Abstract
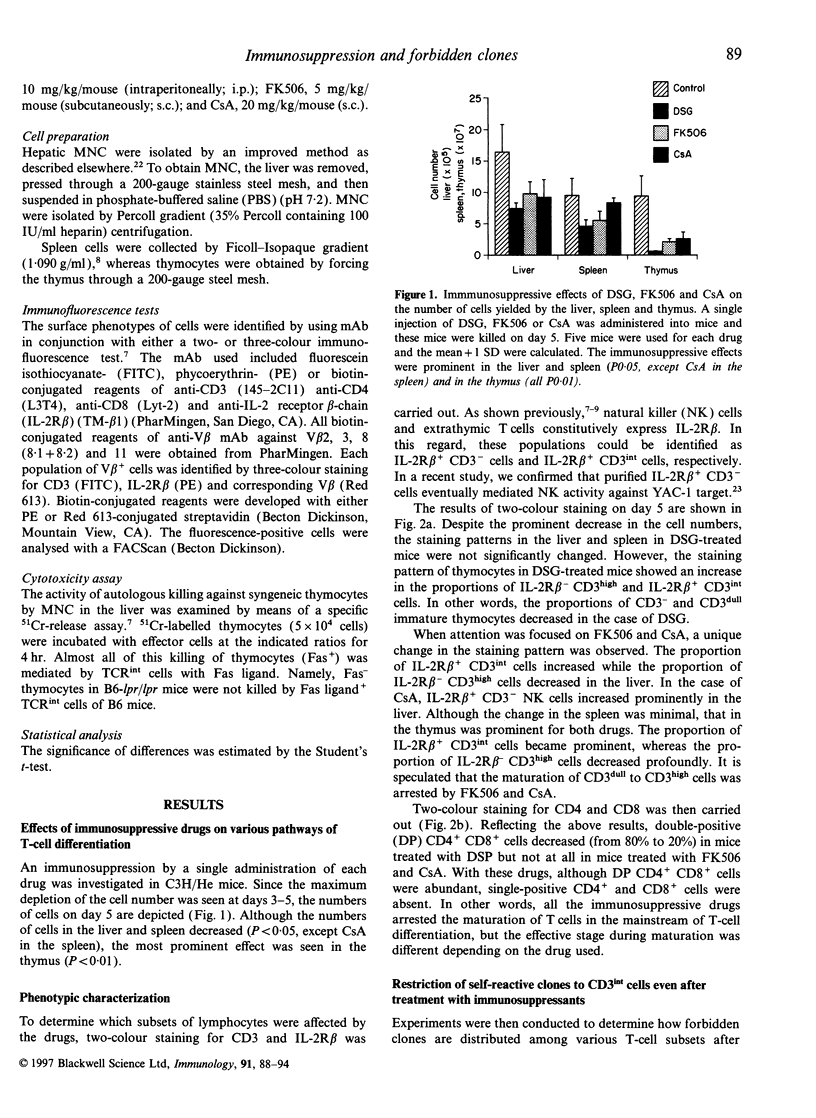
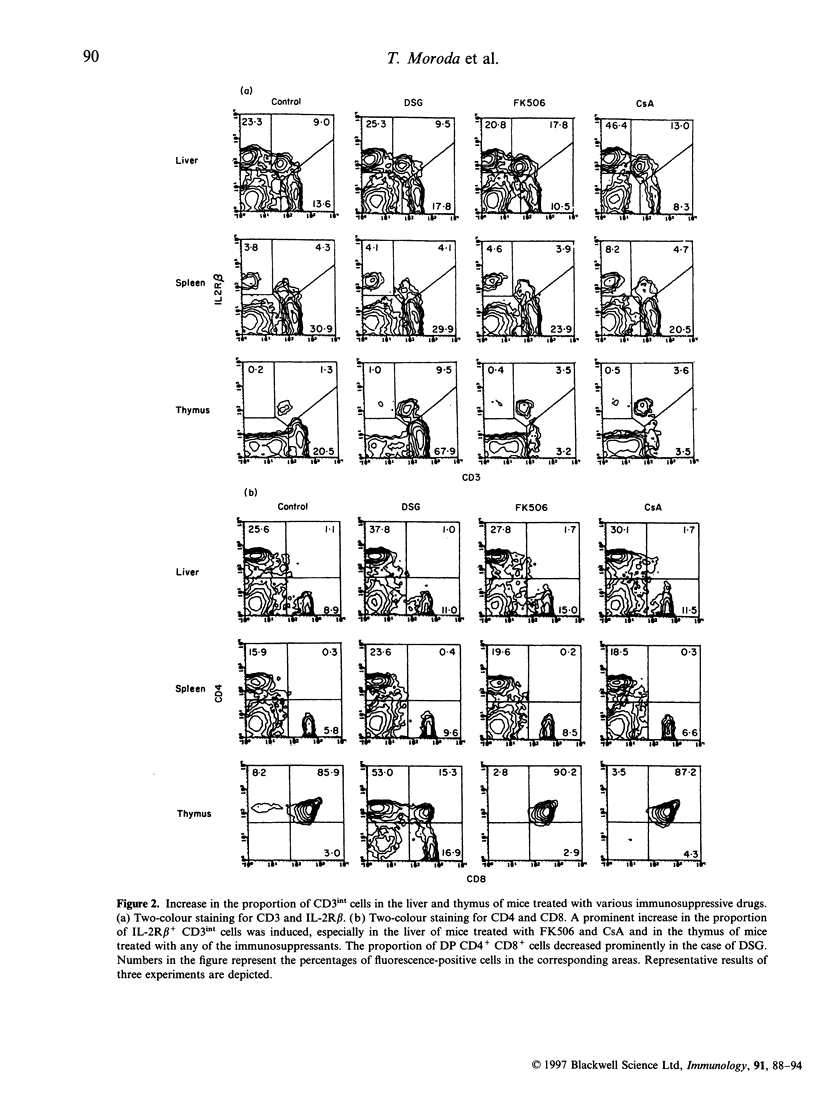
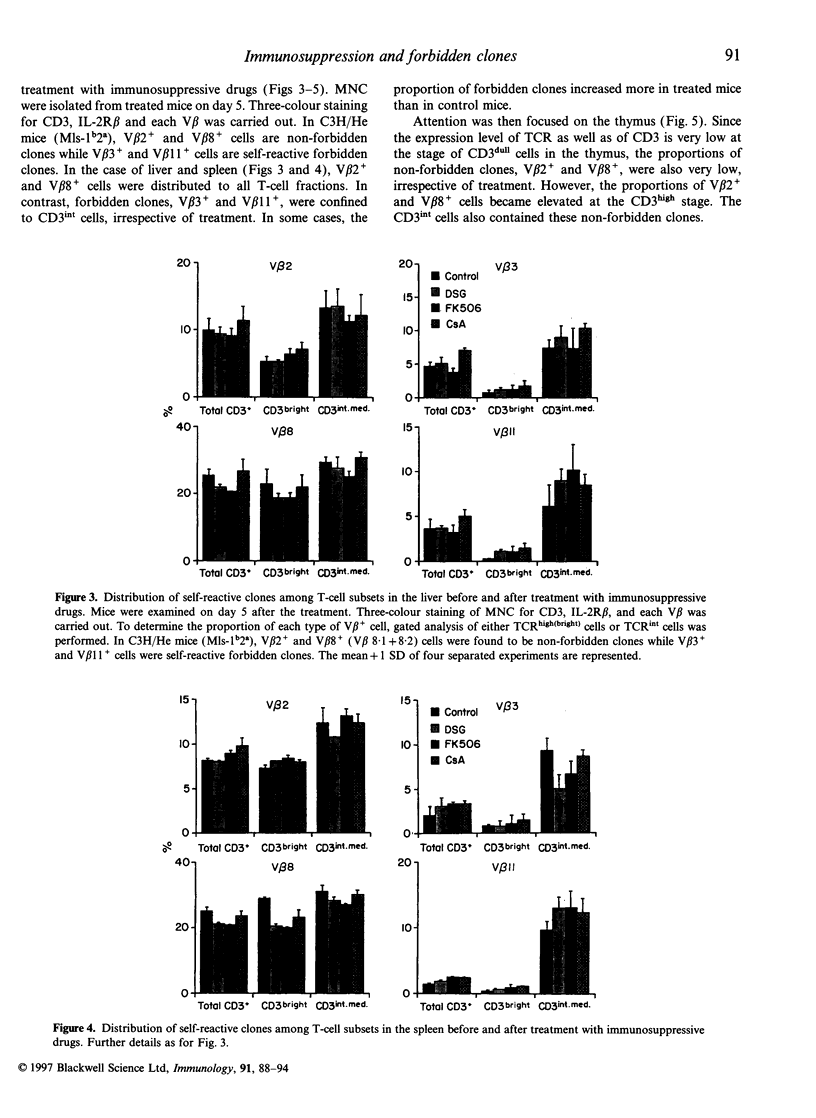
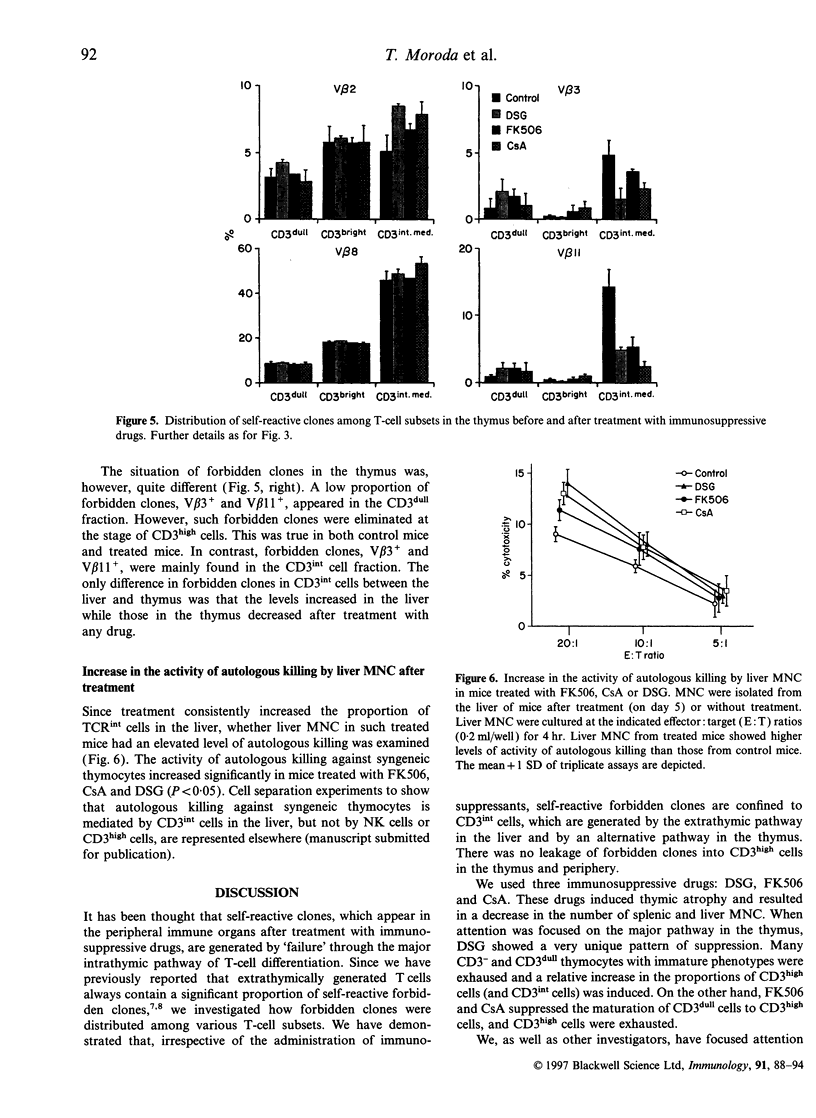
It is believed that self-reactive forbidden T-cell clones are generated by 'failure' of the pathway of T-cell differentiation in the thymus, if it is disturbed. We examined how such forbidden clones are generated under immunosuppressive conditions. Mice were treated with an injection of deoxyspergualin, FK506, or cycloporin A. From day 3, the number of cells yielded by various organs decreased. Because of the resistance of intermediate (int) T-cell receptor (TCR) cells (i.e. TCRint cells), they became more prominent in proportion than TCRhigh cells. TCRhigh cells are conventional T cells generated through the mainstream in the thymus, whereas TCRint cells are primordial T cells generated by the extrathymic pathway or an alternative intrathymic pathway. Similar to untreated mice, forbidden V beta 3+ and V beta 11+ clones in C3H/He (Mls-1b2a) mice were confined to TCRint cells after treatment; there was no leakage of forbidden clones into TCRhigh cells in the thymus and periphery. In parallel with the increase in the proportion of TCRint cells, the proportion of forbidden clones also increased under immunosuppressive states, especially in the liver. Liver mononuclear cells isolated from treated mice still had the potential to mediate autologous killing. The present results suggest that the generation of self-reactive clones is highly restricted to the pathways of TCRint cell differentiation even under immunosuppressive conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arase H., Arase N., Ogasawara K., Good R. A., Onoé K. An NK1.1+ CD4+8- single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor V beta family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6506–6510. doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995 Jun;7(3):367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Budd R. C., Miescher G. C., Howe R. C., Lees R. K., Bron C., MacDonald H. R. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes. J Exp Med. 1987 Aug 1;166(2):577–582. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Egerton M., Scollay R. Intrathymic selection of murine TCR alpha beta+CD4-CD8- thymocytes. Int Immunol. 1990;2(2):157–163. doi: 10.1093/intimm/2.2.157. [DOI] [PubMed] [Google Scholar]
- Finkel T. H., Cambier J. C., Kubo R. T., Born W. K., Marrack P., Kappler J. W. The thymus has two functionally distinct populations of immature alpha beta + T cells: one population is deleted by ligation of alpha beta TCR. Cell. 1989 Sep 22;58(6):1047–1054. doi: 10.1016/0092-8674(89)90503-5. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Kruisbeek A. M., Ton-That H., Weston M. A., Coligan J. E., Schwartz R. H., Pardoll D. M. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987 Sep 17;329(6136):251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Gao E. K., Lo D., Cheney R., Kanagawa O., Sprent J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature. 1988 Nov 10;336(6195):176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- Goossens P. L., Jouin H., Marchal G., Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990 Aug 28;132(1):137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Iiai T., Watanabe H., Seki S., Sugiura K., Hirokawa K., Utsuyama M., Takahashi-Iwanaga H., Iwanaga T., Ohteki T., Abo T. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992 Dec;77(4):556–563. [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H., Pardoll D. M. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988 Sep 23;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kawachi Y., Watanabe H., Moroda T., Haga M., Iiai T., Hatakeyama K., Abo T. Self-reactive T cell clones in a restricted population of interleukin-2 receptor beta+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995 Aug;25(8):2272–2278. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Kawachi Y., Moroda T., Weerasinghe A., Iiai T., Seki S., Tazawa Y., Takada G., Abo T. Cytotoxic activity against tumour cells mediated by intermediate TCR cells in the liver and spleen. Immunology. 1996 Sep;89(1):68–75. doi: 10.1046/j.1365-2567.1996.d01-719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Hanawa H., Watanabe H., Ogawa M., Abo T. Synchronous expansion of intermediate TCR cells in the liver and uterus during pregnancy. Cell Immunol. 1995 Apr 15;162(1):16–25. doi: 10.1006/cimm.1995.1046. [DOI] [PubMed] [Google Scholar]
- Levitsky H. I., Golumbek P. T., Pardoll D. M. The fate of CD4-8- T cell receptor-alpha beta+ thymocytes. J Immunol. 1991 Feb 15;146(4):1113–1117. [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Okuyama R., Seki S., Abo T., Sugiura K., Kusumi A., Ohmori T., Watanabe H., Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992 Sep 1;149(5):1562–1570. [PubMed] [Google Scholar]
- Ohtsuka K., Hasegawa K., Sato K., Arai K., Watanabe H., Asakura H., Abo T. A similar expression pattern of adhesion molecules between intermediate TCR cells in the liver and intraepithelial lymphocytes in the intestine. Microbiol Immunol. 1994;38(8):677–683. doi: 10.1111/j.1348-0421.1994.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K., Sato K., Watanabe H., Kimura M., Asakura H., Abo T. Unique order of the lymphocyte subset induction in the liver and intestine of mice during Listeria monocytogenes infection. Cell Immunol. 1995 Mar;161(1):112–124. doi: 10.1006/cimm.1995.1015. [DOI] [PubMed] [Google Scholar]
- Osman Y., Watanabe T., Kawachi Y., Sato K., Ohtsuka K., Watanabe H., Hashimoto S., Moriyama Y., Shibata A., Abo T. Intermediate TCR cells with self-reactive clones are effector cells which induce syngeneic graft-versus-host disease in mice. Cell Immunol. 1995 Dec;166(2):172–186. doi: 10.1006/cimm.1995.9980. [DOI] [PubMed] [Google Scholar]
- Papiernik M., Pontoux C. In vivo and in vitro repertoire of CD3+CD4-CD8- thymocytes. Int Immunol. 1990;2(5):407–412. doi: 10.1093/intimm/2.5.407. [DOI] [PubMed] [Google Scholar]
- Sato K., Ohtsuka K., Hasegawa K., Yamagiwa S., Watanabe H., Asakura H., Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995 Sep 1;182(3):759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin R., Kimura H., Schroder K., Wilson D. H., Wilson D. B. Cyclosporine-induced autoimmunity. Conditions for expressing disease, requirement for intact thymus, and potency estimates of autoimmune lymphocytes in drug-treated rats. J Exp Med. 1986 Nov 1;164(5):1615–1625. doi: 10.1084/jem.164.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Zlotnik A. Origin, differentiation, and repertoire selection of CD3+CD4-CD8- thymocytes bearing either alpha beta or gamma delta T cell receptors. J Immunol. 1993 Jan 15;150(2):447–455. [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Iiai T., Kimura M., Ohtsuka K., Tanaka T., Miyasaka M., Tsuchida M., Hanawa H., Abo T. Characterization of intermediate TCR cells in the liver of mice with respect to their unique IL-2R expression. Cell Immunol. 1993 Jul;149(2):331–342. doi: 10.1006/cimm.1993.1159. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Kawachi Y., Iiai T., Ohtsuka K., Iwanage T., Takahashi-Iwanaga H., Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995 Sep 15;155(6):2972–2983. [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Seki S., Abo T. c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996 Aug 1;184(2):687–693. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]



