Abstract
Despite eradication attempts, measles remains a global health concern. Here we report results that demonstrate that a single-dose DNA immunization followed by multiple boosters, delivered orally as a plant-derived vaccine, can induce significantly greater quantities of measles virus-neutralizing antibodies than immunization with either DNA or plant-derived vaccines alone. This represents the first demonstration of an enhanced immune response to a prime-boost vaccination strategy combining a DNA vaccine with edible plant technology.
Measles is a highly contagious viral disease that is responsible for over 800,000 deaths every year (11). At particular risk are children in developing countries where the effectiveness of the attenuated vaccine has been compromised by difficulties associated with transportation, storage, and subcutaneous administration (8). Successful control and eradication of the measles virus (MV) will require new vaccines and vaccination strategies that address these problems.
Edible plant technology has the potential to overcome many of the problems associated with the current live attenuated MV vaccine (13). We have recently demonstrated that the MV hemagglutinin protein (MV-H) can be expressed in plants and that oral immunization of mice with plant-derived MV-H results in low titers of MV-specific neutralizing antibodies (5). MV-neutralizing antibodies have been correlated with protection in humans (2, 9). Vaccination strategies that combine two unique vaccines frequently result in improved immune responses, particularly when different routes of administration or types of vaccine are combined (7, 10, 15). Thus, the goal of this study was to induce high-titer MV-neutralizing antibodies by combining our plant-derived MV-H protein vaccine with an MV-H DNA vaccine in a prime-boost vaccination strategy.
The MV-H DNA vaccine encoded a secreted form of the MV-H gene, with the N-terminal transmembrane domain replaced by the secretion signal peptide (SP) of the CD5 gene. The truncated MV-H gene was amplified by PCR with the 5′ primer MTHFwd (5′-CGACGCGTGTAACTAACTCAATCGAGCATCAG-3′) and with the 3′ primer MTHRev (5′-CTAGTCTAGACTATCTGCGATTGGTTCCATCTTC-3′). This PCR product was then ligated in frame with the 3′ end of the pCI-SP-hIg vector (3). An ovalbumin control DNA construct was obtained from A. M. Lew (WEHI, Victoria, Australia). Plasmids containing the DNA constructs were prepared for vaccination by CsCl gradient centrifugation as previously described (4).
Plant extracts were prepared from transgenic tobacco plants expressing either the MV-H or a control gene (5). Batches of frozen leaves were ground to a fine powder and mixed with 4 volumes of extraction buffer (phosphate-buffered saline [PBS], 100 mM ascorbic acid, 20 mM EDTA, 0.1% Triton X-100 [vol/vol], 1 mM phenylmethylsulfonyl flouride [pH 7.2]). The extract was filtered through Miracloth and centrifuged at 100 × g for 5 min (4°C), and then the supernatant was centrifuged again at 32,600 × g for 1 h (4°C). The pellet was resuspended in a minimal volume of PBS containing glycerol (final concentration, 16%). This resulted in extracts containing the equivalent of 3.5 to 4.5 g of tobacco leaves (fresh weight) liter−1.
Experiment 1.
Groups of 10 mice each received a single 50-μg intramuscular dose of MV-H or control DNA in 50 μl of saline solution (0.85% NaCl [wt/vol]). This was followed by four 1-g doses of plant extract delivered orally by gavage on days 21, 28, 35, and 42. Five mice from each DNA vaccine group received MV-H plant extract, and five received control plant extract. Each dose of plant extract was supplemented with mucosal adjuvant (2 μg of cholera toxin [CT] and 10 μg of CT-B Σ).
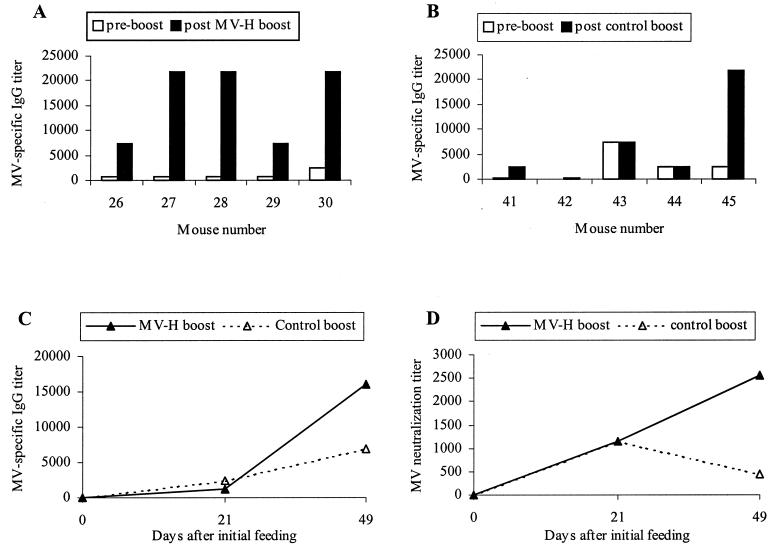
MV-specific serum immunoglobulin G (IgG) was detected in 90% of the mice immunized with MV-H DNA by using Enzygnost MV-coated enzyme-linked immunosorbent assay plates (Dade-Behring, Marburg, Germany). Titers ranged from 270 to 7,290 in sera collected on day 21 (Fig. 1). Following boosting with MV-H plant extract, average MV-specific IgG titers increased from 1,215 to 16,038 (P = 0.04) (Fig. 1C). In contrast, boosting with control plant extract did not result in a significant serum IgG titer increase (P = 0.11). Serum IgG titers for mice boosted with MV-H plant extract were significantly greater than for mice boosted with control plant extract (day 49; P = 0.01). Furthermore, all animals boosted with MV-H plant extract responded, with serum IgG titers increasing between 9- and 27-fold (Fig. 1A). Of the animals boosted with control plant extract, 60% displayed an increase in serum IgG titer (Fig. 1B). However, because the animals vaccinated with control DNA followed by the control plant extract did not produce any MV-specific serum IgG (average titer, <10), it is likely that this increase is due to a continued response to the MV-H DNA vaccination. Mice vaccinated with control DNA followed by MV-H plant extract responded with an average MV-specific serum IgG titer of 130.
FIG. 1.
Experiment 1. Shown are the immune responses of mice immunized with 50 μg of MV-H DNA (day 0) followed by gavage with 1-g doses of MV-H or control plant extract and mucosal adjuvant on days 21, 28, 35, and 42. (A and B) MV-specific serum IgG titers for individual mice boosted with MV-H (A) or control (B) plant extract. (C) Average MV-specific serum IgG titers. (D) MV-neutralization titers of pooled sera.
The humoral response to prime-boost vaccination was dominated by IgG1. An MV-specific IgG1 titer of 6,561 was recorded for pooled sera collected on day 49; the IgG2a titer was <3. This suggests that the response to prime-boost vaccination was mediated by Th2 pathways (12). This is consistent with the response induced following DNA vaccination with a secreted form of MV-H (1).
Experiment 2.
In an additional prime-boost experiment, the time between DNA priming and immunization with plant extract was extended from 21 days to 90 days (Table 1). Mice were immunized intramuscularly with a single 50-μg dose of MV-H DNA. This was followed by four 1-g doses of plant extract delivered by gavage on days 90, 97, 104, and 111. Seven mice received MV-H plant extract, and four mice received control plant extract. Each dose of plant extract was supplemented with mucosal adjuvant.
TABLE 1.
MV-specific serum IgG titers from experiment 2a
| Plant | Mouse no. | MV-specific IgG titer
|
|||
|---|---|---|---|---|---|
| Preboost
|
Day 125 postboost | ||||
| Day 0 | Day 42 | Day 70 | |||
| MV-H | 1 | 0 | 64 | 77 | 730 |
| 2 | 0 | 90 | 3,250 | 10,000 | |
| 3 | 0 | 75 | 850 | 550 | |
| 4 | 0 | 280 | 80 | 730 | |
| 5 | 0 | 990 | 990 | 6,400 | |
| 6 | 0 | 820 | 820 | 460 | |
| 7 | 0 | 700 | 460 | 820 | |
| Avg | 0 | 430 | 932 | 2,813 | |
| Control | 1 | 0 | 460 | 75 | 77 |
| 2 | 0 | 325 | 800 | 280 | |
| 3 | 0 | 75 | 850 | 640 | |
| 4 | 0 | 35 | 700 | 730 | |
| Avg | 0 | 223 | 606 | 432 | |
All mice received a single, 50-μg, intramuscular dose of MV-H DNA on day 0, followed by four 1-g doses of plant extract with a mucosal adjuvant delivered orally by gavage on days 90, 97, 104, and 111.
MV-specific IgG was detected in all mice immunized with MV-H DNA. By day 70, titers had plateaued or declined in 54% of mice, including five of the seven mice to be boosted with MV-H plant extract (Table 1). As with the previous experiment, the average serum IgG titer increased following vaccination with MV-H plant extract. In contrast, the average serum IgG titer decreased following immunization with control plant extract. MV-specific serum IgG titers for mice boosted with MV-H plant extract (titer, 2,813) were significantly greater than for mice boosted with control plant extract (titer, 432; P = 0.05). These results support the findings of the previous experiment.
MV-neutralization titers.
MV-neutralization titers were determined with pooled sera from experiment 1 in plaque-reduction neutralization assays (5). Following immunization with MV-H DNA, the neutralization titer increased from a preimmune titer of 8 to 1,150 (Fig. 1D). Neutralization titers increased from 1,150 to 2,550 following immunization with MV-H plant extract, but decreased to 450 when boosted with control plant extract. An increase in neutralization titer, from 8 to 100, was also observed in pooled day 49 sera from mice vaccinated with control DNA and MV-H plant extract. No MV neutralization was detected in mice vaccinated with control DNA and control plant extract.
The DNA oral prime-boost vaccination strategy outlined in this paper induced MV neutralization titers up to 21 times greater than those considered protective in humans (2). This represents the first demonstration of an enhanced immune response to a prime-boost vaccination strategy combining a DNA vaccine with edible plant technology. It also supports the findings of Kong et al. (7), who demonstrated that edible vaccines can be effectively used in protein-based prime-boost vaccination strategies.
Provision in heat-stable packaging of an MV vaccine that can be directly consumed would significantly increase the availability of viable vaccine doses in countries in which measles is endemic and maintenance of the cold chain for vaccine storage and transport is difficult. Edible vaccines also reduce the need for skilled personnel to administer injections and negate concerns regarding the reuse of needles. A DNA-edible plant prime-boost vaccination strategy may also be suitable for other infectious diseases that require a broad immune response, particularly if frequent booster immunizations or multiple antigens are likely to be required to induce protection, such as, for example, human immunodeficiency virus and malaria, respectively.
Translation of these proof-of-concept types of studies into practical vaccines will require the development of sensible dosing schedules followed by transfer of these results into edible plant species, primate models, and, finally, human trials (13). Recent work in our laboratory has shown that the MV-H protein can be expressed in edible plant species such as lettuce and rice. With further investigation, a prime-boost vaccination strategy, combining advances in the oral delivery of DNA (6, 14) with an edible plant vaccine, could become a feasible alternative to traditional vaccination strategies for measles and other infectious diseases.
Acknowledgments
We thank Terri King and M. Waldhuber for valuable input into the project.
This work was supported by the NHMRC, Australia.
REFERENCES
- 1.Cardoso, A., M. Blixenkrone-Moller, J. Fayolle, M. Liu, R. Buckland, and T. Wild. 1996. Immunization with plasmid DNA encoding for the measles virus hemagglutinin and nucleoprotein leads to humoral and cell-mediated immunity. Virology 225:293-299. [DOI] [PubMed] [Google Scholar]
- 2.Chen, R., L. Markowitz, P. Albrecht, J. Stewart, L. Mofenson, S. Preblud, and W. Orenstein. 1990. Measles antibody: reevaluation of protective titres. J. Infect. Dis. 162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 3.Drew, D., J. Boyle, A. Lew, M. Lightowlers, and R. Strugnell. 2001. The human IgG3 hinge mediates the formation of antigen dimers that enhance humoral immune responses to DNA immunization. Vaccine 19:4115-4120. [DOI] [PubMed] [Google Scholar]
- 4.Drew, D., M. Lightowlers, and R. Strugnell. 2000. Humoral immune responses to DNA vaccines expressing secreted, membrane bound and non-secreted forms of the Taenia ovis 45W antigen. Vaccine 18:2522-2532. [DOI] [PubMed] [Google Scholar]
- 5.Huang, Z., I. Dry, D. Webster, R. Strugnell, and S. Wesselingh. 2001. Plant-derived measles virus hemagglutinin protein induces neutralizing antibodies in mice. Vaccine 19:2163-2171. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko, H., I. Bednarek, A. Wierzbicki, I. Kiszka, M. Dmochowski, T. Wasik, Y. Kaneko, and D. Kozbor. 2000. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology 267:8-16. [DOI] [PubMed] [Google Scholar]
- 7.Kong, Q., L. Richter, Y. F. Yang, C. J. Arntzen, H. S. Mason, and Y. Thanavala. 2001. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 98:11539-11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller, C. 2001. Measles elimination: old and new challenges? Vaccine 19:2258-2261. [DOI] [PubMed] [Google Scholar]
- 9.Polack, F. P., S. Lee, S. Permar, E. Manyara, H. Nousari, Y. Jeng, F. Mustafa, A. Valsamakis, R. Adams, H. Robinson, and D. Griffin. 2000. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat. Med. 6:776-781. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay, A., K. Leong, and I. Ramshaw. 1997. DNA vaccination against virus infection and enhancement of antiviral immunity following consecutive immunisation with DNA and viral vectors. Immunol. Cell Biol. 75:382-388. [DOI] [PubMed] [Google Scholar]
- 11.Shann, F. 1999. A little bit of measles does you good. Br. Med. J. 319:4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snapper, C., and W. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 13.Webster, D., M. Thomas, R. Strugnell, I. Dry, and S. Wesselingh. 2002. Appetising solutions: an edible vaccine for measles. Med. J. Aust. 176:434-437. [PubMed] [Google Scholar]
- 14.Woo, P., L. Wong, B. Zheng, and K. Yuen. 2001. Unique immunogenicity of hepatitis B virus DNA vaccine by live-attenuated Salmonella typhimurium. Vaccine 19:2945-2954. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizawa, I., Y. Soda, T. Mizuochi, S. Yasuda, T. Rizvi, T. Mizuochi, T. Takemori, and Y. Tsunetsugu-Yokota. 2001. Enhancement of mucosal immune response against HIV-1 Gag by DNA immunisation. Vaccine 19:2995-3003. [DOI] [PubMed] [Google Scholar]