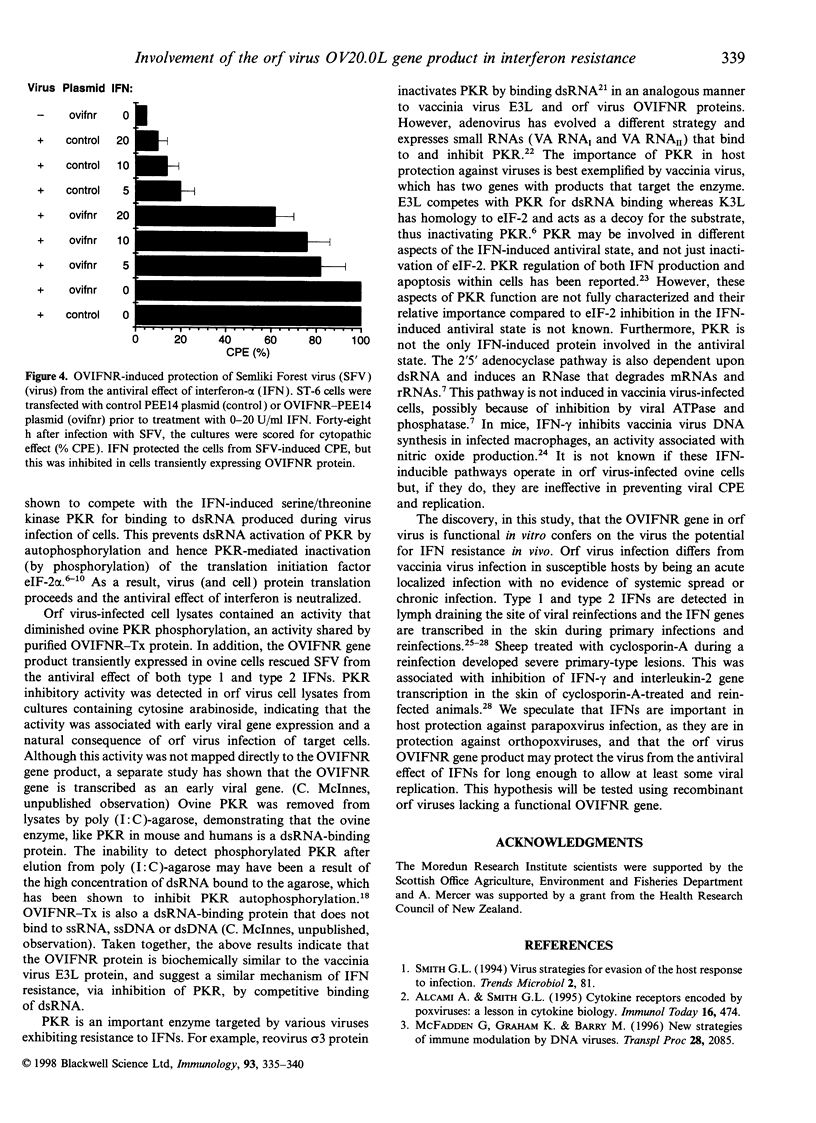
Abstract
The parapoxvirus orf virus was resistant to type 1 (IFN-alpha) and type 2 (IFN-gamma) interferons in cultures of ovine cells. The recently identified orf virus OV20.0L gene exhibits 31% predicted amino acid identity to the vaccinia virus E3L interferon-resistance gene, and is referred to as the (putative) orf virus interferon-resistance gene (OVIFNR). The objective of this study was to determine whether OVIFNR was involved in interferon resistance. Recombinant OVIFNR as a thioredoxin fusion protein (OVIFNR-Tx) inhibited the activation (by autophosphorylation) of an interferon-inducible, double-stranded (ds) RNA-dependent kinase (PKR) of sheep, which was shown to bind dsRNA (poly I:C). PKR in other species is involved in the inhibition of protein synthesis as part of the antiviral state in infected cells. Virus-infected cell lysates, but not control lysates, from cells grown in the presence of cytosine arabinoside also contained PKR inhibitory activity, which indicated that the inhibitory activity was associated with early viral gene expression. Significantly, the OVIFNR gene expressed in interferon-treated ovine fibroblasts protected the unrelated Semliki Forest virus from the antiviral effect of both type 1 and type 2 interferons. Taken together, the results indicate that the OVIFNR gene functions as an interferon-resistance gene, the product of which inhibits PKR in a similar way to the vaccinia virus E3L gene product.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Alcamí A., Smith G. L. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol Today. 1995 Oct;16(10):474–478. doi: 10.1016/0167-5699(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Watson J. C., Jacobs B. L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockett M. I., Bebbington C. R., Yarranton G. T. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology (N Y) 1990 Jul;8(7):662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Davies M. V., Chang H. W., Jacobs B. L., Kaufman R. J. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J Virol. 1993 Mar;67(3):1688–1692. doi: 10.1128/jvi.67.3.1688-1692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. V., Elroy-Stein O., Jagus R., Moss B., Kaufman R. J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol. 1992 Apr;66(4):1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der S. D., Lau A. S. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der S. D., Yang Y. L., Weissmann C., Williams B. R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entrican G., Haig D. M., Norval M. Identification of ovine interferons: differential activities derived from fibroblast and lymphoid cells. Vet Immunol Immunopathol. 1989 Jun;21(2):187–195. doi: 10.1016/0165-2427(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Fleming S. B., McCaughan C. A., Andrews A. E., Nash A. D., Mercer A. A. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J Virol. 1997 Jun;71(6):4857–4861. doi: 10.1128/jvi.71.6.4857-4861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S. P., Jones G. E., MacLean M., Livingstone M., Entrican G. Recombinant ovine interferon gamma inhibits the multiplication of Chlamydia psittaci in ovine cells. J Comp Pathol. 1995 Feb;112(2):185–195. doi: 10.1016/s0021-9975(05)80060-x. [DOI] [PubMed] [Google Scholar]
- Haig D. M., Deane D. L., Myatt N., Thomson J., Entrican G., Rothel J., Reid H. W. The activation status of ovine CD45R+ and CD45R- efferent lymph T cells after orf virus reinfection. J Comp Pathol. 1996 Aug;115(2):163–174. doi: 10.1016/s0021-9975(96)80038-7. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McInnes C. J., Hutchison G., Seow H. F., Reid H. W. Cyclosporin A abrogates the acquired immunity to cutaneous reinfection with the parapoxvirus orf virus. Immunology. 1996 Dec;89(4):524–531. doi: 10.1046/j.1365-2567.1996.940967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. M., McInnes C., Deane D., Reid H., Mercer A. The immune and inflammatory response to orf virus. Comp Immunol Microbiol Infect Dis. 1997 Jun;20(3):197–204. doi: 10.1016/s0147-9571(96)00045-8. [DOI] [PubMed] [Google Scholar]
- Imani F., Jacobs B. L. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7887–7891. doi: 10.1073/pnas.85.21.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Lyttle D. J., Fraser K. M., Fleming S. B., Mercer A. A., Robinson A. J. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J Virol. 1994 Jan;68(1):84–92. doi: 10.1128/jvi.68.1.84-92.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden G., Graham K., Barry M. New strategies of immune modulation by DNA viruses. Transplant Proc. 1996 Aug;28(4):2085–2088. [PubMed] [Google Scholar]
- Melková Z., Esteban M. Interferon-gamma severely inhibits DNA synthesis of vaccinia virus in a macrophage cell line. Virology. 1994 Feb;198(2):731–735. doi: 10.1006/viro.1994.1087. [DOI] [PubMed] [Google Scholar]
- Mercer A. A., Fraser K., Barns G., Robinson A. J. The structure and cloning of orf virus DNA. Virology. 1987 Mar;157(1):1–12. doi: 10.1016/0042-6822(87)90307-2. [DOI] [PubMed] [Google Scholar]
- Mercer A. A., Lyttle D. J., Whelan E. M., Fleming S. B., Sullivan J. T. The establishment of a genetic map of orf virus reveals a pattern of genomic organization that is highly conserved among divergent poxviruses. Virology. 1995 Oct 1;212(2):698–704. doi: 10.1006/viro.1995.1527. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991 Jul;183(1):1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- Smith G. L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994 Mar;2(3):81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]