of anti-HCMV immunity may account for differences in the extent of HCMV replication. It is likely that those with HCMV disease have HCMV immune responses that fall below minimum thresholds required to control replication of the virus, leading to fulminant infection. A fundamental question for understanding HCMV pathogenesis is what level and type of anti-HCMV immune responses are required to restrict HCMV disease potential. To further investigate parameters of protective immunity, a nonhuman primate model of HCMV was used to investigate how differences in antiviral immune status influenced the course of viral infection.
The experimental design for this study was based on a finding from a previous experiment. Briefly, a rhesus cytomegalovirus (RhCMV)-seronegative macaque was inoculated with simian immunodeficiency virus (SIV) 6 weeks after the serological screen for RhCMV. The animal died 15 weeks later with clinical signs of simian AIDS (SAIDS) and weak anti-SIV antibody responses. Numerous cells containing cytoplasmic and nuclear inclusions characteristic of RhCMV were observed in multiple tissues. It was subsequently determined that this animal had become naturally infected with RhCMV by an unknown route of exposure approximately 2 to 4 weeks prior to SIV inoculation. The rapid onset of RhCMV disease following SIV infection in this sentinel animal differed from our previous observations. RhCMV infection alone in healthy animals is distinguished by an absence of clinical signs of disease (14). Acute RhCMV disease suggested that an immature immune response to RhCMV at the time of SIV infection might have predisposed the animal to increased risk of RhCMV sequelae during SAIDS pathogenesis.
We report here that animals inoculated with SIV 2 weeks after inoculation with RhCMV had meager antibody responses to both RhCMV and SIV, succumbed quickly to SAIDS, and often had histopathological evidence of activated RhCMV. In addition, elevated copy numbers of RhCMV DNA were detected in plasma and tissue in all of these animals. In contrast, none of the animals inoculated with SIV 11 weeks after RhCMV died with acute RhCMV disease or SAIDS. These animals developed stronger antibody responses to both RhCMV and SIV and had lower RhCMV genome copy numbers in plasma and tissues than animals inoculated with SIV 2 weeks after RhCMV inoculation. The pattern of RhCMV infection and antibody responses following SIV inoculation implied that protective immune responses to RhCMV were generated within 11 weeks of RhCMV inoculation.
MATERIALS AND METHODS
Animal inoculations.
Healthy colony-bred juvenile rhesus macaques (Macaca mulatta) from the California National Primate Research Center were used for this study. All animal protocols were approved in advance by the University of California, Davis (UC Davis), Animal Use and Care Committee. UC Davis is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animals were serologically negative for RhCMV and SIV prior to inoculation. Pathogenic isolates of RhCMV (strain 68-1 [23]) and molecularly cloned SIV (SIVmac239 nef open reading frame [11]) were used for this study. Fourteen macaques were inoculated intravenously (i.v.) with 1 ml containing 106 50% tissue culture infectious doses (TCID50) of RhCMV 68-1 at week 0. Animals were subsequently inoculated with SIV (106 TCID50) and/or RhCMV as follows. Animals were inoculated i.v. with SIV either at 2 (n = 4; group I) or 11 weeks (n = 4; group II) after RhCMV inoculation. Four animals (group III) were inoculated with both SIV and RhCMV at 11 weeks after RhCMV inoculation. Two animals (group IV) received a second inoculum of RhCMV but no SIV at 11 weeks. Longitudinal plasma samples were collected for immunological and molecular analysis. Animals were euthanized upon signs of SAIDS or 67 to 76 weeks after RhCMV inoculation. At necropsy, tissues and plasma were collected for histopathological, immunological, and molecular analyses. The tissues examined for histopathology included lymph nodes (axillary, inguinal, mesenteric), spleen, thymus, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, kidney, adrenal, lung, bone marrow, tonsil, salivary gland (parotid and submandibular), and brain, unless otherwise noted (Table 1).
TABLE 1.
Inoculation groups and outcomes
| Group | Animal | Age (mos) at RhCMV inoculation (wk 0) | Inoculation with:
|
Cull (wks ARI)f
|
SIV histopathology | Tissue(s) with RhCMV histopathology | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SIV at 2 wks | SIV at 11 wks | SIV + RhCMV at 11 wks | RhCMV at 11 wks | Medical | Elective | |||||
| 29863 | 8a | 15a | SAIDSd | Lung, submandibular lymph nodee | ||||||
| I | 30435 | 7 | + | 29 | SAIDS | None | ||||
| 30437 | 7 | + | 13 | SAIDS | Duodenum, kidney, thymus, brain | |||||
| 30458 | 6 | + | 12 | SAIDS | Ileocolic lymph node, brain, thymus | |||||
| 30462 | 6 | + | 19 | SAIDS | Nonee | |||||
| II | 30396 | 9 | + | 65 | Lymphoproliferation | Nonee | ||||
| 30397 | 9 | + | 68 | Lymphoproliferation | Nonee | |||||
| 30402 | 8 | + | 14b | Lymphoproliferation | Nonee | |||||
| 30409 | 8 | + | 69 | Lymphoproliferation | Nonee | |||||
| III | 30317 | 10 | + | 38 | SAIDS | Thymuse | ||||
| 30349 | 9 | + | 70 | Lymphoproliferation | Nonee | |||||
| 30351 | 9 | + | 67 | Lymphoproliferation | Nonee | |||||
| 30367 | 9 | + | 53c | Lymphoproliferation | Meninges (spinal core) | |||||
| IV | 30217 | 11 | + | 76 | Nonee | |||||
| 30218 | 11 | + | 76 | Nonee | ||||||
RhCMV infection estimated to be 2 to 4 weeks prior to SIV inoculation (see text).
Cause of death is unknown.
Animal exhibited paraparesis of legs with no other clinical signs of SAIDS.
SAIDS: profound generalized lymphoid depletion of lymph nodes and spleen, often accompanied by opportunistic infections.
Brain not examined.
ARI, after RhCMV inoculation.
ELISA and avidity assays.
Anti-RhCMV immunoglobulin G (IgG) responses to total viral antigens were evaluated by enzyme-linked immunosorbent assay (ELISA) as previously described (14). All samples were assayed at 1:100 dilutions. The antibody avidity indices (AI) of plasma samples were determined as previously described (14). Plasma samples (diluted 1:100) were assayed in increasing concentrations (0, 1, 2, 3, 4, and 5 M) of sodium thiocyanate (NaSCN) diluted in phosphate-buffered saline. AI was calculated as the molar concentration of NaSCN that was required to reduce antigen-antibody binding by 50%, compared to incubation in the absence of NaSCN. SIV IgG was quantified by end point ELISA by the Core Immunology Laboratory at the California National Primate Research Center (15).
Real-time PCR.
A real-time PCR assay was developed to quantify RhCMV DNA copy numbers in plasma and tissues. Primer and probe sequences were designed to the glycoprotein B gene (UL55) of RhCMV (accession no. U41526) (12) by using the Primer Express program (version 1.0). The forward and reverse primer sequences were 5′-TGC GTA CTA TGG AAG AGA CAA TGC-3′ and 5′-ACA TCT GGC CGT TCA AAA AAA C-3′, respectively. Probe sequence CCA GAA GTT GCG CAT CCG CTT GT contained 5′ TET (tetrachloro-6-carboxyfluorescein) as the reporter dye and 3′ TAMRA (6-carboxytetramethylrhodamine) as the quencher dye (PE Applied Biosystems, Foster City, Calif.). PCR was performed with 1× Taqman universal PCR master mixture (PE Applied Biosystems), 17.5 pmol of each primer, 2.5 pmol of probe, and template DNA (either 500 ng of tissue DNA or 5 μl of DNA extracted from plasma) in a 25-μl reaction volume. DNA templates were purified from tissues and plasma as previously described (14). DNA was amplified (1 cycle of 50°C for 2 min and 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min) with the ABI Prism 7700 (PE Applied Biosystems). A standard curve was generated by using 10-fold serial dilutions of a plasmid (106 to 100 copies per reaction) containing the gB amplicon. Results were analyzed with Sequence Detection System software (version 1.6.3; Perkin-Elmer). Fluorescence intensity in each well was measured, and result was considered positive when intensity exceeded 10 times the baseline fluorescence. The limit of sensitivity was reproducibly between 1 and 10 copies of the template. Tissue RhCMV DNA loads were calculated as copy number per 106 cells, and plasma DNA loads were calculated as copy number per milliliter of plasma. SIV copy number was quantified according to published protocols (Lucy Whittier Molecular Core Facility, UC Davis) (13).
Histopathology and immunohistochemistry.
Formalin-fixed, paraffin-embedded tissues were analyzed for histopathology and expression of the RhCMV IE1 gene according to published protocols (14).
Statistics.
Mean values for anti-RhCMV IgG response and AI for different inoculation groups were compared by using a one-way analysis of variance and Duncan's post hoc test with the SPSS for Windows data analysis software (SPSS, Inc., Chicago, Ill.).
RESULTS
Animal inoculation.
Two groups of animals were inoculated with RhCMV followed by SIV inoculation at either 2 (group I; n = 4) or 11 weeks (group II; n = 4) later. The goal was to use SIV infection to perturb the development of normal anti-RhCMV immune responses. Thus, it would be possible to determine whether arrest of anti-RhCMV immune development after 2 or 11 weeks would alter the pathogenesis of RhCMV infection. The time point of 2 weeks after RhCMV inoculation was chosen to recapitulate the conditions that resulted in the rapid demise of the sentinel animal, which prompted this study (animal 29863; Table 1). The time point of 11 weeks after RhCMV inoculation was chosen because anti-RhCMV immune responses are more developed than those at 2 weeks but not yet fully matured (14). A third group of animals (group III; n = 4) was inoculated with RhCMV followed by inoculation with SIV at 11 weeks together with a second inoculation of RhCMV. The inoculation protocol for group III was chosen to test whether the level of anti-RhCMV immunity at 11 weeks after primary RhCMV exposure was sufficient to protect from a second RhCMV challenge given simultaneously with SIV. A fourth group of animals (n = 2) was inoculated with only RhCMV at weeks 2 and 11.
The different inoculation protocols resulted in a range of clinical outcomes distinguished by the kinetics and frequency of SAIDS and RhCMV disease. In addition, animals inoculated with SIV at 2 and 11 weeks after RhCMV inoculation yielded distinct molecular and immunological parameters of infection. Detailed results are presented for each inoculation group below.
Group I.
Rapid onset of disease occurred in the four monkeys inoculated with SIV 2 weeks after RhCMV infection. Two of the four animals died at 11 and 13 weeks after RhCMV infection and exhibited marked lymphoid depletion consistent with SAIDS and histopathological evidence of RhCMV disease in multiple tissues (Fig. 1B and C). All tissues with inclusion-bearing cells were immunohistochemically positive for RhCMV IE1 expression (presented only in Fig. 1C). The acute onset of SAIDS and RhCMV disease in these two animals was similar to the course of disease in the index animal (29863). Of the other two animals of group I, one died at 29 weeks with SAIDS while the other died at 19 weeks with lymphadenopathy and the early stages of lymphoid depletion (Table 1). Neither of these two animals had evidence of active RhCMV.
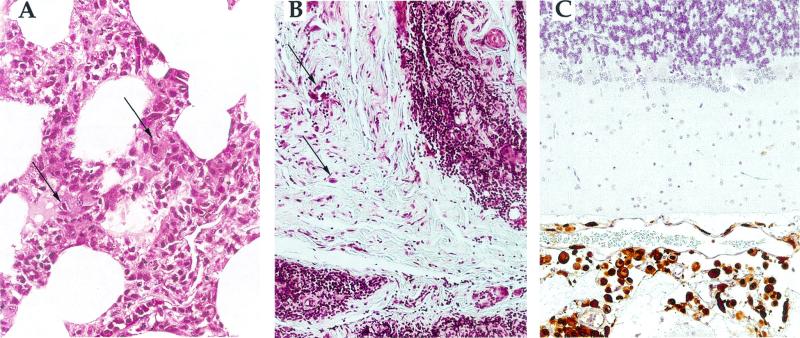
FIG. 1.
Representative examples of RhCMV histopathology in macaques coinfected with RhCMV and SIV. (A) Tissue from the sentinel monkey (29863) showing evidence of multifocal interstitial pneumonia and containing cells with intranuclear and intracytoplasmic RhCMV inclusions (arrows). (B) Markedly atrophic thymus from 30458 (group I), in which fibrous connective tissue has replaced much of the thymus and in which large numbers of large cells containing RhCMV inclusions were observed (arrows). (C) Disseminated meningitis in the brain of 30437 (group I). Multiple cells were positive (brown) for RhCMV IE1 expression, including both cells with inclusions and histologically normal cells. The morphology of the IE1-positive cells was consistent with that of macrophages.
Each animal displayed one or more hematological abnormalities, such as lymphopenia, lymphocytosis, neutropenia, monocytosis, and thrombocytopenia (data not shown). Deviations from normative parameters were mild to moderate, and the frequency of observation ranged from once to multiple times for some animals. There was no apparent relation between the pattern of hematologic abnormalities and clinical outcome. Similarly, absolute CD4+ T-cell number did not correlate with SAIDS or RhCMV disease outcome (data not shown). Most animals of groups I to III exhibited at least one instance of marked CD4+ T-cell depletion (<500 cells/μl).
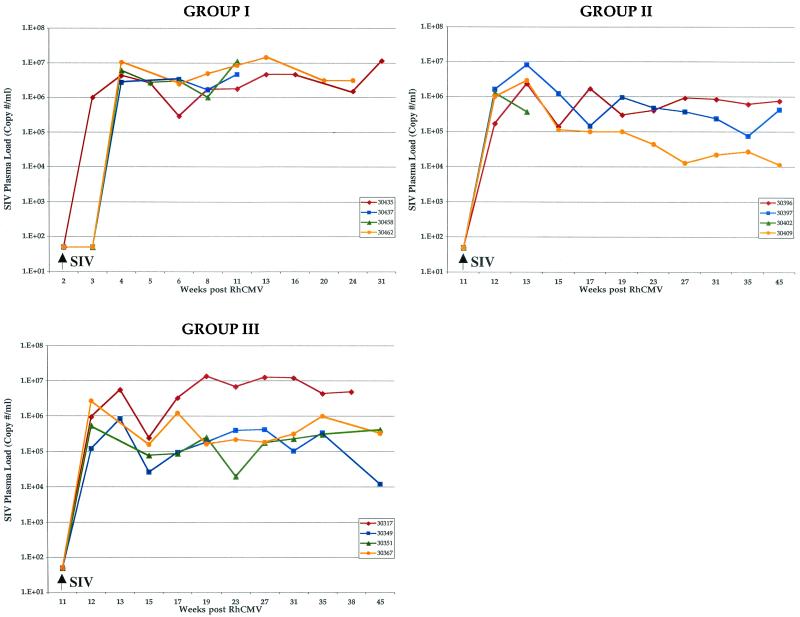
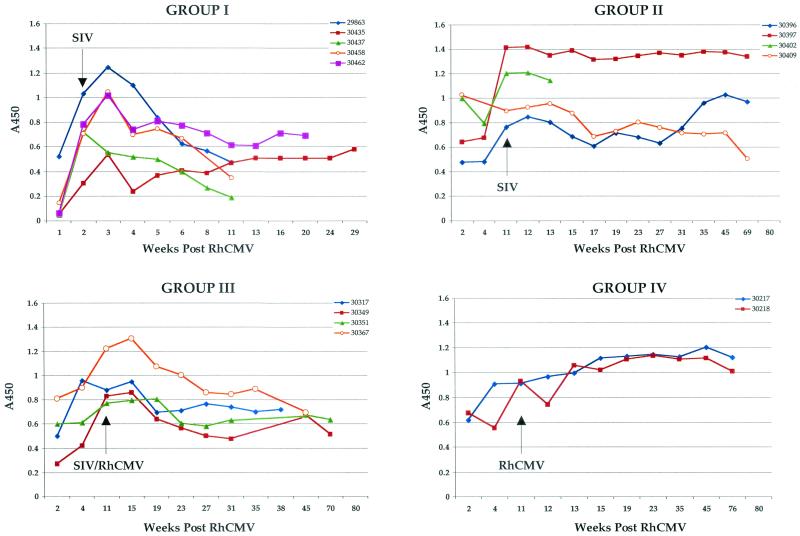
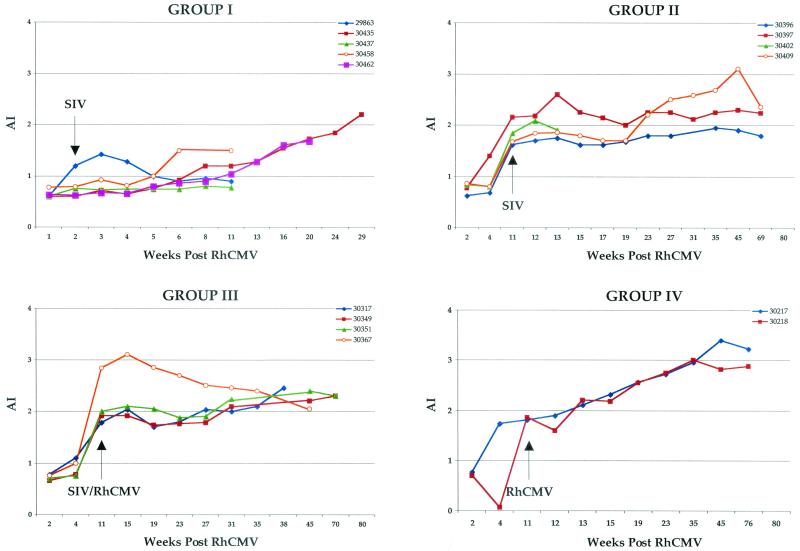
Development of antibodies against SIV was either very weak or undetectable in all four animals (Table 2), similar to the phenotype for the sentinel animal (29863). SIV plasma loads reached levels between 1 × 106 and 10 × 106 copies/ml of plasma within 2 weeks of SIV inoculation and remained mostly within this range for all animals until the time of necropsy (Fig. 2). Similarly, anti-RhCMV antibody responses were severely attenuated following SIV inoculation. Both IgG levels (Fig. 3) and AI (Fig. 4) were markedly depressed compared to those for control animals (group IV). Mean IgG responses and AI for group I monkeys at 11 weeks after RhCMV inoculation were significantly lower than those for control animals (P = 0.008 and 0.01, respectively), and the difference was nearly significant at 13 weeks (P = 0.06 for both). The effects of SIV infection on RhCMV antibody development were observed within 2 weeks of SIV inoculation.
TABLE 2.
Anti-SIV IgG antibody titers
| Wks ARIa | Titer for:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 29863 | Group I
|
Group II
|
Group III
|
||||||||||
| 30435 | 30437 | 30458 | 30462 | 30396 | 30397 | 30402 | 30409 | 30317 | 30349 | 30351 | 30367 | ||
| 2 | Negb | Neg | Neg | Neg | Neg | ||||||||
| 5 | Neg | 100 | Neg | Neg | Neg | ||||||||
| 8 | 100 | ||||||||||||
| 11 | 100 | 400 | Neg | Neg | 100 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 14 | Neg | Neg | |||||||||||
| 17 | 4,000 | 3,200 | 32,000 | 1,600 | 8,000 | 8,000 | 3,200 | ||||||
| 19 | Neg | 16,000 | |||||||||||
| 29 | 800 | ||||||||||||
| 35 | 8,000 | 8,000 | 200,000 | 100 | 8,000 | 32,000 | |||||||
| 38 | 100 | ||||||||||||
| 53 | |||||||||||||
| 67 | |||||||||||||
| 58 | |||||||||||||
| 67 | 8,000 | 32,000 | |||||||||||
| 68 | 16,000 | ||||||||||||
| 69 | 16,000 | 32,000 | |||||||||||
| 70 | 80,000 | ||||||||||||
ARI, after RhCMV inoculation.
Neg, <100.
FIG. 2.
Longitudinal analysis of SIV plasma viral load. The copy numbers of SIV genomes (expressed as copy numbers per milliliter of plasma) for each monkey, grouped according to inoculation protocol, are plotted relative to time after RhCMV inoculation (week 0). Arrows, times of SIV inoculation. The limit of detection was 50 copies.
FIG. 3.
Longitudinal analysis of RhCMV IgG immune responses. Individual RhCMV IgG immune responses (A450), grouped according to inoculation protocol, are plotted relative to time after RhCMV inoculation (week 0). Inoculation with SIV and/or RhCMV at week 11 is indicated.
FIG. 4.
Longitudinal analysis of RhCMV IgG AI. Individual RhCMV AI, grouped according to inoculation protocol, are plotted relative to weeks after RhCMV inoculation (week 0). Inoculation with SIV and/or RhCMV at week 11 is indicated.
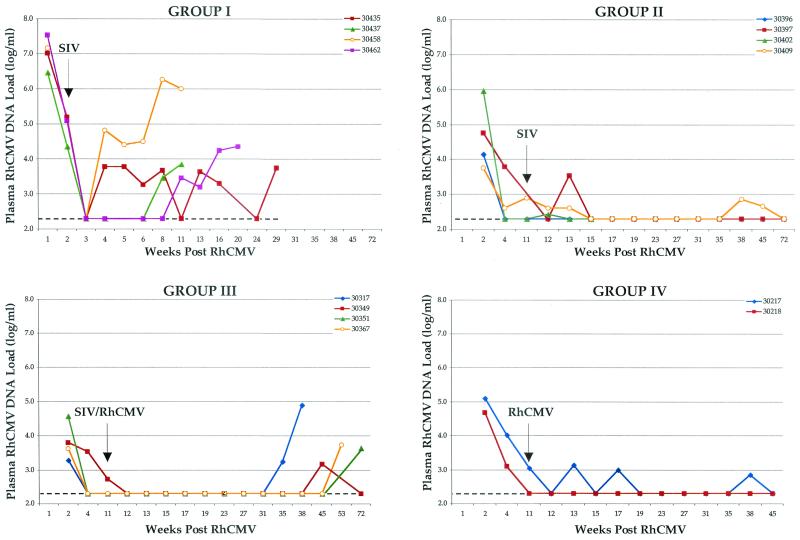
Attenuation of the anti-RhCMV immune response was accompanied by high copy numbers of RhCMV DNA in the plasma of all group I animals. RhCMV DNA was transiently cleared from plasma by 3 weeks of RhCMV inoculation following a peak in DNA loads at 1 week (Fig. 5). The pattern of RhCMV detection in plasma at the earliest stages of coinfection with RhCMV and SIV was similar to that observed for control animals (group IV) (14). Unlike control animals, however, all group I animals soon became persistently positive for RhCMV DNA in plasma and maintained high copy numbers until death or necropsy. In addition, RhCMV DNA was readily detected in almost every tissue from each coinfected animal (Table 3). Both the tissue distribution and quantity of RhCMV DNA in tissues of group I animals were greater than those observed in control animals (group IV) (14). The temporal pattern of RhCMV DNA in plasma and the RhCMV load in tissues for group I monkeys were similar to those observed for the sentinel animal (29863; only tissue copy numbers are presented in Table 3).
FIG. 5.
Longitudinal analysis of RhCMV plasma DNA copy number. Individual RhCMV plasma DNA copy numbers (log copy number per milliliter), grouped according to inoculation protocol, are plotted relative to time after RhCMV inoculation (week 0). Inoculation with SIV and/or RhCMV at week 11 is indicated. The limit of sensitivity was 2.3 (dashed lines).
TABLE 3.
RhCMV copy numbers in tissues
| Tissuea | Log copy no/106 cells for:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 29863 | Group I
|
Group II
|
Group III
|
Group IV
|
||||||||||
| 30435 | 30437 | 30462 | 30396 | 30397 | 40402 | 30409 | 30317 | 30349 | 30351 | 30367 | 30217 | 30218 | ||
| Spleen | 4.40 | 2.34 | 2.95 | 2.51 | 1.11 | 0.00 | 2.59 | 0.30 | 3.65 | 1.92 | 0.78 | 1.93 | 1.15 | 1.20 |
| Thymus | 4.97 | 3.32 | 2.16 | 3.82 | 1.83 | 0.00 | 2.10 | 0.00 | 5.26 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 |
| Axillary LN | 4.77 | 3.57 | 3.38 | 1.89 | 1.48 | 0.30 | 1.95 | 0.00 | 1.43 | 0.00 | 0.30 | 0.48 | 0.90 | 0.85 |
| Inguina LN | 5.27 | 1.20 | 4.47 | 2.52 | 0.00 | 0.00 | 1.80 | 0.00 | 0.00 | 0.48 | 1.30 | 0.90 | 0.85 | 0.48 |
| Duodenum | 3.69 | 1.04 | 5.41 | 2.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.48 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ileum | 4.87 | 0.00 | 0.78 | 0.78 | 0.00 | 0.00 | 1.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.90 | 0.00 |
| Liver | 4.75 | 1.20 | 2.69 | 2.20 | 1.32 | 1.51 | 1.67 | 0.30 | 1.11 | 0.85 | 0.95 | 0.48 | 1.92 | 0.48 |
| Kidney | 4.96 | 0.48 | 5.17 | 3.67 | 0.30 | 0.30 | 0.78 | 0.00 | 1.28 | 1.99 | 0.30 | 0.00 | 0.50 | 0.78 |
| Bone marrow | 3.43 | 0.78 | 1.28 | 2.74 | 0.00 | 0.85 | 1.91 | 0.00 | 0.70 | 0.00 | 0.70 | 0.78 | 1.23 | 0.60 |
| Lung | 3.34 | 0.00 | 0.30 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
LN, lymph nodes.
Group II.
The frequency and kinetics of SAIDS and RhCMV disease in monkeys that were coinfected with SIV 11 weeks after RhCMV inoculation were markedly different from those in monkeys receiving SIV inoculations at 2 weeks after RhCMV inoculation (group I). Of the four monkeys inoculated with only SIV at 11 weeks (group II; Table 1), none exhibited clinical or histological signs of SAIDS, including three that were selectively euthanized after more than 1 year of observation. One animal died at 14 weeks of unknown causes. All were in the lymphoproliferative phase of SIV infection at the time of necropsy, with large germinal centers in secondary lymphoid follicles. There was a complete absence of RhCMV histopathology in all of the tissues analyzed.
SIV plasma loads were consistent with the absence of SAIDS in this group of monkeys. Peak copy numbers were observed within 1 to 2 weeks of SIV inoculation and were comparable to those observed for group I animals (1 × 106 to 8 × 106 copies/ml; Fig. 2). Unlike what was found for group I monkeys, however, SIV loads declined and remained within a range of 104 to 106 copies/ml for the three long-term-surviving animals of group II.
Immune responses to both SIV and RhCMV were robust and sustained in the three long-term survivors (Table 2 and Fig. 3 and 4). However, SIV infection at 11 weeks immediately blunted further development of IgG and avidity against RhCMV (Fig. 3 and 4). Anti-RhCMV immune responses remained relatively level following SIV inoculation. Antibody and avidity levels were below those noted for control animals (group IV), although statistical significance was not observed. The profile of RhCMV DNA in plasma was very similar to that for control animals (Fig. 5). RhCMV DNA was mostly cleared from plasma by 4 to 11 weeks at the time of SIV infection. There was only sporadic detection in two of the three long-term-surviving animals during the next year, a frequency similar to that for control animals (group IV) (14). SIV infection at 11 weeks after RhCMV inoculation did not alter the distribution or copy number of RhCMV in tissues from those observed in animals infected with RhCMV alone (group IV) (Table 3).
Group III.
Of the four animals inoculated with both SIV and RhCMV 11 weeks after the primary exposure to RhCMV (group III), one was euthanized with SAIDS at 35 weeks and another animal was euthanized at 53 weeks due to paraparesis of the lower limbs (Table 1). The latter monkey did not display any clinical or histopathological signs of SAIDS. RhCMV inclusions were confined to a single tissue in each animal. The other two animals of group III were euthanized for nonmedical reasons at 67 and 69 weeks. Both animals were in the lymphoproliferative stage of SIV infection, and neither had histological evidence of RhCMV infection. The temporal pattern of SIV in plasma was almost identical to that for group II monkeys, with one exception. All four animals exhibited peak loads 1 to 2 weeks after SIV (0.9 × 106 to 6 × 106 copies/ml; Fig. 2). SIV loads subsequently declined in three monkeys (to 104 to 106 copies/ml), while the fourth (30317) had an elevated SIV load (106 to 107 copies/ml), similar to group I animals. Animal 30317 was noted for profound immunodeficiency (Table 1).
Immune responses to SIV and RhCMV were generally similar to those for group II monkeys. Three of four animals developed persistently strong IgG responses to SIV (Table 2). The one animal that died at 35 weeks with SAIDS had very low anti-SIV antibody titers. Anti-RhCMV IgG and avidity steadily increased until shortly after SIV infection (Fig. 3 and 4). Humoral responses to RhCMV remained relatively flat or exhibited slight increases by the time of necropsy for three of four animals, including the monkey that died with SAIDS and RhCMV disease. Values for all animals remained below those for control animals (group IV), but not at statistically significant levels. The other animal that died with RhCMV histopathology (30367; Table 1) exhibited steadily declining RhCMV IgG and avidity, beginning soon after SIV infection, until the time of necropsy.
The temporal detection of RhCMV in plasma was very similar to that for control animals (Fig. 5). RhCMV DNA was undetectable in plasma in three animals by 4 weeks after RhCMV inoculation and by 11 weeks for the remaining animal. All group III animals remained persistently negative for RhCMV DNA for an extended period of time following SIV inoculation at 11 weeks even though they were also reinoculated with RhCMV. Plasma from all four monkeys became RhCMV DNA positive between 35 and 72 weeks. Both monkeys with histological evidence of activated RhCMV infection were positive for DNA in plasma for the terminal sample. The distribution of RhCMV was similar to that for control animals (Table 3), in terms of tissues that were RhCMV DNA positive and genomic copy number. The notable exception to the general pattern of low RhCMV copy number in tissues was the thymus of the animal that had histologically apparent RhCMV disease.
Group IV.
Animals challenged with RhCMV 11 weeks after primary RhCMV inoculation (group IV) exhibited no clinical abnormalities, and histological examinations of tissues revealed no evidence of RhCMV infection. There was no apparent difference in the immunological and molecular parameters of infection (Fig. 3 to 5; Table 3) between the animals inoculated twice with RhCMV (group IV) and those inoculated once (14).
DISCUSSION
The innate and adaptive immunity responses that prevent acute and recurrent HCMV pathogenesis offer a paradigm of protective immunity. Two issues that remain unresolved are the immunological markers of protection and the temporal kinetics of their development. The data from this study indicate that two measures of protection developed in a relatively short time following i.v. inoculation of macaques with RhCMV. Development of an anti-RhCMV immune response for a period of time between 2 to 11 weeks was sufficient to (i) protect animals from acute RhCMV disease and (ii) significantly limit viral replication as measured in both blood and tissues. This conclusion is based on the clinical, virological, and immunological distinctions between the groups following infection with SIV. Attenuation of the RhCMV immune response at 2 weeks by SIV resulted in sustained high loads of RhCMV DNA in plasma, elevated copy numbers of RhCMV in tissues, and increased risk of early RhCMV sequelae. None of these phenotypes was observed in animals infected with SIV at 11 weeks. The absence of large amounts in RhCMV DNA in plasma after SIV inoculation at 11 weeks indicated that anti-RhCMV immunity controlled both the reactivation of the primary RhCMV inoculum (group II) and any detectable replication following secondary infection with RhCMV (group III). In addition, similar tissue distributions and RhCMV genome copy numbers for SIV-inoculated (groups II and III) and control (group IV) animals demonstrated that, for the most part, the anti-RhCMV immune responses at 11 weeks limited dissemination and local amplification of RhCMV replication after SIV infection.
Our results also indicated that the relative timing of RhCMV and SIV infections may have determined the course of SIV pathogenesis. All animals inoculated with SIV 2 weeks after RhCMV (group I and animal 29863) rapidly developed SAIDS or early signs of immune compromise. In contrast, only one animal inoculated with SIV at 11 weeks developed SAIDS, and then only after 35 weeks. HCMV has long been implicated in hastening the development of AIDS (20, 25). Our results suggest a synergistic interaction between RhCMV and SIV, particularly during concurrent primary infections. It should be noted, however, that acceleration of immunodeficiency in group I animals was not accompanied by early increases in SIV plasma burden. SIV copy numbers within 2 weeks of SIV infection in groups I, II, and III were comparable. It is unlikely, therefore, that CD4+ T cells were primed by RhCMV infection 2 weeks earlier to support a more robust infection by SIV.
Four monkeys in this study had histologically apparent RhCMV disease in addition to our initial observation of activated RhCMV (animal 29863). Additional lesions may have been present in tissues that either were not examined or were in locations other than those processed for fixation. It is also possible that more animals would have presented with RhCMV disease had they been observed longer, particularly with the later onset of SAIDS. Currently, the best predictor for HCMV disease in compromised individuals is evidence of activated HCMV replication. Four assays that have been commonly employed to detect systemic HCMV activation are assays for (i) viruria (22), (ii) viremia (7), (iii) pp65 antigenemia (24), and (iv) plasma DNAemia (20, 21). Positive results by any of these assays are infrequently observed in chronically infected, healthy hosts. Our results suggest that a combination of quantifiable measures may be diagnostic for predicting those at risk for disease. Four of five animals with RhCMV sequelae (30437, 30458, 30367, and 29863) exhibited at least two of the following characteristics: (i) a failure to develop an increase in the AI or a progressive decline in the AI of RhCMV antibodies, (ii) a progressive decline in anti-RhCMV IgG response, (iii) prolonged detection of RhCMV DNA in plasma. Animals without RhCMV sequelae exhibited no more than one of these phenotypes. Of note, the decline for the two RhCMV humoral parameters for animal 30367 (group III) preceded the detection of RhCMV DNA in plasma by many weeks. The fifth monkey with fulminant RhCMV infection (30317; group III) had dramatically elevated numbers of RhCMV genomes in the plasma at 35 weeks and at necropsy, although both elevated RhCMV IgG levels and AI were maintained until the time of necropsy.
Quantification of both anti-RhCMV IgG development and AI provides two markers of humoral development. Innate and cellular immunity and the specificity of the antibody responses were not evaluated as part of this study. Further study of both is essential for the definition of any correlates of protective immunity. Previous studies have demonstrated that RhCMV antigen-specific CD4+ proliferation and precursor frequency and cytotoxic T-lymphocyte responses are diminished during SIV infection (8-10). We observed that SIV infection produced a rapid attenuation of anti-RhCMV antibody production and maturation. This effect allowed us to investigate how the course of RhCMV infection differed in animals with different levels of RhCMV immunity. The mechanism responsible for disruption of IgG responsiveness and avidity maturation is unknown. The phenotype may have been due to direct effects on B cells or possibly an indirect effect on accessory cells, such as disruption of antigen presentation by dendritic cells. Human immunodeficiency virus (HIV) is known to alter B-cell function in HIV-infected individuals (16). An important downstream consequence of HIV and SIV infection is that immune responses to heterologous pathogens may be permanently weakened during the early stage of immunodeficiency virus infection, not just during the late stage. This scenario appears to have been especially relevant for animal 30367. As noted above, immune responses to RhCMV steadily declined in this animal soon after it was inoculated with SIV and RhCMV at 11 weeks (group III). Detection of RhCMV DNA in plasma and the presence of RhCMV histopathology in the absence of any evidence of SAIDS imply that very early insults to the immune system by SIV can result in long-term ramifications.
RhCMV infections in juvenile monkeys differed from those observed in experimentally inoculated fetuses (23; K. M. Lockridge, A. F. Tarantal, and P. A. Barry, unpublished data). Intrauterine RhCMV disease can be extensive in some fetuses in which RhCMV lesions are detected in most tissues. The dissemination of RhCMV in fetuses indicates that, in the absence of immunological restraints, RhCMV will readily produce fulminant disease. A restricted profile of tissues with RhCMV lesions in the juvenile animals of this study suggests that there were different levels of anti-RhCMV immunity in different tissues. According to this scenario, sufficient levels of anti-RhCMV immunity were present in most tissues to have prevented the accumulation of high RhCMV loads and onset of disease. The lack of detectable RhCMV DNA in some tissues (e.g., lung and thymus) of control animals supports the notion that normal immune responses prevent the establishment of large reservoirs of persistent viral genomes. The mechanism by which these same tissues support RhCMV replication during SIV infection is not known.
The nonhuman primate model of HCMV persistence and pathogenesis offers a highly relevant system with which to investigate viral and host factors involved in the establishment, maintenance, and loss of a stable virus-host relationship. Our results demonstrate that the first few weeks of RhCMV infection constitute a critical period for development of protective antiviral immune responses. If this immune maturation is never established (group I) or is not maintained (group III), animals are at risk for RhCMV disease.
Acknowledgments
We thank James R. Carlson, William Chang, Jennifer Loomis-Huff, and Yujuan Yue for insightful discussions, Dorie Borjesson and Kate Wasson for expert statistical analysis, and Joanne Higgins and Christian Leutenegger for determination of SIV plasma loads.
This work was supported by grants from the National Institutes of Health (HL 57883 to P.A.B.) and the California National Primate Research Center (RR 00169).
REFERENCES
- 1.Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, Ltd., New York, N.Y.
- 2.Boppana, S. B., and W. J. Britt. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 171:1115-1121. [DOI] [PubMed] [Google Scholar]
- 3.Boppana, S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. [DOI] [PubMed] [Google Scholar]
- 4.Boppana, S. B., R. F. Pass, and W. J. Britt. 1993. Virus-specific antibody responses in mothers and their newborn infants with asymptomatic congenital cytomegalovirus infections. J. Infect. Dis. 167:72-77. [DOI] [PubMed] [Google Scholar]
- 5.Bowen, E. F., P. D. Griffiths, C. C. Davey, V. C. Emery, and M. A. Johnson. 1996. Lessons from the natural history of cytomegalovirus. AIDS 10:S37-S41. [PubMed] [Google Scholar]
- 6.Fowler, K. B., S. Stagno, R. F. Pass, W. J. Britt, T. J. Boll, and C. A. Alford. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663-667. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths, P. D., A. V. Cope, A. F. Hassan-Walker, and V. C. Emery. 1999. Diagnostic approaches to cytomegalovirus infection in bone marrow and organ transplantation. Transpl. Infect. Dis. 1:179-186. [DOI] [PubMed] [Google Scholar]
- 8.Kaur, A., M. D. Daniel, D. Hempel, D. Lee-Parritz, M. S. Hirsch, and R. P. Johnson. 1996. Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected macaques. J. Virol. 70:7725-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur, A., C. L. Hale, B. Noren, N. Kassis, M. A. Simon, and R. P. Johnson. 2002. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J. Virol. 76:3646-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur, A., M. Rosenzweig, and R. P. Johnson. 2000. Immunological memory and acquired immunodeficiency syndrome pathogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 12.Kravitz, R. H., K. S. Sciabica, K. Cho, P. A. Luciw, and P. A. Barry. 1997. Cloning and characterization of the rhesus cytomegalovirus glycoprotein B. J. Gen. Virol. 78:2009-2013. [DOI] [PubMed] [Google Scholar]
- 13.Leutenegger, C. M., J. Higgins, T. B. Matthews, A. F. Tarantal, P. A. Luciw, N. C. Pedersen, and T. W. North. 2001. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res. Hum. Retroviruses 17:243-251. [DOI] [PubMed] [Google Scholar]
- 14.Lockridge, K. M., G. Sequar, S. S. Zhou, Y. Yue, C. M. Mandell, and P. A. Barry. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu, X., H. Kiyono, D. Lu, S. Kawabata, J. Torten, S. Srinivasan, P. J. Dailey, J. R. McGhee, T. Lehner, and C. J. Miller. 1998. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS 12:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moir, S., A. Malaspina, K. M. Ogwaro, E. T. Donoghue, C. W. Hallahan, L. A. Ehler, S. Liu, J. Adelsberger, R. Lapointe, P. Hwu, M. Baseler, J. M. Orenstein, T. W. Chun, J. A. Mican, and A. S. Fauci. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl. Acad. Sci. USA 98:10362-10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pass, R. F., S. Stagno, G. J. Myers, and C. A. Alford. 1980. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758-762. [PubMed] [Google Scholar]
- 18.Rentenaar, R. J., L. E. Gamadia, N. van DerHoek, F. N. van Diepen, R. Boom, J. F. Weel, P. M. Wertheim-van Dillen, R. A. van Lier, and I. J. ten Berge. 2000. Development of virus-specific CD4+ T cells during primary cytomegalovirus infection. J. Clin. Investig. 105:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sia, I. G., and R. Patel. 2000. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin. Microbiol. Rev. 13:83-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spector, S. A., K. Hsia, M. Crager, M. Pilcher, S. Cabral, and M. J. Stempien. 1999. Cytomegalovirus (CMV) DNA load is an independent predictor of CMV disease and survival in advanced AIDS. J. Virol. 73:7027-7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spector, S. A., R. Wong, K. Hsia, M. Pilcher, and M. J. Stempien. 1998. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J. Clin. Investig. 101:497-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stagno, S., D. W. Reynolds, A. Tsiantos, D. A. Fuccillo, W. Long, and C. A. Alford. 1975. Comparative serial virologic and serologic studies of symptomatic and subclinical congenitally and natally acquired cytomegalovirus infections. J. Infect. Dis. 132:568-577. [DOI] [PubMed] [Google Scholar]
- 23.Tarantal, A. F., S. Salamat, W. J. Britt, P. A. Luciw, A. G. Hendrickx, and P. A. Barry. 1998. Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta). J. Infect. Dis. 177:446-450. [DOI] [PubMed] [Google Scholar]
- 24.van der Bij, W., J. Schirm, R. Torensma, W. J. van Son, A. M. Tegzess, and T. H. The. 1988. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J. Clin. Microbiol. 26:2531-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster, A. 1991. Cytomegalovirus as a possible cofactor in HIV disease progression. J. Acquir. Immune Defic. Syndr. 4(Suppl. 1):S47-S52. [PubMed] [Google Scholar]
- 26.Zanghellini, F., S. B. Boppana, V. C. Emery, P. D. Griffiths, and R. F. Pass. 1999. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J. Infect. Dis. 180:702-707. [DOI] [PubMed] [Google Scholar]