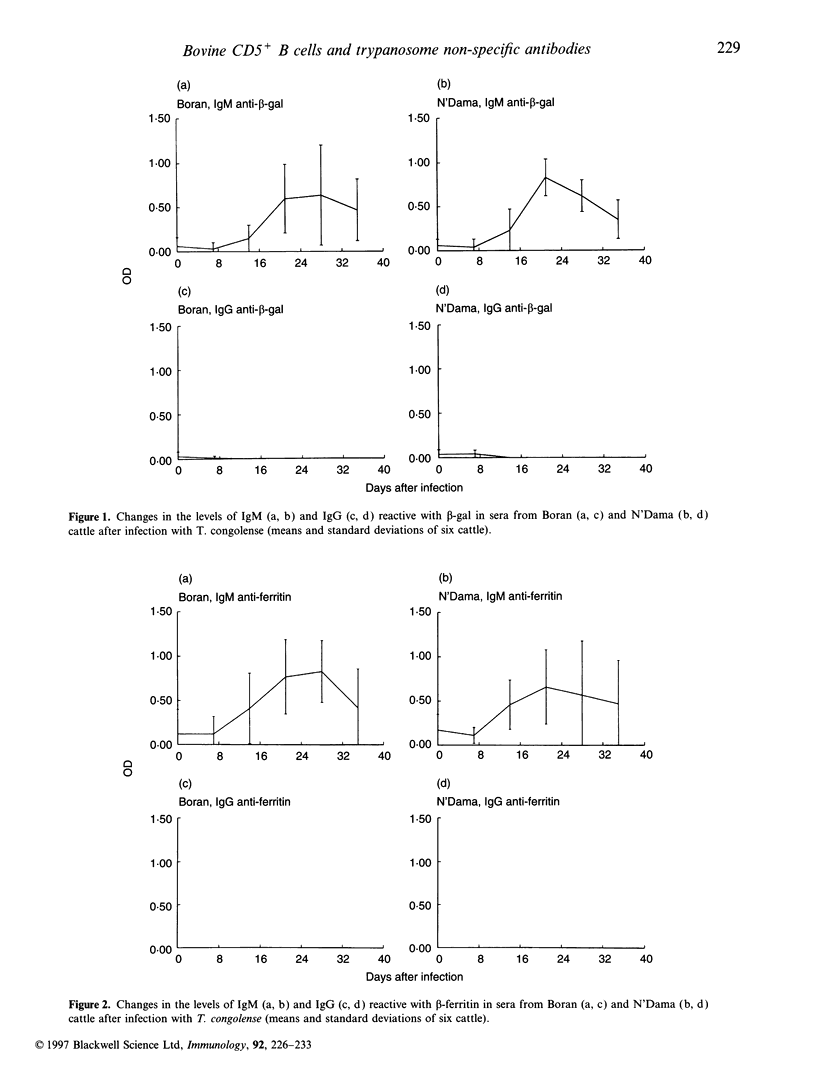
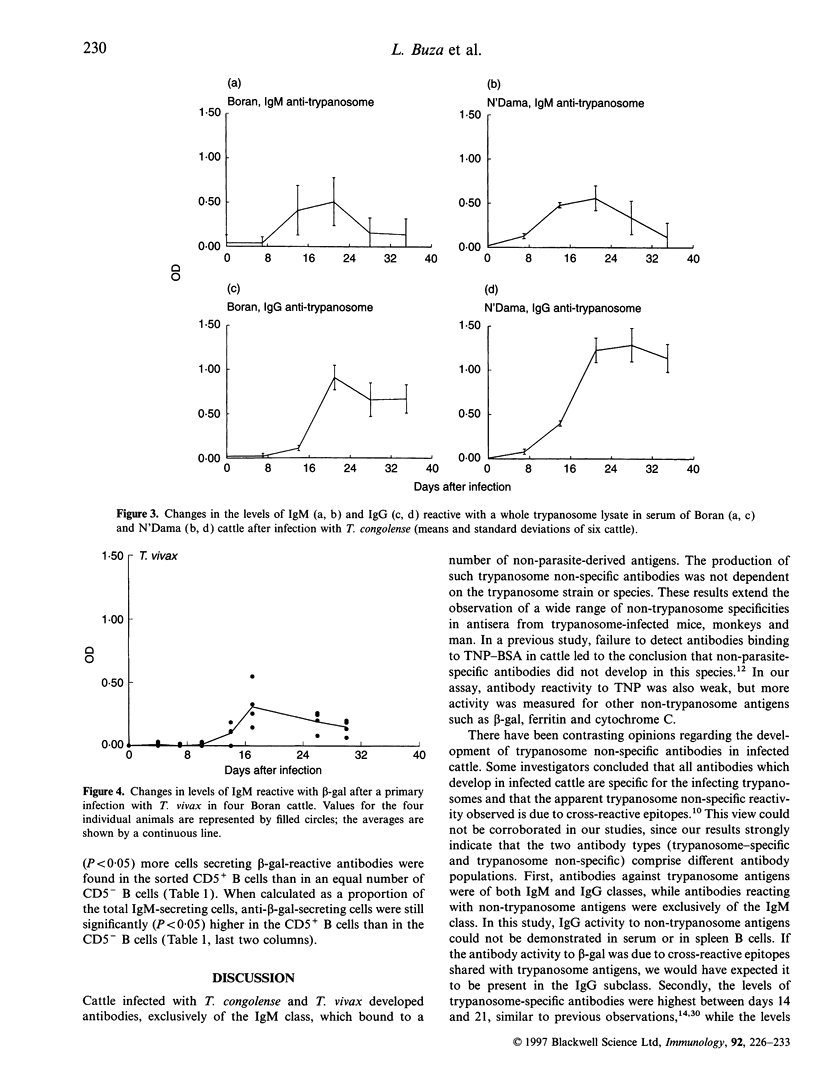
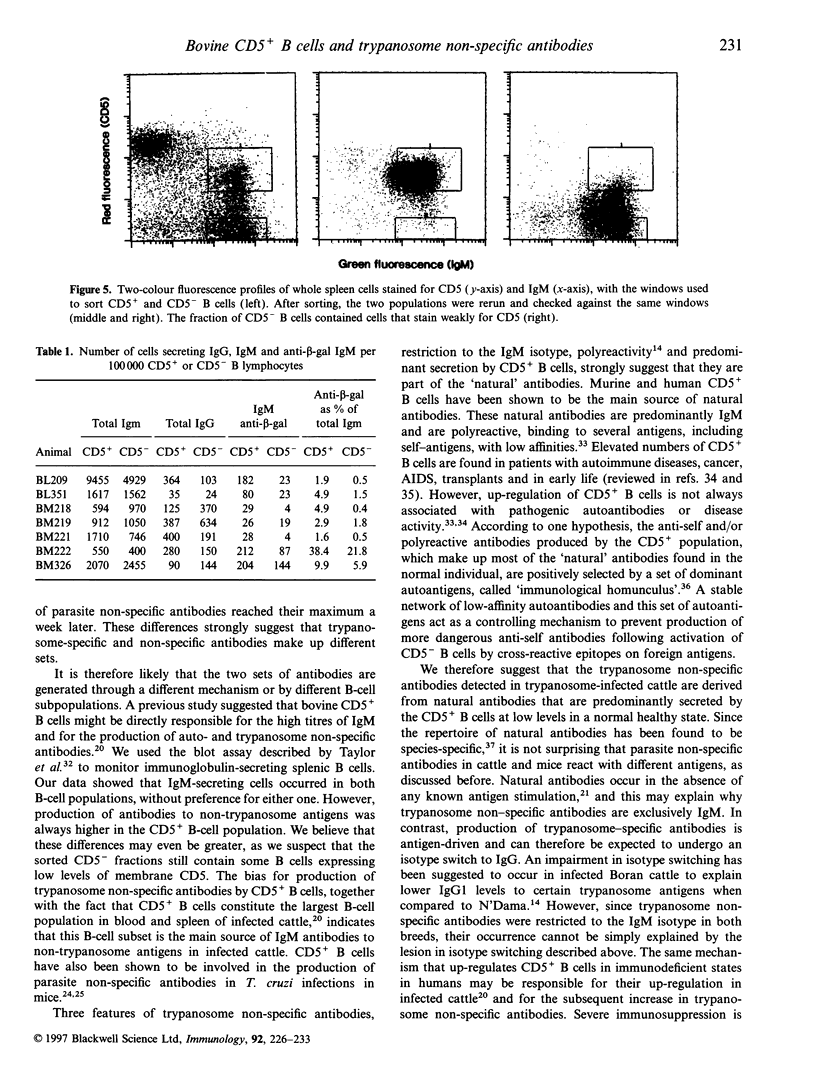
Abstract
Mice infected with African trypanosomes produce exceptionally large amounts of serum IgM, a major part of which binds to non-trypanosome antigens such as trinitrophenol and single-strand DNA. In this paper, we describe that in cattle infected with Trypanosoma congolense and T. vivax, similar antibodies are found, although they bind mainly to protein antigens, such as beta-galactosidase, ovalbumin and ferritin. The parasite non-specific IgM antibodies appear around the same time as the parasite-specific antibodies, but their origin and function are not clear. We tested the hypothesis that CD5+ B cells (or B-1 cells), which increase during trypanosome infections in cattle, are responsible for production of antibodies to non-trypanosome antigens. Splenic CD5+ and CD5- B cells from infected cattle were sorted and tested in a single cell blot assay. The numbers of immunoglobulin-secreting cells were similar in both B-cell populations. However, antibodies with reactivity for non-trypanosome antigens were significantly more prevalent in the CD5+ B-cell fraction and were exclusively IgM. The preference for production of these antibodies by CD5+ B cells and the expansion of this subpopulation during infections in cattle, strongly suggest that CD5+ B cells are the main source of trypanosome non-specific antibodies. We propose that these antibodies are natural, polyreactive antibodies that are predominantly secreted by CD5+ B cells. Since B-1 cells are up-regulated in many states of immune insufficiency, the immunosuppression associated with trypanosome infections may be responsible for the increase of this subset and the concomitant increase in trypanosome non-specific antibodies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoku R. K., Gardiner P. R. Detection of antibodies to platelets and erythrocytes during infection with haemorrhage-causing Trypanosoma vivax in Ayrshire cattle. Vet Parasitol. 1989 Jun;31(3-4):199–216. doi: 10.1016/0304-4017(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Authié E., Duvallet G., Robertson C., Williams D. J. Antibody responses to a 33 kDa cysteine protease of Trypanosoma congolense: relationship to 'trypanotolerance' in cattle. Parasite Immunol. 1993 Aug;15(8):465–474. doi: 10.1111/j.1365-3024.1993.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989 Nov;10(11):364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Casali P., Schettino E. W. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Sacks D. L., Ogilvie B. M., Askonas B. A. Membrane fractions of trypanosomes mimic the immunosuppressive and mitogenic effects of living parasites on the host. Parasite Immunol. 1979 Autumn;1(3):241–249. doi: 10.1111/j.1365-3024.1979.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Cunha-Neto E., Duranti M., Gruber A., Zingales B., De Messias I., Stolf N., Bellotti G., Patarroyo M. E., Pilleggi F., Kalil J. Autoimmunity in Chagas disease cardiopathy: biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3541–3545. doi: 10.1073/pnas.92.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinger R. H., Murray M., Moloo S. K. Potential value of localized skin reactions (chancres) induced by Trypanosoma congolense transmitted by Glossina morsitans centralis for the analysis of metacyclic trypanosome populations. Parasite Immunol. 1987 May;9(3):353–362. doi: 10.1111/j.1365-3024.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Gardiner P. R., Assoku R. K., Whitelaw D. D., Murray M. Haemorrhagic lesions resulting from Trypanosoma vivax infection in Ayrshire cattle. Vet Parasitol. 1989 Jun;31(3-4):187–197. doi: 10.1016/0304-4017(89)90069-1. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Kantor A. B., Herzenberg L. A. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992 May 4;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- Houba V., Allison A. C. M-antiglobulins (rheumatoid-factor-like globulins) and other gamma-globulins in relation to tropical parasitic infections. Lancet. 1966 Apr 16;1(7442):848–852. doi: 10.1016/s0140-6736(66)90186-3. [DOI] [PubMed] [Google Scholar]
- Houba V., Brown K. N., Allison A. C. Heterophile antibodies, M-antiglobulins and immunoglobulins in experimental trypanosomiasis. Clin Exp Immunol. 1969 Jan;4(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T., Louis J., Izui S., Lambert P. H. Autoimmune response to DNA, red blood cells, and thymocyte antigens in association with polyclonal antibody synthesis during experimental African trypanosomiasis. J Immunol. 1979 Jan;122(1):296–301. [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Lindsley H. B., Kysela S., Steinberg A. D. Nucleic acid antibodies in African trypanosomiasis: studies in Rhesus monkeys and man. J Immunol. 1974 Dec;113(6):1921–1927. [PubMed] [Google Scholar]
- MacKenzie A. R., Boreham P. F. Autoimmunity in trypanosome infections. I. Tissue autoantibodies in Trypanosoma (Trypanozoon) brucei infections of the rabbit. Immunology. 1974 Jun;26(6):1225–1238. [PMC free article] [PubMed] [Google Scholar]
- Mansfield J. M., Craig S. A., Stelzer G. T. Lymphocyte function in experimental african trypanosomiasis: mitogenic effects of trypanosome extracts in vitro. Infect Immun. 1976 Oct;14(4):976–981. doi: 10.1128/iai.14.4.976-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masake R. A., Musoke A. J., Nantulya V. M. Specific antibody responses to the variable surface glycoproteins of Trypanosoma congolense in infected cattle. Parasite Immunol. 1983 Jul;5(4):345–355. doi: 10.1111/j.1365-3024.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Bandeira A., Pereira P., Mota Santos T., Coutinho A. Preferential expansion of Ly-1 B and CD4- CD8- T cells in the polyclonal lymphocyte responses to murine T. cruzi infection. Int Immunol. 1989;1(2):176–184. doi: 10.1093/intimm/1.2.176. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Coutinho A., Spinella S., Hontebeyrie-Joskowicz M. Xid immunodeficiency imparts increased parasite clearance and resistance to pathology in experimental Chagas' disease. Int Immunol. 1991 May;3(5):427–433. doi: 10.1093/intimm/3.5.427. [DOI] [PubMed] [Google Scholar]
- Naessens J., Newson J., Williams D. J., Lutje V. Identification of isotypes and allotypes of bovine immunoglobulin M with monoclonal antibodies. Immunology. 1988 Apr;63(4):569–574. [PMC free article] [PubMed] [Google Scholar]
- Naessens J., Williams D. J. Characterization and measurement of CD5+ B cells in normal and Trypanosoma congolense-infected cattle. Eur J Immunol. 1992 Jul;22(7):1713–1718. doi: 10.1002/eji.1830220708. [DOI] [PubMed] [Google Scholar]
- Nobrega A., Haury M., Grandien A., Malanchère E., Sundblad A., Coutinho A. Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological "homunculus" of antibodies in normal serum. Eur J Immunol. 1993 Nov;23(11):2851–2859. doi: 10.1002/eji.1830231119. [DOI] [PubMed] [Google Scholar]
- Raveche E. S. Possible immunoregulatory role for CD5 + B cells. Clin Immunol Immunopathol. 1990 Aug;56(2):135–150. doi: 10.1016/0090-1229(90)90136-e. [DOI] [PubMed] [Google Scholar]
- Rurangirwa F. R., Musoke A. J., Nantulya V. M., Tabel H. Immune depression in bovine trypanosomiasis: effects of acute and chronic Trypanosoma congolense and chronic Trypanosoma vivax infections on antibody response to Brucella abortus vaccine. Parasite Immunol. 1983 May;5(3):267–276. doi: 10.1111/j.1365-3024.1983.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Seed J. R., Cornille R. L., Risby E. L., Gam A. A. The presence of agglutinating antibody in the IgM immunoglobulin fraction of rabbit antiserum during experimental African trypanosomiasis. Parasitology. 1969 May;59(2):283–292. doi: 10.1017/s003118200008224x. [DOI] [PubMed] [Google Scholar]
- Sileghem M., Flynn J. N. Suppression of interleukin 2 secretion and interleukin 2 receptor expression during tsetse-transmitted trypanosomiasis in cattle. Eur J Immunol. 1992 Mar;22(3):767–773. doi: 10.1002/eji.1830220321. [DOI] [PubMed] [Google Scholar]
- Tabel H., Losos G. J., Maxie M. G., Minder C. E. Experimental bovine trypanosomiasis (Trypanosoma vivax and T. congolense). III. Serum levels of immunoglobulins, heterophile antibodies, and antibodies to T. vivax. Tropenmed Parasitol. 1981 Sep;32(3):149–153. [PubMed] [Google Scholar]
- Talal N., Dauphinee M., Ahmed S. A. CD5 B cells in autoimmunity. Ann N Y Acad Sci. 1992 May 4;651:551–556. doi: 10.1111/j.1749-6632.1992.tb24661.x. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Lutje V., Kennedy D., Authié E., Boulangé A., Logan-Henfrey L., Gichuki B., Gettinby G. Trypanosoma congolense: B-lymphocyte responses differ between trypanotolerant and trypanosusceptible cattle. Exp Parasitol. 1996 Jun;83(1):106–116. doi: 10.1006/expr.1996.0054. [DOI] [PubMed] [Google Scholar]
- Williams D. J., Taylor K., Newson J., Gichuki B., Naessens J. The role of anti-variable surface glycoprotein antibody responses in bovine trypanotolerance. Parasite Immunol. 1996 Apr;18(4):209–218. doi: 10.1046/j.1365-3024.1996.d01-76.x. [DOI] [PubMed] [Google Scholar]



