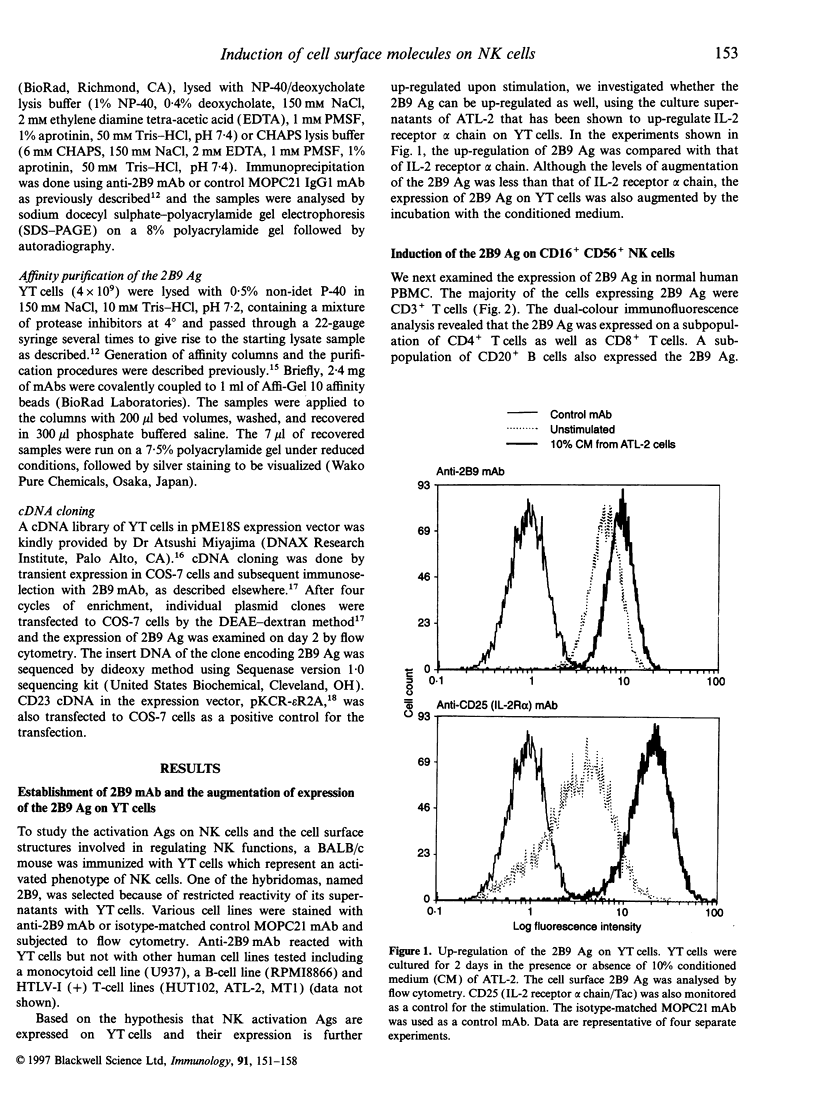
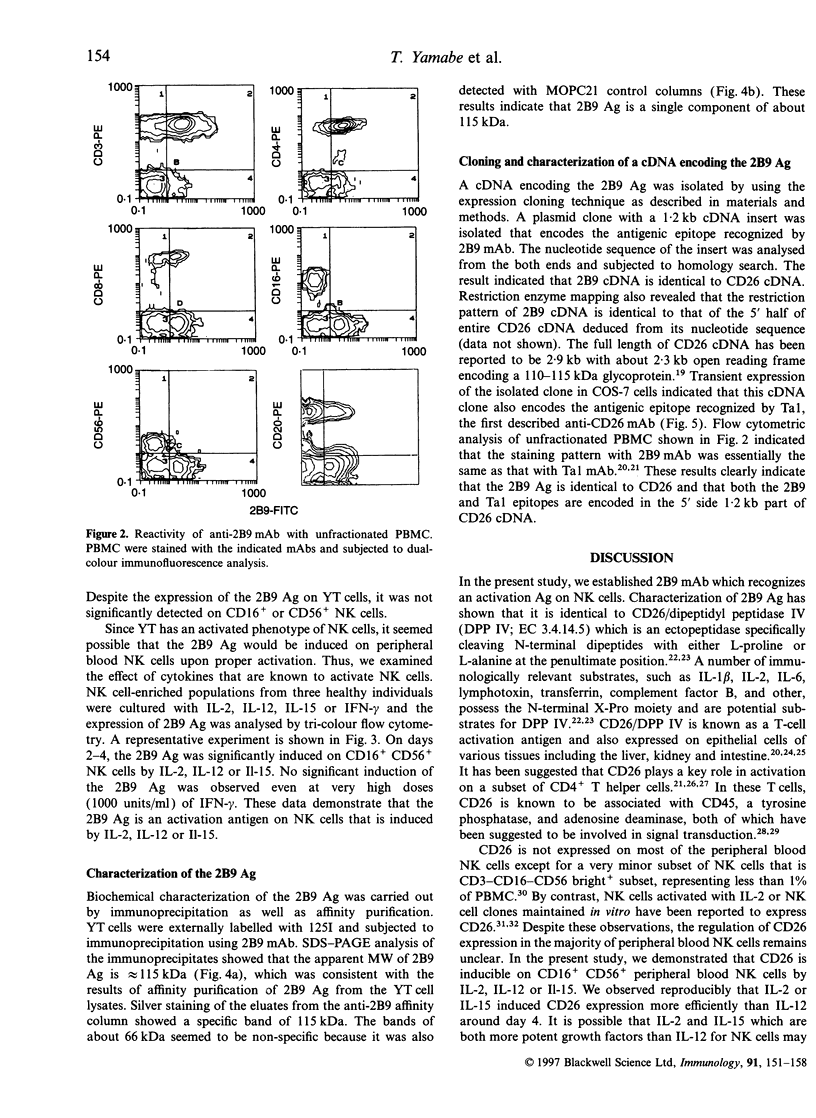
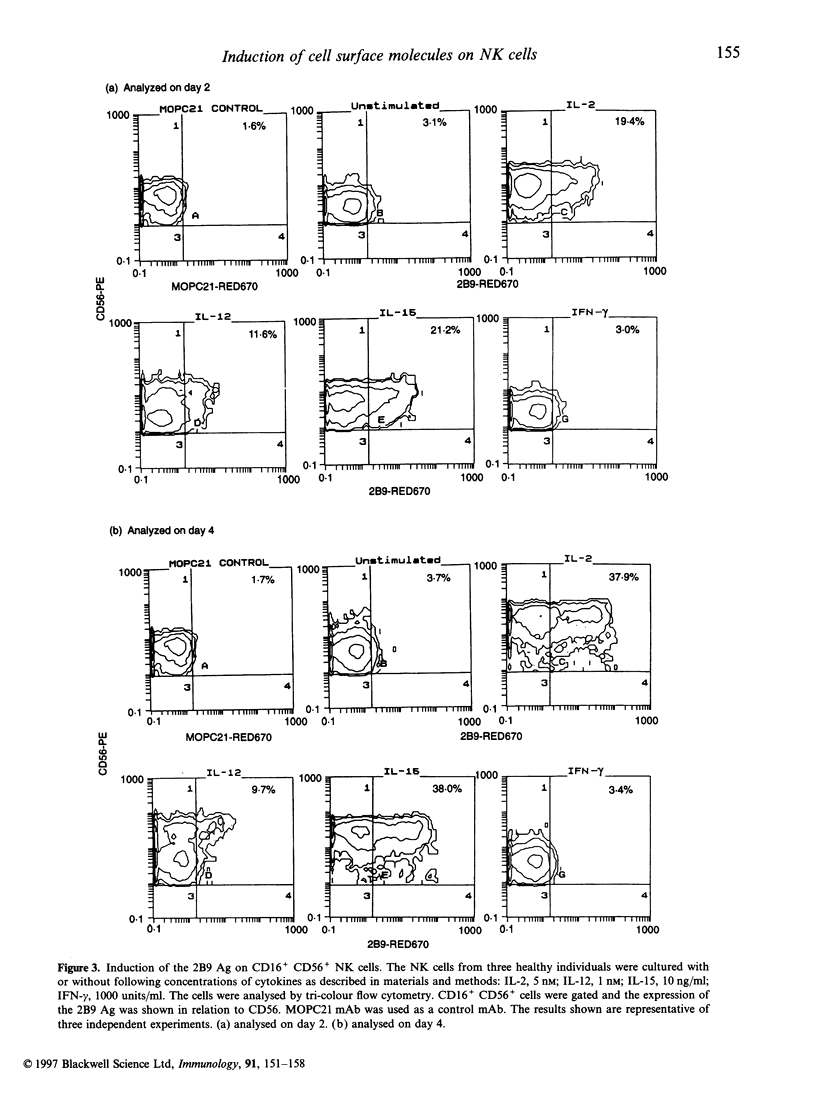
Abstract
Activation of human natural killer (NK) cells involves sequential events including cytokine production and induction of cell surface molecules, resulting in the enhancement of cytolytic activity. To delineate the activation process of NK cells, we generated murine monoclonal antibodies (mAbs) against YT, a human large granular lymphocyte/natural killer (LGL/NK) cell line. Among the mAbs reactive with YT cells, one mAb, termed 2B9, was noted because of the lack of reactivity with most of the human T- and B-cell lines tested. In fresh peripheral blood mononuclear cells (PBMC), however, the majority of cells expressing this antigen (Ag) were T cells but not CD16+ nor CD56+ NK cells. Since YT cells showed an activated phenotype expressing interleukin-2 (IL-2) receptor alpha chain, we examined whether 2B9 Ag could be induced on normal human peripheral blood NK cells by cytokines known to activate NK cells. The 2B9 Ag was induced on NK cells by IL-2, IL-12 or IL-15 while no induction was observed by interferon-gamma (IFN-gamma). Biochemical analysis showed that anti-2B9 mAb recognized a 115 kDa molecule in YT cells. A cDNA clone encoding the 2B9 Ag was isolated from a cDNA expression library of YT cells and its sequence was identical to CD26 cDNA although it was not of full length. Transient expression of the 2B9 cDNA on COS-7 cells revealed that this cDNA encodes the antigenic epitope(s) recognized by anti-2B9 mAb as well as Ta1, an anti-CD26 mAb. These results showed that the 2B9 Ag is identical to CD26, and demonstrated that CD26 is an activation antigen on CD16+ CD56+ NK cells inducible by IL-2, IL-12 or IL-15.
Full text
PDF


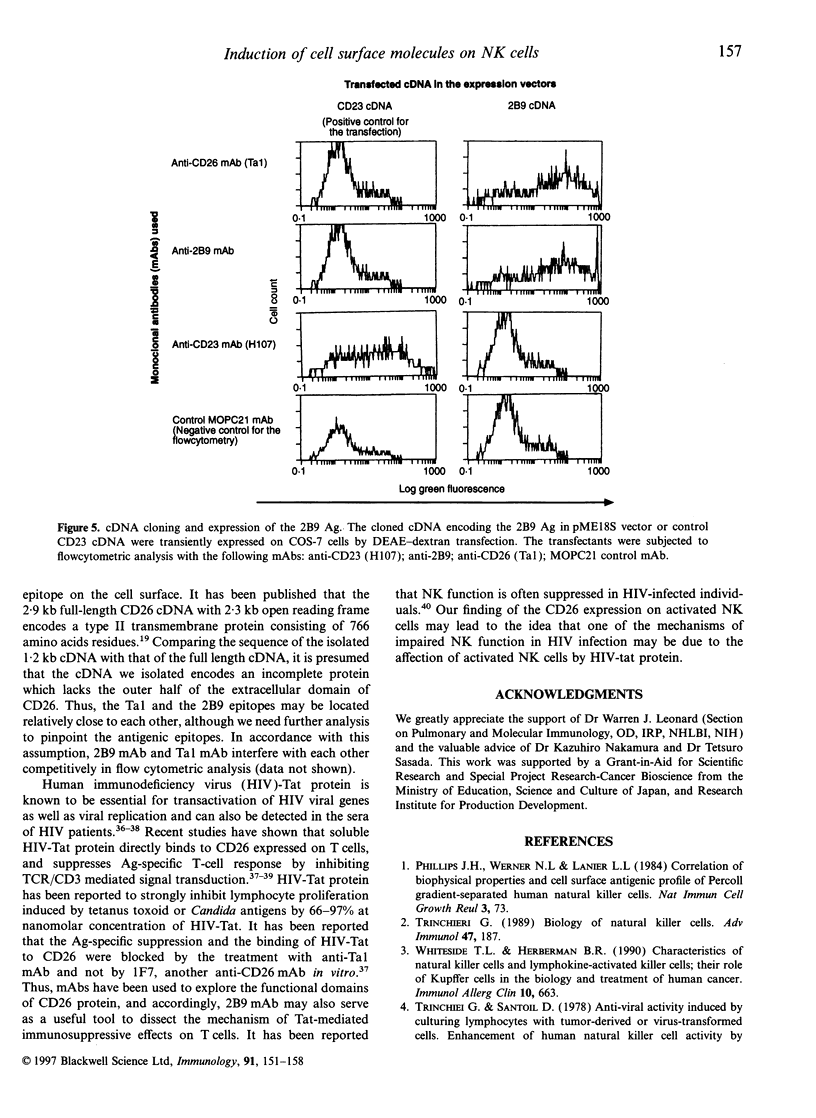

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B. A collagen-binding glycoprotein on the surface of mouse fibroblasts is identified as dipeptidyl peptidase IV. Biochem J. 1988 Jun 15;252(3):723–731. doi: 10.1042/bj2520723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnema J. D., Rivlin K. A., Ting A. T., Schoon R. A., Abraham R. T., Leibson P. J. Cytokine-enhanced NK cell-mediated cytotoxicity. Positive modulatory effects of IL-2 and IL-12 on stimulus-dependent granule exocytosis. J Immunol. 1994 Mar 1;152(5):2098–2104. [PubMed] [Google Scholar]
- Brenner B. G., Gornitsky M., Wainberg M. A. Interleukin-2-inducible natural immune (lymphokine-activated killer cell) responses as a functional correlate of progression to AIDS. Clin Diagn Lab Immunol. 1994 Sep;1(5):538–544. doi: 10.1128/cdli.1.5.538-544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühling F., Kunz D., Reinhold D., Ulmer A. J., Ernst M., Flad H. D., Ansorge S. Expression and functional role of dipeptidyl peptidase IV (CD26) on human natural killer cells. Nat Immun. 1994 Sep-Oct;13(5):270–279. [PubMed] [Google Scholar]
- Cullen B. R. Does HIV-1 Tat induce a change in viral initiation rights? Cell. 1993 May 7;73(3):417–420. doi: 10.1016/0092-8674(93)90126-b. [DOI] [PubMed] [Google Scholar]
- Dang N. H., Torimoto Y., Deusch K., Schlossman S. F., Morimoto C. Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol. 1990 Jun 1;144(11):4092–4100. [PubMed] [Google Scholar]
- Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J Immunol. 1987 Mar 1;138(5):1346–1350. [PubMed] [Google Scholar]
- Fleischer B., Sturm E., De Vries J. E., Spits H. Triggering of cytotoxic T lymphocytes and NK cells via the Tp103 pathway is dependent on the expression of the T cell receptor/CD3 complex. J Immunol. 1988 Aug 15;141(4):1103–1107. [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Acuto O., Poole C., Palley L., Daley J. F., Schlossman S. F., Reinherz E. L. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984 Sep;133(3):1250–1256. [PubMed] [Google Scholar]
- Grabstein K. H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M. A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994 May 13;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Gutheil W. G., Subramanyam M., Flentke G. R., Sanford D. G., Munoz E., Huber B. T., Bachovchin W. W. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6594–6598. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. T., Travis W. W., Koren H. S. Induction of activation antigens on human natural killer cells mediated through the Fc-gamma receptor. J Immunol. 1989 Oct 1;143(7):2401–2406. [PubMed] [Google Scholar]
- Hegen M., Niedobitek G., Klein C. E., Stein H., Fleischer B. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990 Apr 15;144(8):2908–2914. [PubMed] [Google Scholar]
- Heike M., Möbius U., Knuth A., Meuer S., Meyer zum Büschenfelde K. H. Tissue distribution of the T cell activation antigen Ta1. Serological, immunohistochemical and biochemical investigations. Clin Exp Immunol. 1988 Dec;74(3):431–434. [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Takami M., Kim C. W., Honjo T., Miyoshi T., Tagaya Y., Kawabe T., Yodoi J. Human lymphocyte Fc receptor for IgE: sequence homology of its cloned cDNA with animal lectins. Proc Natl Acad Sci U S A. 1987 Feb;84(3):819–823. doi: 10.1073/pnas.84.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R., Stoll M., Stratmann G., Leo R., Link H., Schmidt R. E. CD16- CD56+ natural killer cells after bone marrow transplantation. Blood. 1992 Jun 15;79(12):3239–3244. [PubMed] [Google Scholar]
- Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Kitaoka Y., Sorachi K., Nakamura H., Masutani H., Mitsui A., Kobayashi F., Mori T., Yodoi J. Detection of adult T-cell leukemia-derived factor/human thioredoxin in human serum. Immunol Lett. 1994 Jul;41(2-3):155–161. doi: 10.1016/0165-2478(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Torimoto Y., Levinson G., Rudd C. E., Schrieber M., Dang N. H., Letvin N. L., Schlossman S. F. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989 Dec 1;143(11):3430–3439. [PubMed] [Google Scholar]
- Nagatsu T., Hino M., Fuyamada H., Hayakawa T., Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem. 1976 Aug;74(2):466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Inamoto T., Sugie K., Masutani H., Shindo T., Tagaya Y., Yamauchi A., Ozawa K., Yodoi J. Mitogenicity and down-regulation of high-affinity interleukin 2 receptor by YTA-1 and YTA-2, monoclonal antibodies that recognize 75-kDa molecules on human large granular lymphocytes. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1318–1322. doi: 10.1073/pnas.86.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H., Harada M., Nagatsu T. Peptidase activity of glycylprolyl beta-naphthylamidase from human submaxillary gland. Arch Oral Biol. 1974 Jun;19(6):489–491. doi: 10.1016/0003-9969(74)90158-7. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Le A. M., Lanier L. L. Natural killer cells activated in a human mixed lymphocyte response culture identified by expression of Leu-11 and class II histocompatibility antigens. J Exp Med. 1984 Apr 1;159(4):993–1008. doi: 10.1084/jem.159.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Warner N. L., Lanier L. L. Correlation of biophysical properties and cell surface antigenic profile of Percoll gradient-separated human natural killer cells. Nat Immun Cell Growth Regul. 1983;3(2):73–86. [PubMed] [Google Scholar]
- Subramanyam M., Gutheil W. G., Bachovchin W. W., Huber B. T. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J Immunol. 1993 Mar 15;150(6):2544–2553. [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Torimoto Y., Dang N. H., Vivier E., Tanaka T., Schlossman S. F., Morimoto C. Coassociation of CD26 (dipeptidyl peptidase IV) with CD45 on the surface of human T lymphocytes. J Immunol. 1991 Oct 15;147(8):2514–2517. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi R. P., Mayur K., Lederman H. M., Frankel A. D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989 Dec 22;246(4937):1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]