Abstract
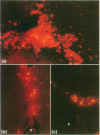
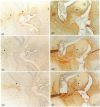
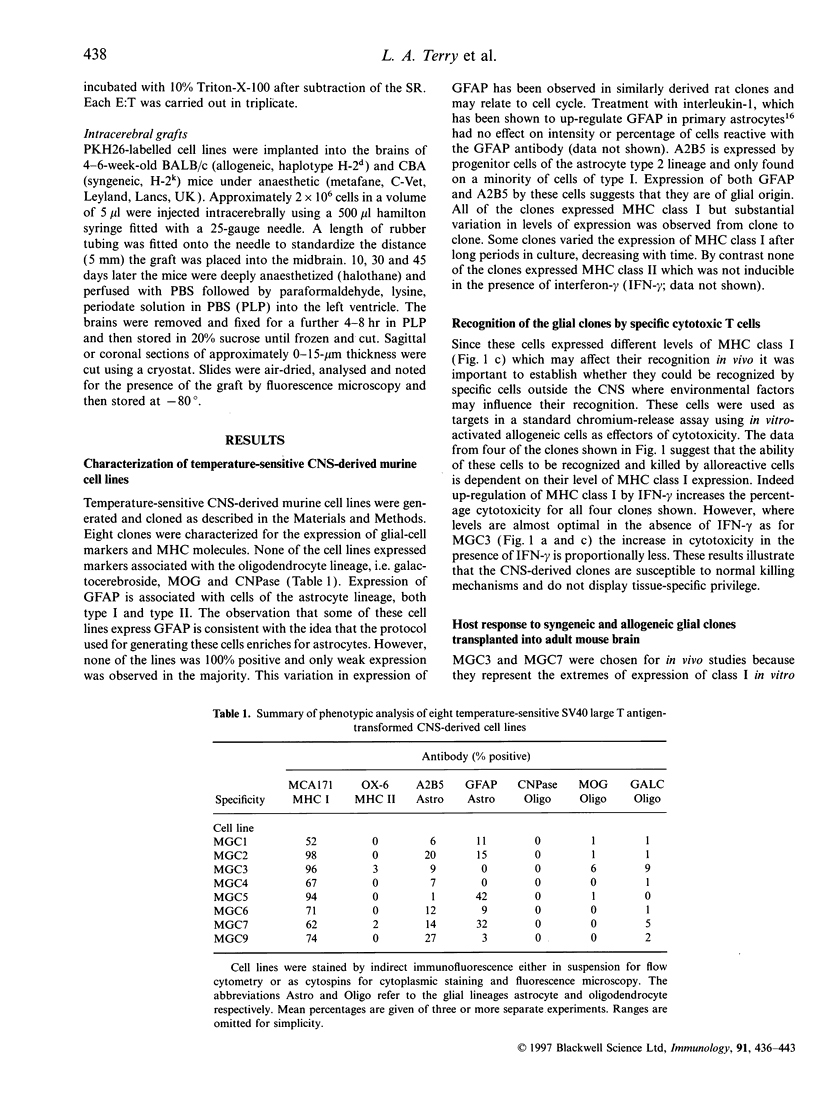
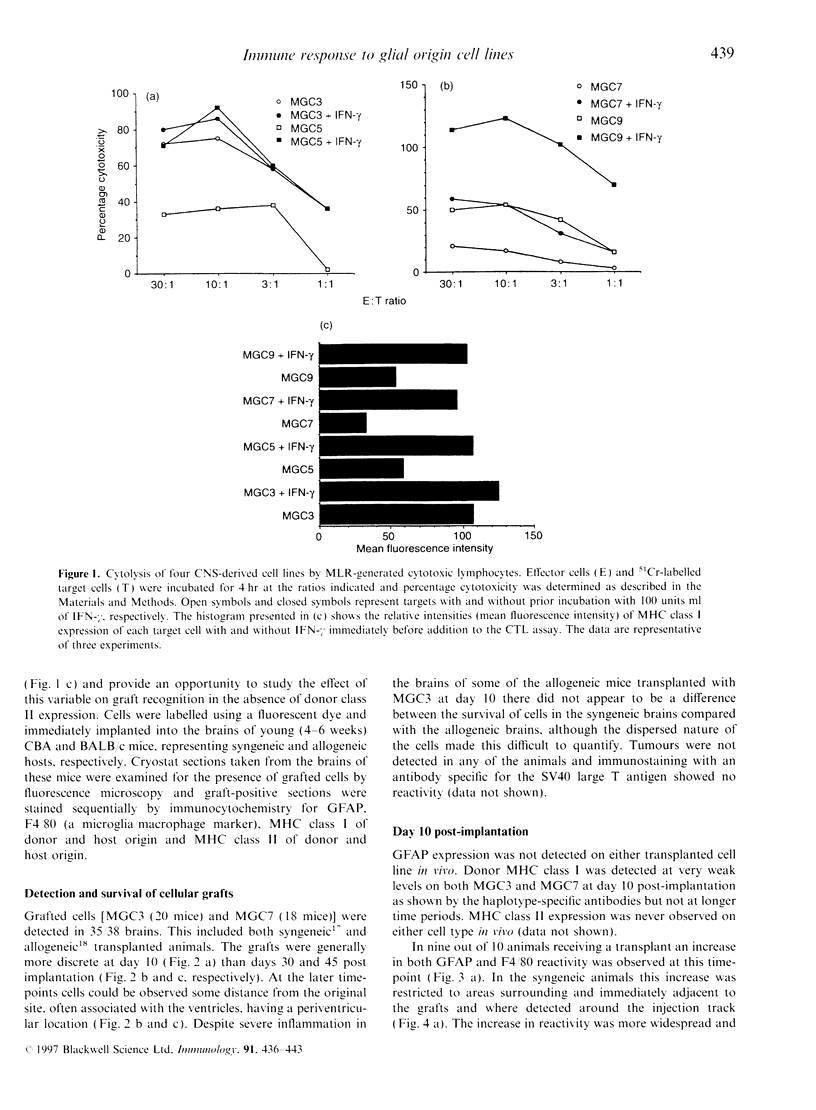
Temperature-sensitive simian virus 40 (SV40) T antigen-transformed central nervous system (CNS)-derived murine cell lines were used to analyse the host response to transplantation in the mouse adult brain. The cell lines were shown to be susceptible to immune recognition in vitro by cytotoxic effector cells indicating that tissue-specific privilege was not in operation. Histological examination at time points post-implantation showed characteristic responses similar to those seen during graft rejection. Astrocytosis and up-regulation of major histocompatibility complex (MHC) class I and MHC class II activation of resident microglia and recruitment of macrophages were observed in both allogeneic and syngeneic hosts 10 days post-implantation suggesting a trauma-induced response. However, the response in allogeneic hosts was more widespread and evident when the syngeneic responses had returned to normal levels. Evidence of T-cell infiltration was also more pronounced in the allogeneic hosts. Despite quite extensive host reactions to these cellular grafts at early time-points the implants appeared to survive in the host CNS long after the responses had abated and could be detected at the maximum time-point studied of 40 days.
Full text
PDF

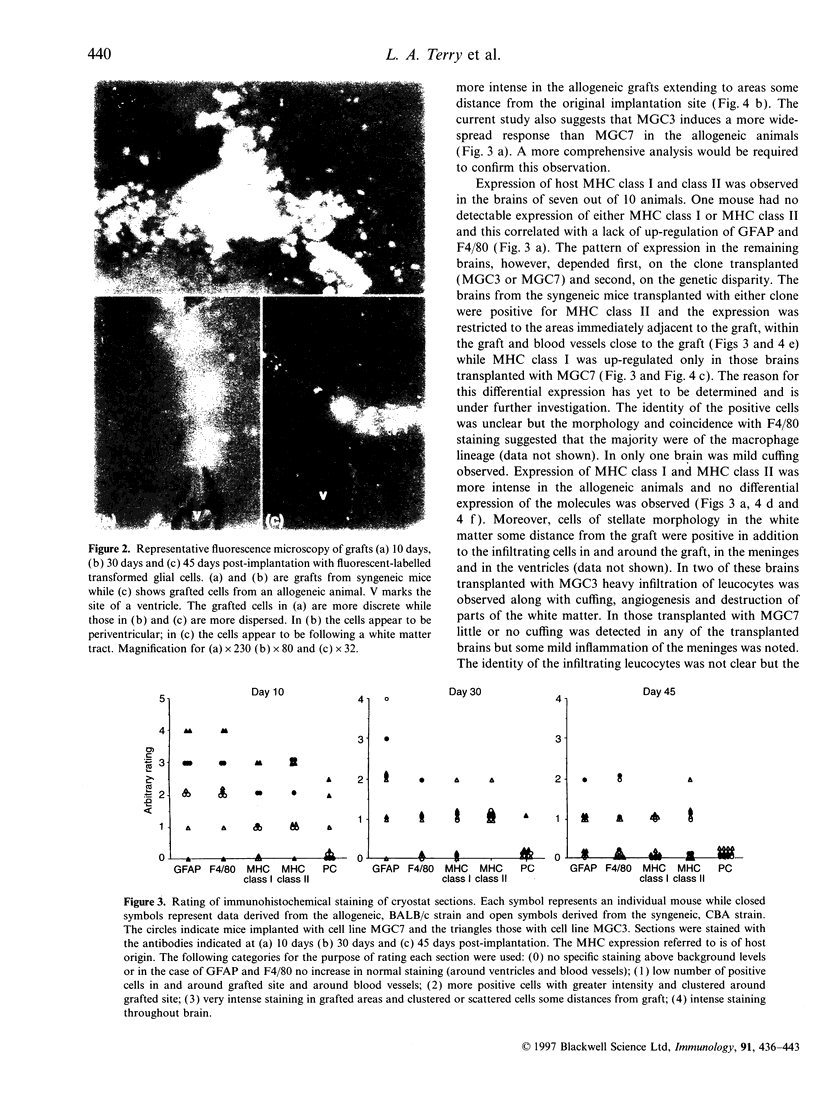
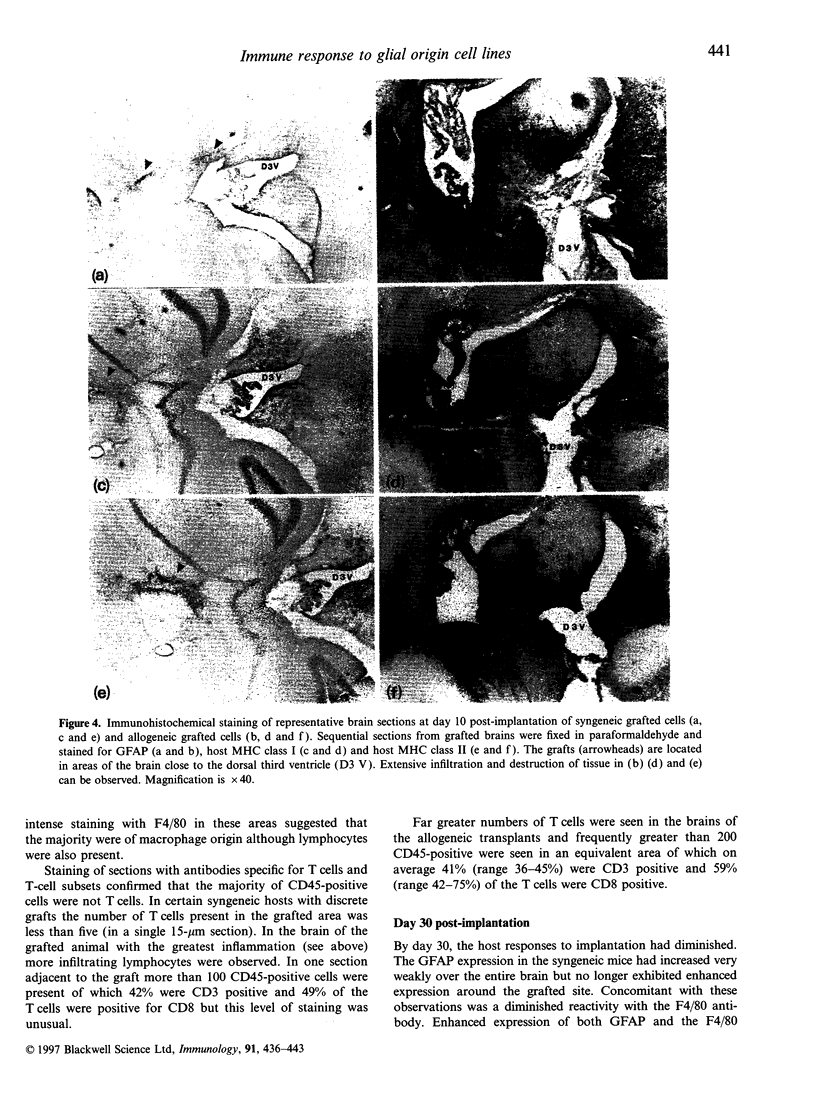


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee R., Lund R. D., Radel J. D. Anatomical and functional consequences of induced rejection of intracranial retinal transplants. Neuroscience. 1993 Oct;56(4):939–953. doi: 10.1016/0306-4522(93)90140-b. [DOI] [PubMed] [Google Scholar]
- Barker C. F., Billingham R. E. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Björklund A. Neurobiology. Better cells for brain repair. Nature. 1993 Apr 1;362(6419):414–415. doi: 10.1038/362414a0. [DOI] [PubMed] [Google Scholar]
- Compston A. Brain repair. J R Coll Physicians Lond. 1994 Mar-Apr;28(2):107–120. [PMC free article] [PubMed] [Google Scholar]
- Date I., Kawamura K., Nakashima H. Histological signs of immune reactions against allogeneic solid fetal neural grafts in the mouse cerebellum depend on the MHC locus. Exp Brain Res. 1988;73(1):15–22. doi: 10.1007/BF00279656. [DOI] [PubMed] [Google Scholar]
- Davis A. A., Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994 Nov 17;372(6503):263–266. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- Duan W. M., Widner H., Brundin P. Temporal pattern of host responses against intrastriatal grafts of syngeneic, allogeneic or xenogeneic embryonic neuronal tissue in rats. Exp Brain Res. 1995;104(2):227–242. doi: 10.1007/BF00242009. [DOI] [PubMed] [Google Scholar]
- Dunnett S. Cholinergic grafts, memory and ageing. Trends Neurosci. 1991 Aug;14(8):371–376. doi: 10.1016/0166-2236(91)90166-r. [DOI] [PubMed] [Google Scholar]
- Fabry Z., Raine C. S., Hart M. N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994 May;15(5):218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- Frisa P. S., Goodman M. N., Smith G. M., Silver J., Jacobberger J. W. Immortalization of immature and mature mouse astrocytes with SV40 T antigen. J Neurosci Res. 1994 Sep 1;39(1):47–56. doi: 10.1002/jnr.490390107. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Kawaja M. D., Fisher L. J. Genetically modified cells: applications for intracerebral grafting. Trends Neurosci. 1991 Aug;14(8):328–333. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- Giulian D., Lachman L. B. Interleukin-1 stimulation of astroglial proliferation after brain injury. Science. 1985 Apr 26;228(4698):497–499. doi: 10.1126/science.3872478. [DOI] [PubMed] [Google Scholar]
- Groves A. K., Barnett S. C., Franklin R. J., Crang A. J., Mayer M., Blakemore W. F., Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993 Apr 1;362(6419):453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Hitchcock E. R., Clough C., Hughes R., Kenny B. Embryos and Parkinson's disease. Lancet. 1988 Jun 4;1(8597):1274–1274. doi: 10.1016/s0140-6736(88)92088-0. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989 Apr;9(4):1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M., Crang A. J., Blakemore W. F., Hoppe D., Kettenmann H., Trotter J. In vitro and in vivo characterisation of glial cells immortalised with a temperature sensitive SV40 T antigen-containing retrovirus. J Neurosci Res. 1994 Feb 1;37(2):182–196. doi: 10.1002/jnr.490370204. [DOI] [PubMed] [Google Scholar]
- Lawrence J. M., Morris R. J., Wilson D. J., Raisman G. Mechanisms of allograft rejection in the rat brain. Neuroscience. 1990;37(2):431–462. doi: 10.1016/0306-4522(90)90413-x. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Prospects of transplantation in human neurodegenerative diseases. Trends Neurosci. 1991 Aug;14(8):376–384. doi: 10.1016/0166-2236(91)90167-s. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Charlton H. M., Jones A. J., Lavy C. B., Puklavec M., Simmonds S. J. The fate of allogeneic and xenogeneic neuronal tissue transplanted into the third ventricle of rodents. Neuroscience. 1986 Nov;19(3):685–694. doi: 10.1016/0306-4522(86)90292-7. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas M. K., Antel J. P., Stefansson K., Arnason B. G. Rejection of fetal neocortical neural transplants by H-2 incompatible mice. J Immunol. 1987 Oct 1;139(7):2275–2283. [PubMed] [Google Scholar]
- Pakzaban P., Deacon T. W., Burns L. H., Dinsmore J., Isacson O. A novel mode of immunoprotection of neural xenotransplants: masking of donor major histocompatibility complex class I enhances transplant survival in the central nervous system. Neuroscience. 1995 Apr;65(4):983–996. doi: 10.1016/0306-4522(94)00626-g. [DOI] [PubMed] [Google Scholar]
- Paterson P. Y. Experimental allergic encephalomyelitis and autoimmune disease. Adv Immunol. 1966;5:131–208. doi: 10.1016/s0065-2776(08)60273-4. [DOI] [PubMed] [Google Scholar]
- Sloan D. J., Baker B. J., Puklavec M., Charlton H. M. The effect of site of transplantation and histocompatibility differences on the survival of neural tissue transplanted to the CNS of defined inbred rat strains. Prog Brain Res. 1990;82:141–152. doi: 10.1016/s0079-6123(08)62599-6. [DOI] [PubMed] [Google Scholar]
- Snyder E. Y., Taylor R. M., Wolfe J. H. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995 Mar 23;374(6520):367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Widner H., Brundin P. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res. 1988 Nov;472(3):287–324. doi: 10.1016/0165-0173(88)90010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]