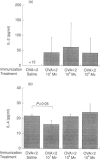
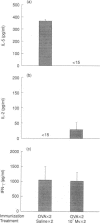
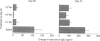Abstract
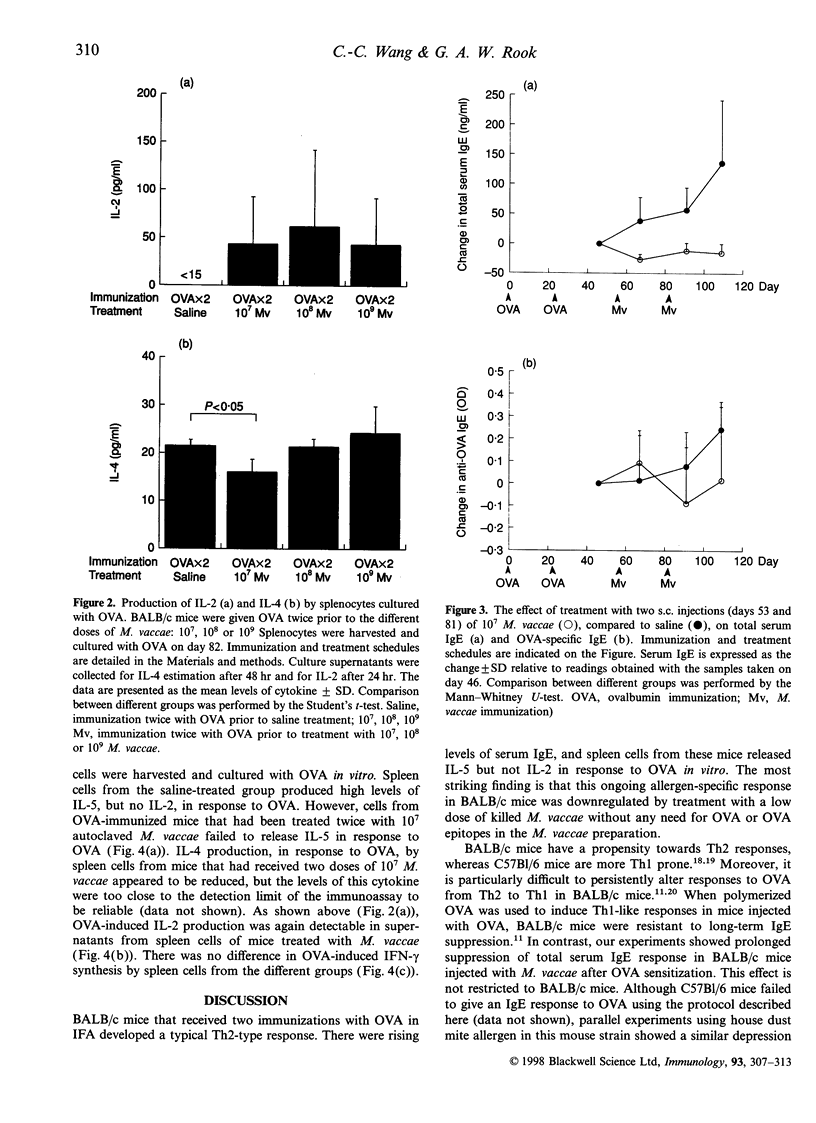
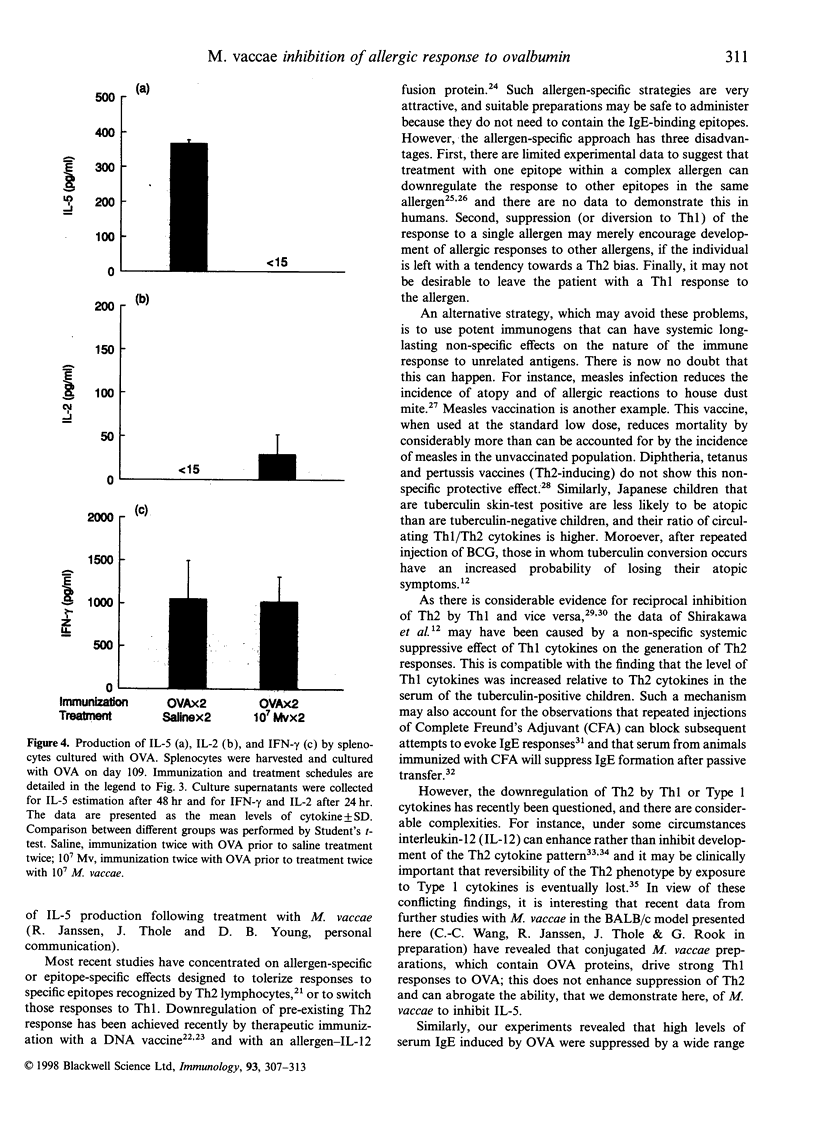
Allergic disorders are mediated by T lymphocytes secreting T helper 2 (Th2) cytokines, interleukin-4 (IL-4) and interleukin-5 (IL-5), resulting in high levels of serum immunoglobulin E (IgE) and recruitment of eosinophils. One of the treatment strategies is to downregulate the Th2 component by inducing a T helper 1 (Th1) response to the relevant allergen, because Th1 and Th2 cytokines are thought to be mutually antagonistic. In this study, we examined the effects of Mycobacterium vaccae, a potent inducer of Th1 immunity, on allergic responses in a murine model. A single injection of M. vaccae into ovalbumin (OVA)-preimmunized BALB/c mice suppressed serum IgE over a wide dose range (10(7), 10(8) or 10(9) M. vaccae). Further experiments, using 10(7) M. vaccae injected twice, showed that this treatment inhibited not only serum IgE, but also the potential for ovalbumin-induced IL-5 production by spleen cells. This non-specific ability of a mycobacterium to decrease Th2 activity, even when not presented together with the allergen, is in agreement with recent epidemiological studies on the impact of bacillus Calmette-Guérin (BCG) vaccination, and of other potent Th1 stimuli, on the incidence of atopy. The suppression of serum IgE and allergen-specific IL-5 synthesis by M. vaccae suggest that this organism is likely to have clinical application in the immunotherapy of allergy.
Full text
PDF

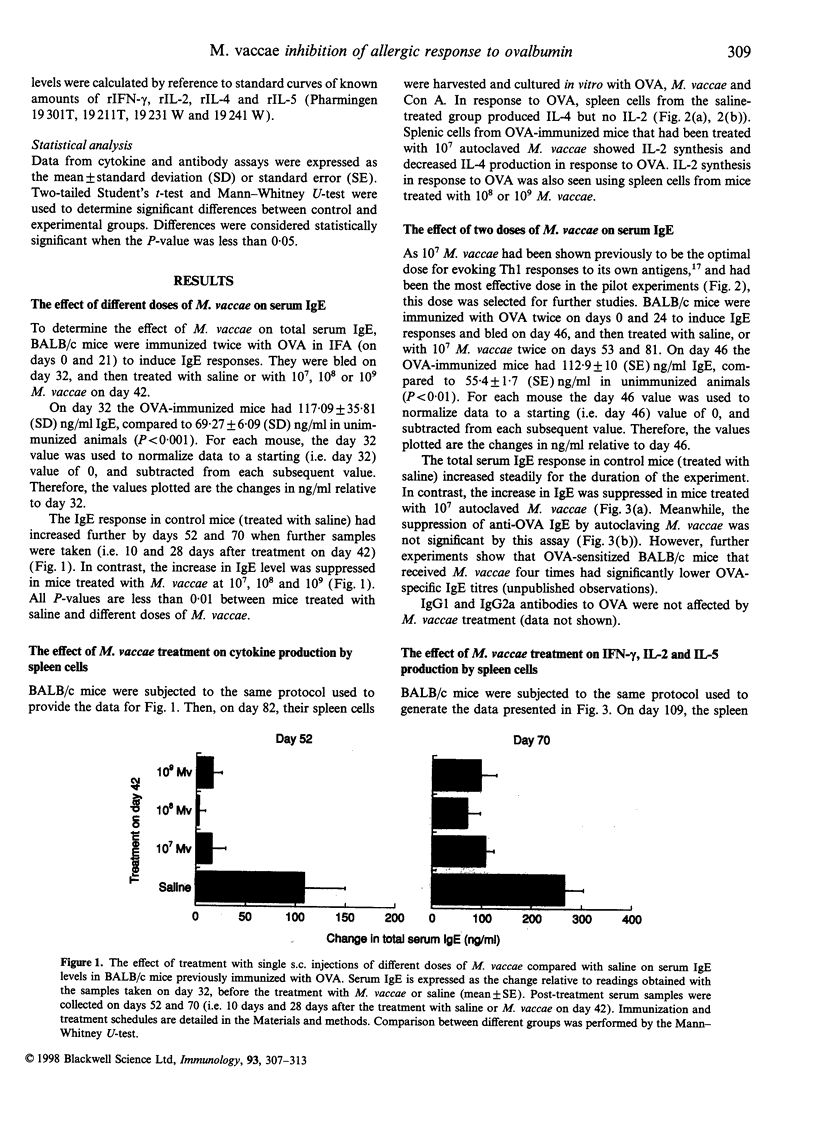


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaby P., Samb B., Simondon F., Seck A. M., Knudsen K., Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. 1995 Aug 19;311(7003):481–485. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner T. J., Kuo M. C., Keating K. M., Rogers B. L., Greenstein J. L. Peripheral T-cell tolerance induced in naive and primed mice by subcutaneous injection of peptides from the major cat allergen Fel d I. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7608–7612. doi: 10.1073/pnas.90.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A. J., Wagner K., Bertrand C., Tsuyuki S., Bews J., Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996 Apr 1;183(4):1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daser A., Meissner N., Herz U., Renz H. Role and modulation of T-cell cytokines in allergy. Curr Opin Immunol. 1995 Dec;7(6):762–770. doi: 10.1016/0952-7915(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Del Prete G. Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy. 1992 Oct;47(5):450–455. doi: 10.1111/j.1398-9995.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Delespesse G., Sarfati M., Hofstetter H. Human IgE-binding factors. Immunol Today. 1989 May;10(5):159–164. doi: 10.1016/0167-5699(89)90173-4. [DOI] [PubMed] [Google Scholar]
- Eum S. Y., Hailé S., Lefort J., Huerre M., Vargaftig B. B. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12290–12294. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieni R. S., HayGlass K. T. Regulation of murine IgE responses: induction of long-lived inhibition of allergen-specific responses is genetically restricted. Cell Immunol. 1991 Nov;138(1):64–78. doi: 10.1016/0008-8749(91)90133-v. [DOI] [PubMed] [Google Scholar]
- Gieni R. S., Yang X., HayGlass K. T. Allergen-specific modulation of cytokine synthesis patterns and IgE responses in vivo with chemically modified allergen. J Immunol. 1993 Jan 1;150(1):302–310. [PubMed] [Google Scholar]
- Hernandez-Pando R., Rook G. A. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994 Aug;82(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Hoyne G. F., O'Hehir R. E., Wraith D. C., Thomas W. R., Lamb J. R. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993 Nov 1;178(5):1783–1788. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., O'Garra A., Murphy K. M. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995 Feb 1;181(2):713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Chua K. Y., Tao M. H., Lai Y. L., Wu H. D., Huang S. K., Hsieh K. H. Immunoprophylaxis of allergen-induced immunoglobulin E synthesis and airway hyperresponsiveness in vivo by genetic immunization. Nat Med. 1996 May;2(5):540–544. doi: 10.1038/nm0596-540. [DOI] [PubMed] [Google Scholar]
- Ishizaka K. Regulation of immunoglobin E biosynthesis. Adv Immunol. 1989;47:1–44. [PubMed] [Google Scholar]
- Jeannin P., Delneste Y., Seveso M., Life P., Bonnefoy J. Y. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996 May 1;156(9):3159–3165. [PubMed] [Google Scholar]
- Kapsenberg M. L., Hilkens C. M., Jansen H. M., Bos J. D., Snijders A., Wierenga E. A. Production and modulation of T-cell cytokines in atopic allergy. Int Arch Allergy Immunol. 1996 Jun;110(2):107–113. doi: 10.1159/000237274. [DOI] [PubMed] [Google Scholar]
- Kato Y., Muto T., Tomura T., Tsumura H., Watarai H., Mikayama T., Ishizaka K., Kuroki R. The crystal structure of human glycosylation-inhibiting factor is a trimeric barrel with three 6-stranded beta-sheets. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):3007–3010. doi: 10.1073/pnas.93.7.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. S., DeKruyff R. H., Rupper R., Maecker H. T., Levy S., Umetsu D. T. An ovalbumin-IL-12 fusion protein is more effective than ovalbumin plus free recombinant IL-12 in inducing a T helper cell type 1-dominated immune response and inhibiting antigen-specific IgE production. J Immunol. 1997 May 1;158(9):4137–4144. [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Murphy E., Shibuya K., Hosken N., Openshaw P., Maino V., Davis K., Murphy K., O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996 Mar 1;183(3):901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K. M., Jaunin F., Masouyé I., Saurat J. H., Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol. 1993 Jun 15;150(12):5576–5584. [PubMed] [Google Scholar]
- Onyebujoh P. C., Abdulmumini T., Robinson S., Rook G. A., Stanford J. L. Immunotherapy with Mycobacterium vaccae as an addition to chemotherapy for the treatment of pulmonary tuberculosis under difficult conditions in Africa. Respir Med. 1995 Mar;89(3):199–207. doi: 10.1016/0954-6111(95)90248-1. [DOI] [PubMed] [Google Scholar]
- Raz E., Tighe H., Sato Y., Corr M., Dudler J. A., Roman M., Swain S. L., Spiegelberg H. L., Carson D. A. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci U S A. 1996 May 14;93(10):5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Bradley K., Larsen G. L., McCall C., Gelfand E. W. Comparison of the allergenicity of ovalbumin and ovalbumin peptide 323-339. Differential expansion of V beta-expressing T cell populations. J Immunol. 1993 Dec 15;151(12):7206–7213. [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98(4):279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Saban R., Haak-Frendscho M., Zine M., Ridgway J., Gorman C., Presta L. G., Bjorling D., Saban M., Jardieu P. Human FcERI-IgG and humanized anti-IgE monoclonal antibody MaE11 block passive sensitization of human and rhesus monkey lung. J Allergy Clin Immunol. 1994 Nov;94(5):836–843. doi: 10.1016/0091-6749(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Schmitt E., Hoehn P., Germann T., Rüde E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994 Feb;24(2):343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- Secrist H., DeKruyff R. H., Umetsu D. T. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995 Mar 1;181(3):1081–1089. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen S. O., Aaby P., Hall A. J., Barker D. J., Heyes C. B., Shiell A. W., Goudiaby A. Measles and atopy in Guinea-Bissau. Lancet. 1996 Jun 29;347(9018):1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- Shirakawa T., Enomoto T., Shimazu S., Hopkin J. M. The inverse association between tuberculin responses and atopic disorder. Science. 1997 Jan 3;275(5296):77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Bahr G. M., Rook G. A., Shaaban M. A., Chugh T. D., Gabriel M., al-Shimali B., Siddiqui Z., Ghardani F., Shahin A. Immunotherapy with Mycobacterium vaccae as an adjunct to chemotherapy in the treatment of pulmonary tuberculosis. Tubercle. 1990 Jun;71(2):87–93. doi: 10.1016/0041-3879(90)90002-p. [DOI] [PubMed] [Google Scholar]
- Stämpfli M. R., Rudolf M., Miescher S., Pachlopnik J. M., Stadler B. M. Antigen-specific inhibition of IgE binding to the high-affinity receptor. J Immunol. 1995 Sep 15;155(6):2948–2954. [PubMed] [Google Scholar]
- Tung A. S., Chiorazzi N., Katz D. H. Regulation of IgE antibody production by serum molecules. I. Serum from complete Freund's adjuvant-immune donors suppresses irradiation-enhanced IgE production in low responder mouse strains. J Immunol. 1978 Jun;120(6):2050–2059. [PubMed] [Google Scholar]
- van Neerven R. J., Ebner C., Yssel H., Kapsenberg M. L., Lamb J. R. T-cell responses to allergens: epitope-specificity and clinical relevance. Immunol Today. 1996 Nov;17(11):526–532. doi: 10.1016/0167-5699(96)10058-x. [DOI] [PubMed] [Google Scholar]
- von Reyn C. F., Barber T. W., Arbeit R. D., Sox C. H., O'Connor G. T., Brindle R. J., Gilks C. F., Hakkarainen K., Ranki A., Bartholomew C. Evidence of previous infection with Mycobacterium avium-Mycobacterium intracellulare complex among healthy subjects: an international study of dominant mycobacterial skin test reactions. J Infect Dis. 1993 Dec;168(6):1553–1558. doi: 10.1093/infdis/168.6.1553. [DOI] [PubMed] [Google Scholar]