Abstract
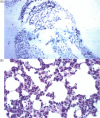
The mouse model was used to study the pathogenesis of equine herpesvirus type 1 (EHV-1) after primary and secondary intranasal infections. Within a few hours after infection, EHV-1 was found in nasal and olfactorial epithelium and sub-epithelial cells of the respiratory mucosa, but antigen-specific immune cells were never detected. Next to the lung, EHV-1 was transmitted early and directly to the brain, both via the olfactory route and the trigeminal nerve, but traces of degenerative or inflammatory processes were not detected there. In the lung, the immune cells residing or invading the parenchyma did not contain viral DNA or proteins. The primary immune response in the lungs was an alveolar and interstitial inflammation, dominated by the sequential appearance of neutrophils and macrophages, while the number of T and B lymphocytes remained unaltered. Within 24 hr after re-infection, lymphocytes accumulated around the blood vessels, outnumbering monocytes more than twofold, without neutrophils appearing. The lymphocytes comprised of little more B than T cells and the T cells were predominantly CD8+ cells. Those and B cells infiltrated the parenchyma. These results show the route of virus distribution and demonstrate the lack of antigen-specific immune cells in the lungs of mice after primary intranasal infection with EHV-1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber D. G., Greensill J., Killington R. A., Stokes A. Role of T-cells, virus neutralising antibodies and complement-mediated antibody lysis in the immune response against equine herpesvirus type-1 (EHV-1) infection of C3H (H-2k) and BALB/c (H-2d) mice. Res Vet Sci. 1995 Nov;59(3):205–213. doi: 10.1016/0034-5288(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Allen G. P., Bryans J. T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Awan A. R., Chong Y. C., Field H. J. The pathogenesis of equine herpesvirus type 1 in the mouse: a new model for studying host responses to the infection. J Gen Virol. 1990 May;71(Pt 5):1131–1140. doi: 10.1099/0022-1317-71-5-1131. [DOI] [PubMed] [Google Scholar]
- Baxi M. K., Borchers K., Bartels T., Schellenbach A., Baxi S., Field H. J. Molecular studies of the acute infection, latency and reactivation of equine herpesvirus-1 (EHV-1) in the mouse model. Virus Res. 1996 Jan;40(1):33–45. doi: 10.1016/0168-1702(95)01255-9. [DOI] [PubMed] [Google Scholar]
- Borchers K., Slater J. A nested PCR for the detection and differentiation of EHV-1 and EHV-4. J Virol Methods. 1993 Dec 31;45(3):331–336. doi: 10.1016/0166-0934(93)90117-a. [DOI] [PubMed] [Google Scholar]
- Burrows R., Goodridge D., Denyer M. S. Trials of an inactivated equid herpesvirus 1 vaccine: challenge with a subtype 1 virus. Vet Rec. 1984 Apr 14;114(15):369–374. doi: 10.1136/vr.114.15.369. [DOI] [PubMed] [Google Scholar]
- Crabb B. S., Studdert M. J. Equine herpesviruses 4 (equine rhinopneumonitis virus) and 1 (equine abortion virus). Adv Virus Res. 1995;45:153–190. doi: 10.1016/s0065-3527(08)60060-3. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Campbell D. L. Cell mediated immunity in equine herpesvirus type 1 infection I. In vitro lymphocyte blastogenesis and serum neutralization antibody in normal parturient and aborting mares. Can J Comp Med. 1977 Oct;41(4):404–408. [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Awan A. R., de la Fuente R. Reinfection and reactivation of equine herpesvirus-1 in the mouse. Arch Virol. 1992;123(3-4):409–419. doi: 10.1007/BF01317274. [DOI] [PubMed] [Google Scholar]
- Gibson J. S., Slater J. D., Awan A. R., Field H. J. Pathogenesis of equine herpesvirus-1 in specific pathogen-free foals: primary and secondary infections and reactivation. Arch Virol. 1992;123(3-4):351–366. doi: 10.1007/BF01317269. [DOI] [PubMed] [Google Scholar]
- Inazu M., Tsuha O., Kirisawa R., Kawakami Y., Iwai H. Equid herpesvirus 1 infection in mice. J Vet Med Sci. 1993 Feb;55(1):119–121. doi: 10.1292/jvms.55.119. [DOI] [PubMed] [Google Scholar]
- Kritas S. K., Nauwynck H. J., Pensaert M. B. Dissemination of wild-type and gC-, gE-and gI-deleted mutants of Aujeszky's disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995 Aug;76(Pt 8):2063–2066. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- Kritas S. K., Pensaert M. B., Mettenleiter T. C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994 Sep;75(Pt 9):2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- Kuper C. F., Koornstra P. J., Hameleers D. M., Biewenga J., Spit B. J., Duijvestijn A. M., van Breda Vriesman P. J., Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992 Jun;13(6):219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- Kydd J. H., Hannant D., Mumford J. A. Residence and recruitment of leucocytes to the equine lung after EHV-1 infection. Vet Immunol Immunopathol. 1996 Jun 15;52(1-2):15–26. doi: 10.1016/0165-2427(95)05533-9. [DOI] [PubMed] [Google Scholar]
- Kydd J. H., Smith K. C., Hannant D., Livesay G. J., Mumford J. A. Distribution of equid herpesvirus-1 (EHV-1) in respiratory tract associated lymphoid tissue: implications for cellular immunity. Equine Vet J. 1994 Nov;26(6):470–473. doi: 10.1111/j.2042-3306.1994.tb04052.x. [DOI] [PubMed] [Google Scholar]
- Scott J. C., Dutta S. K., Myrup A. C. In vivo harboring of equine herpesvirus-1 in leukocyte populations and subpopulations and their quantitation from experimentally infected ponies. Am J Vet Res. 1983 Jul;44(7):1344–1348. [PubMed] [Google Scholar]
- Slater J. D., Borchers K., Thackray A. M., Field H. J. The trigeminal ganglion is a location for equine herpesvirus 1 latency and reactivation in the horse. J Gen Virol. 1994 Aug;75(Pt 8):2007–2016. doi: 10.1099/0022-1317-75-8-2007. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Welch H. M., Bridges C. G., Lyon A. M., Griffiths L., Edington N. Latent equid herpesviruses 1 and 4: detection and distinction using the polymerase chain reaction and co-cultivation from lymphoid tissues. J Gen Virol. 1992 Feb;73(Pt 2):261–268. doi: 10.1099/0022-1317-73-2-261. [DOI] [PubMed] [Google Scholar]