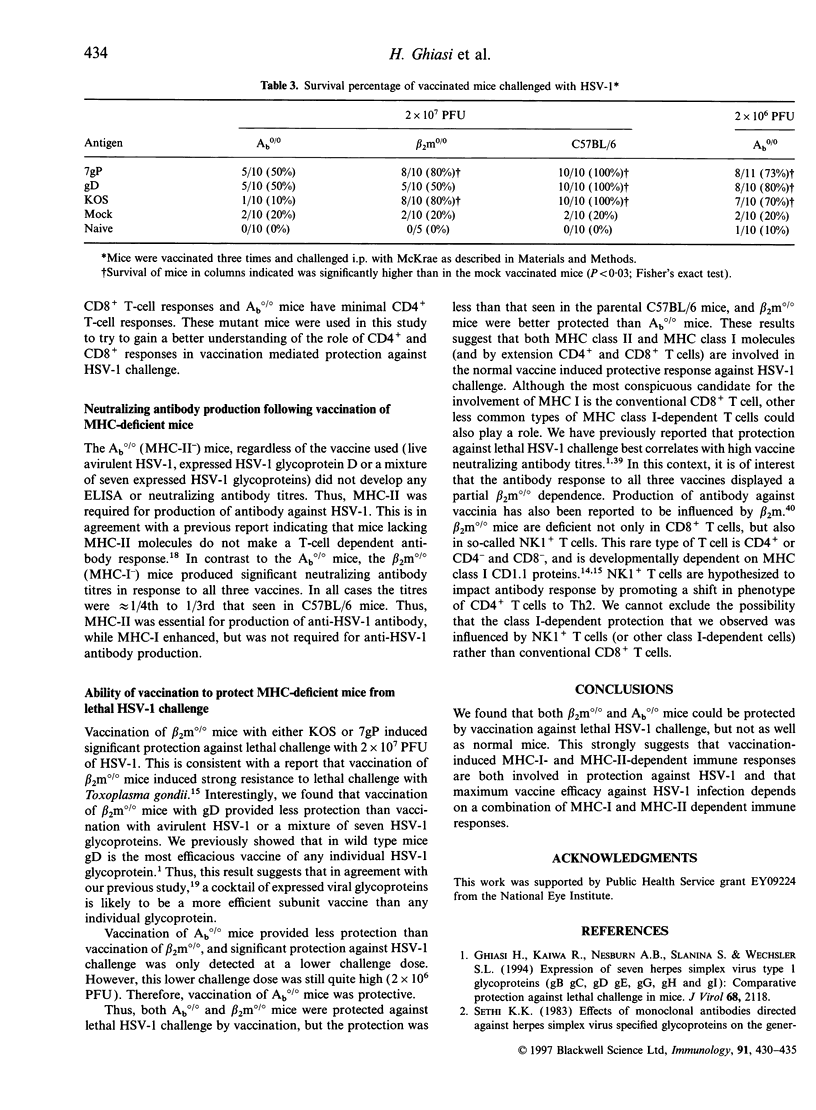
Abstract
To investigate the importance of major histocompatability complex (MHC) class I- and MHC class II-dependent immune responses in herpes simplex virus-1 (HSV-1) vaccine efficacy, groups of beta 2% (MHC I-) and Ab% (MHC II-) mice were inoculated with various vaccines, and then challenged intraperitoneally with HSV-1. Following vaccination with either live avirulent HSV-1, expressed HSV-1 glycoprotein D (gD), or a mixture of seven expressed HSV-1 glycoproteins (7gPs), Ab% (MHC-II-) mice developed no enzyme-linked immunosorbent assay (ELISA) or neutralizing antibody titres. In contrast, significant ELISA and neutralizing antibody titres were induced in beta 2m% (MHC-I-) mice by all three vaccines. The neutralizing antibody titres were similar for all three vaccines, but were only approximately 1/4 to 1/3 of that developed in C57BL/6 (parental) mice vaccinated with the same antigens. All three vaccines protected 100% of the wild-type C57BL/6 mice against lethal challenge with 2 x 10(7) plaque-forming units (PFU) of HSV-1. The live virus vaccine and the 7gPs vaccine also protected 80% of the beta 2m% mice against the same lethal HSV-1 challenge dose. In contrast, in Abo/o mice, none of the vaccines provided significant protection against the same lethal challenge dose of HSV-1. However, at a lower challenge dose of 2 x 10(6) PFU, all three vaccines protected 70-80% of the vaccinated Ab% mice (compared to only 10% survival in mock vaccinated controls). Thus, vaccination provided some protection against lethal HSV-1 challenge in both beta 2m% and Ab% mice; however, the protection was less than that seen in the parental C57BL/6 mice. In addition, Ab% mice were less well protected by vaccination than were beta 2m% mice. Our results suggest that (1) both MHC-I and MHC-II are involved in vaccine efficacy against HSV-1 challenge; (2) both types of responses must be present for maximum vaccine efficacy: and (3) the MHC-II-dependent immune response appeared to be more important than the MHC-I-dependent immune response for vaccine efficacy against HSV-I challenge.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995 Jun;7(3):367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Blacklaws B. A., Krishna S., Minson A. C., Nash A. A. Immunogenicity of herpes simplex virus type 1 glycoproteins expressed in vaccinia virus recombinants. Virology. 1990 Aug;177(2):727–736. doi: 10.1016/0042-6822(90)90539-4. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Herpes simplex virus-specific cytolytic T lymphocytes restricted to a normally low responder H-2 allele are protective in vivo. Virology. 1990 Feb;174(2):599–604. doi: 10.1016/0042-6822(90)90113-6. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989 Mar;63(3):1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. D., Paveloff M. J., Doucet J. J., Cottingham A. J., Sedarati F., Hill J. M. Ocular herpes simplex virus reactivation in mice latently infected with latency-associated transcript mutants. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1558–1561. [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Davis W. B., Taylor J. A., Oakes J. E. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J Infect Dis. 1979 Oct;140(4):534–540. doi: 10.1093/infdis/140.4.534. [DOI] [PubMed] [Google Scholar]
- Denkers E. Y., Gazzinelli R. T., Martin D., Sher A. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993 Nov 1;178(5):1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck B. K., Yung C. M., Carteron N. L., Wofsy D. The role of T-cell subsets in the response to anti-CD3 monoclonal antibodies. Clin Immunol Immunopathol. 1992 Dec;65(3):234–241. doi: 10.1016/0090-1229(92)90152-e. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Cai S., Slanina S., Nesburn A. B., Wechsler S. L. Vaccination of mice with herpes simplex virus type 1 glycoprotein D DNA produces low levels of protection against lethal HSV-1 challenge. Antiviral Res. 1995 Oct;28(2):147–157. doi: 10.1016/0166-3542(95)00045-n. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Slanina S., Wechsler S. L. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J Virol. 1994 Apr;68(4):2118–2126. doi: 10.1128/jvi.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H., Nesburn A. B., Kaiwar R., Wechsler S. L. Immunoselection of recombinant baculoviruses expressing high levels of biologically active herpes simplex virus type 1 glycoprotein D. Arch Virol. 1991;121(1-4):163–178. doi: 10.1007/BF01316752. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Nesburn A. B., Wechsler S. L. Vaccination with a cocktail of seven recombinantly expressed HSV-1 glycoproteins protects against ocular HSV-1 challenge more efficiently than vaccination with any individual glycoprotein. Vaccine. 1996 Feb;14(2):107–112. doi: 10.1016/0264-410x(95)00169-2. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990 Oct;31(10):1929–1939. [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M., Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992 Nov 1;149(9):3023–3028. [PubMed] [Google Scholar]
- Hill T. J., Blyth W. A., Harbour D. A. Recurrence of herpes simplex in the mouse requires an intact nerve supply to the skin. J Gen Virol. 1983 Dec;64(Pt 12):2763–2765. doi: 10.1099/0022-1317-64-12-2763. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Kapoor A. K., Buckmaster A., Nash A. A., Field H. J., Wildy P. Role of neutralizing antibodies and T-cells in pathogenesis of herpes simplex virus infection in congenitally athymic mice. Immunol Lett. 1982 Nov;5(5):259–265. doi: 10.1016/0165-2478(82)90109-2. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel C. H., Daugelat S., Kaufmann S. H. Immune response to Mycobacterium bovis bacille Calmette Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995 Feb;25(2):377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- Manickan E., Rouse B. T. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J Virol. 1995 Dec;69(12):8178–8179. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Rouse B. T. The mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: clearance of local infection. J Immunol. 1987 May 15;138(10):3431–3437. [PubMed] [Google Scholar]
- Nagafuchi S., Hayashida I., Higa K., Wada T., Mori R. Role of Lyt-1 positive immune T cells in recovery from herpes simplex virus infection in mice. Microbiol Immunol. 1982;26(4):359–362. doi: 10.1111/j.1348-0421.1982.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Newell C. K., Martin S., Sendele D., Mercadal C. M., Rouse B. T. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989 Feb;63(2):769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng G. C., Thompson R. L., Sawtell N. M., Taylor W. E., Slanina S. M., Ghiasi H., Kaiwar R., Nesburn A. B., Wechsler S. L. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J Virol. 1995 May;69(5):3033–3041. doi: 10.1128/jvi.69.5.3033-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman M. B., Foster C. S. Passive transfer of anti-HSV-1 IgG protects against stromal keratitis in mice. Curr Eye Res. 1988 Aug;7(8):823–829. doi: 10.3109/02713688809033214. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Type-specific delayed hypersensitivity and protective immunity induced by isolated herpes simplex virus glycoprotein. J Immunol. 1983 Mar;130(3):1413–1418. [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Schneweis K. E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983 Feb;64(Pt 2):443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Koller B. H., Sato T., Morrissey P. J., Fanslow W. C., Smithies O., Voice R. F., Widmer M. B., Maliszewski C. R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats H. F., Oakes J. E., Lausch R. N. Anti-glycoprotein D monoclonal antibody protects against herpes simplex virus type 1-induced diseases in mice functionally depleted of selected T-cell subsets or asialo GM1+ cells. J Virol. 1991 Nov;65(11):6008–6014. doi: 10.1128/jvi.65.11.6008-6014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo A. B., Shimeld C., Blyth W. A., Hill T. J., Easty D. L. Ocular infection with herpes simplex virus in nonimmune and immune mice. Arch Ophthalmol. 1983 Jun;101(6):961–964. doi: 10.1001/archopht.1983.01040010961023. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]



