Abstract
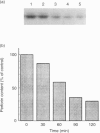
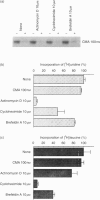
In our recent studies, an inhibitor of vacuolar-type H(+)-ATPase, concanamycin A (CMA) has been shown to neutralize acidic pH in vacuolar organelles, including lytic granules, and to decrease the perforin content markedly. In the present paper, we have further investigated the role of acidification in perforin storage by using CMA. In CD8+ cytotoxic T-lymphocyte (CTL) clones, the amount of perforin decreased rapidly at 30-90 min but no more decrease occurred at 90-120 min after the addition of CMA. Since exposure to actinomycin D, cycloheximide, or brefeldin A failed to reduce the perforin content, the perforin decrease in CMA-treated cells seems to be largely due to a reduction in the perforin already stored in lytic granules, rather than to the inhibition of the de novo synthesis or the intracellular glycoprotein transport of perforin. Diisopropylfluorophosphoridate (DFP) markedly antagonized the decrease in the perforin content in CMA-treated cells, while other protease inhibitors, i.e. antipain, E-64, leupeptin, pepstatin A and phenylmethylsulphonyl fluoride, did not. Nevertheless, DFP hardly reversed the abrogation of the killing activity by CMA. Indeed, the lytic granules prepared from DFP plus CMA-treated cells showed only a marginal level of haemolytic activity. In cell-free experiments using perforin-enriched granule fractions, acidic pH completely blocked the perforin activity. Under the acidic conditions, perforin was more resistant to an inactivation by calcium when exposed to calcium prior to the haemolysis test. Thus, these data suggest that perforin is primarily inactivated, possibly in a calcium-dependent manner, and is subsequently hydrolysed by DFP-sensitive proteases in the lytic granules at neutral pH. We conclude that acidic pH plays an essential role to maintain the integrity of perforin within the lytic granules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Menestrina G., Henkart P. A., Pasternak C. A. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988 Dec 1;141(11):3965–3974. [PubMed] [Google Scholar]
- Burkhardt J. K., Hester S., Lapham C. K., Argon Y. The lytic granules of natural killer cells are dual-function organelles combining secretory and pre-lysosomal compartments. J Cell Biol. 1990 Dec;111(6 Pt 1):2327–2340. doi: 10.1083/jcb.111.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M., Lindstrom J. M., Anderson C. G., Dennert G. Cytotoxic granules from killer cells: specificity of granules and insertion of channels of defined size into target membranes. J Immunol. 1985 Dec;135(6):4245–4251. [PubMed] [Google Scholar]
- Dröse S., Bindseil K. U., Bowman E. J., Siebers A., Zeeck A., Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry. 1993 Apr 20;32(15):3902–3906. doi: 10.1021/bi00066a008. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sudo T., Iizuka M., Kobayashi S., Nishio S., Kano S., Minato N. Generation of continuous large granular lymphocyte lines by interleukin 2 from the spleen cells of mice infected with Moloney leukemia virus. Involvement of interleukin 3. J Exp Med. 1987 Oct 1;166(4):833–849. doi: 10.1084/jem.166.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudig D., Allison N. J., Pickett T. M., Winkler U., Kam C. M., Powers J. C. The function of lymphocyte proteases. Inhibition and restoration of granule-mediated lysis with isocoumarin serine protease inhibitors. J Immunol. 1991 Aug 15;147(4):1360–1368. [PubMed] [Google Scholar]
- Hudig D., Ewoldt G. R., Woodard S. L. Proteases and lymphocyte cytotoxic killing mechanisms. Curr Opin Immunol. 1993 Feb;5(1):90–96. doi: 10.1016/0952-7915(93)90086-8. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Sato M., Kondo S., Nagai K. Estimation of pH and the number of lytic granules in a CD8+ CTL clone treated with an inhibitor of vacuolar type H(+)-ATPase concanamycin A. Biosci Biotechnol Biochem. 1996 Oct;60(10):1729–1731. doi: 10.1271/bbb.60.1729. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Shinohara N., Takayama H., Takaku K., Kondo S., Yonehara S., Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996 May 15;156(10):3678–3686. [PubMed] [Google Scholar]
- Kataoka T., Takaku K., Magae J., Shinohara N., Takayama H., Kondo S., Nagai K. Acidification is essential for maintaining the structure and function of lytic granules of CTL. Effect of concanamycin A, an inhibitor of vacuolar type H(+)-ATPase, on CTL-mediated cytotoxicity. J Immunol. 1994 Nov 1;153(9):3938–3947. [PubMed] [Google Scholar]
- Kataoka T., Taniguchi M., Yamada A., Suzuki H., Hamada S., Magae J., Nagai K. Identification of low molecular weight probes on perforin- and Fas-based killing mediated by cytotoxic T lymphocytes. Biosci Biotechnol Biochem. 1996 Oct;60(10):1726–1728. doi: 10.1271/bbb.60.1726. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Shinkai Y., Kuwana Y., Furuya A., Iigo Y., Hanai N., Itoh S., Yagita H., Okumura K. Perforin, a pore-forming protein detectable by monoclonal antibodies, is a functional marker for killer cells. Int Immunol. 1990;2(7):677–684. doi: 10.1093/intimm/2.7.677. [DOI] [PubMed] [Google Scholar]
- Kendall J. M., Badminton M. N., Dormer R. L., Campbell A. K. Changes in free calcium in the endoplasmic reticulum of living cells detected using targeted aequorin. Anal Biochem. 1994 Aug 15;221(1):173–181. doi: 10.1006/abio.1994.1394. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Schmidt R. E., Caulfield J. P., Hein A., Bartley G. T., Ritz J., Schlossman S. F., Austen K. F., Stevens R. L. Proteoglycans in cell-mediated cytotoxicity. Identification, localization, and exocytosis of a chondroitin sulfate proteoglycan from human cloned natural killer cells during target cell lysis. J Exp Med. 1985 Dec 1;162(6):1771–1787. doi: 10.1084/jem.162.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. 1987 Jun 5;49(5):679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Peters P. J., Borst J., Oorschot V., Fukuda M., Krähenbühl O., Tschopp J., Slot J. W., Geuze H. J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991 May 1;173(5):1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Smyth M. J., Trapani J. A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995 Apr;16(4):202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Kanagawa O., Bevan M. J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Apr 18;314(6012):628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Otsu K., Weis J. H., Tantravahi R. V., Austen K. F., Henkart P. A., Galli M. C., Reynolds C. W. Co-sedimentation of chondroitin sulfate A glycosaminoglycans and proteoglycans with the cytolytic secretory granules of rat large granular lymphocyte (LGL) tumor cells, and identification of a mRNA in normal and transformed LGL that encodes proteoglycans. J Immunol. 1987 Aug 1;139(3):863–868. [PubMed] [Google Scholar]
- Takayama H., Shinohara N., Kawasaki A., Someya Y., Hanaoka S., Kojima H., Yagita H., Okumura K., Shinkai Y. Antigen-specific directional target cell lysis by perforin-negative T lymphocyte clones. Int Immunol. 1991 Nov;3(11):1149–1156. doi: 10.1093/intimm/3.11.1149. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Masson D. Inhibition of the lytic activity of perforin (cytolysin) and of late complement components by proteoglycans. Mol Immunol. 1987 Sep;24(9):907–913. doi: 10.1016/0161-5890(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Woo J. T., Shinohara C., Sakai K., Hasumi K., Endo A. Isolation, characterization and biological activities of concanamycins as inhibitors of lysosomal acidification. J Antibiot (Tokyo) 1992 Jul;45(7):1108–1116. doi: 10.7164/antibiotics.45.1108. [DOI] [PubMed] [Google Scholar]
- Young J. D., Damiano A., DiNome M. A., Leong L. G., Cohn Z. A. Dissociation of membrane binding and lytic activities of the lymphocyte pore-forming protein (perforin). J Exp Med. 1987 May 1;165(5):1371–1382. doi: 10.1084/jem.165.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]