Abstract
By virtue of their strong bias towards production of interferon-gamma (IFN-gamma), CD8+ T cells have the potential to promote the development of type 1 immune responses. We have previously shown that the CD4+ T-cell response to immunization with the protein antigen keyhole limpet haemocyanin (KLH) has a mixed interleukin-4 (IL-4)/IFN-gamma production profile. Here we show that this immunization regimen also stimulates accumulation in the draining lymph nodes of CD8+ T cells, which preferentially contain IFN-gamma mRNA ex vivo and secrete IFN-gamma protein in vitro. This provides a model to test whether CD8+ cell-derived IFN-gamma participates in the normal control of the immune response to a non-viable exogenous antigen. To investigate regulation of the anti-KLH response by the CD8+ population or IFN-gamma produced by this or other cell types, mice were administered depleting antibodies. Depletion of CD8+ cells had no effect on the frequency of clonogenic KLH-specific CD4+ T cells, the IL-4/IFN-gamma profiles of their progeny, or the isotype profiles of the serum antibody response to KLH. In contrast, IFN-gamma neutralization diminished cell accumulation in the lymph nodes and reduced both the frequency of KLH-specific CD4+ T cells that gave rise to IFN-gamma-producing clones and serum titres of KLH-specific IgG2a and IgG3. Therefore, despite the potential for cross-regulation, the CD4+ T-cell response to this immunogen is independent of the IFN-gamma-skewed CD8+ response.
Full text
PDF

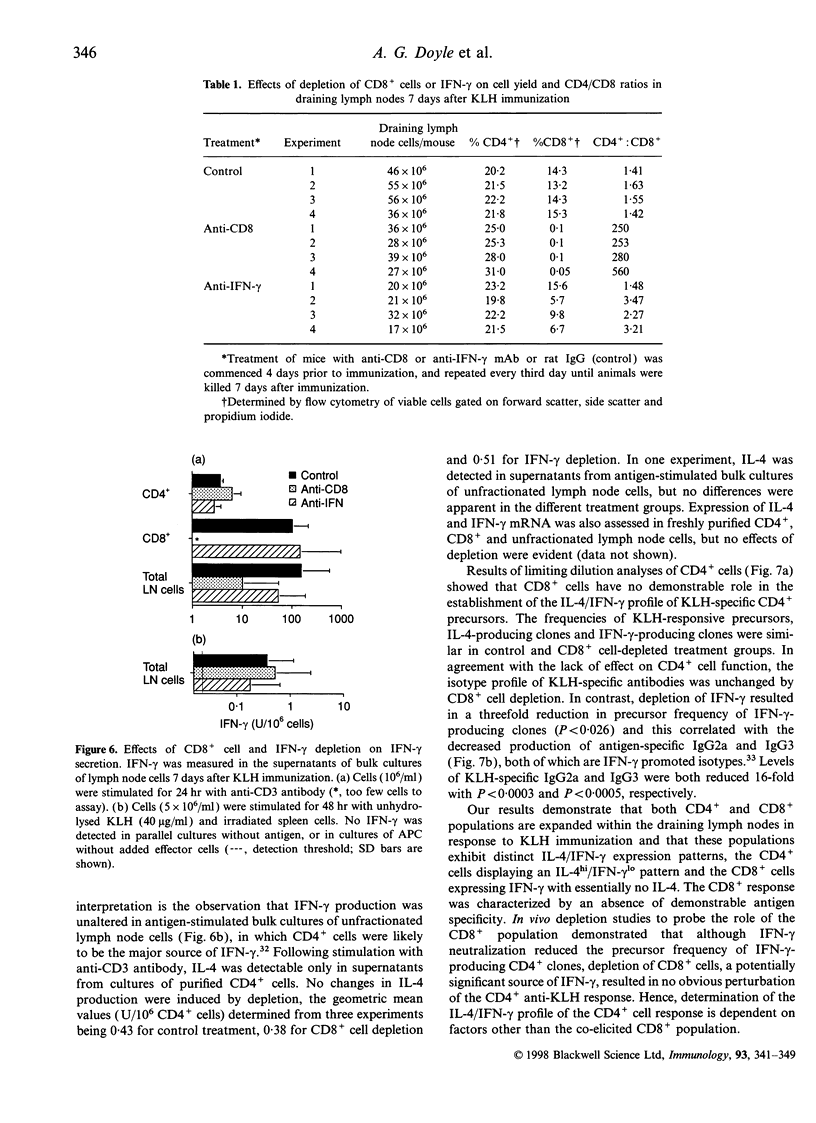
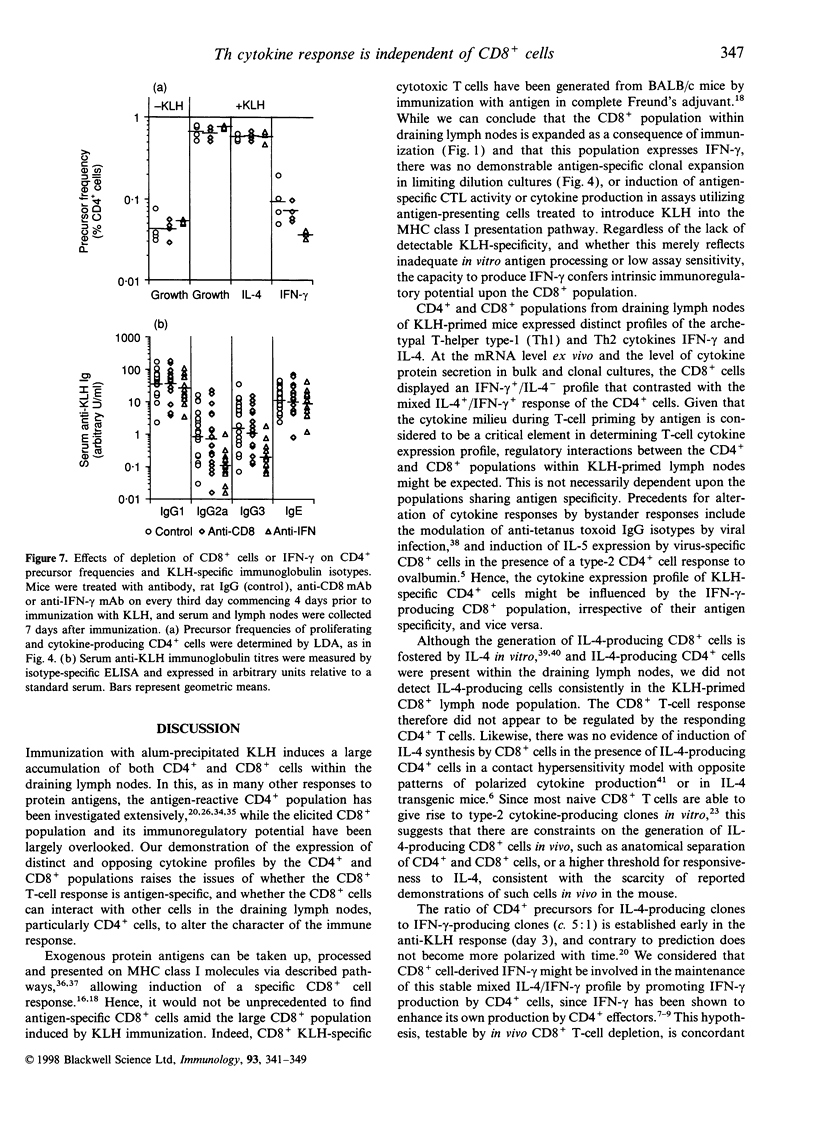


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarth N., Brown L., Jackson D., Kelso A. Novel features of the respiratory tract T-cell response to influenza virus infection: lung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J Virol. 1994 Nov;68(11):7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995 Sep 1;182(3):639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L. M., Duncan D. D., Tonkonogy S., Swain S. L. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med. 1991 Sep 1;174(3):547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. Y., DeBruyne L. A., Goodman R. E., Eichwald E. J., Bishop D. K. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995 Apr 27;59(8):1155–1161. [PubMed] [Google Scholar]
- Chen W., Carbone F. R., McCluskey J. Electroporation and commercial liposomes efficiently deliver soluble protein into the MHC class I presentation pathway. Priming in vitro and in vivo for class I-restricted recognition of soluble antigen. J Immunol Methods. 1993 Mar 15;160(1):49–57. doi: 10.1016/0022-1759(93)90007-t. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Vink A., van Snick J. Virally induced modulation of murine IgG antibody subclasses. J Exp Med. 1988 Dec 1;168(6):2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A. J., Erard F., Bertrand C., Walti S., Pircher H., Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. 1995 Mar 1;181(3):1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M., Carter L., Swain S. L., Dutton R. W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994 Nov 1;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez D., Lee T. H., Kemeny D. M. Ricin enhances IgE responses by inhibiting a subpopulation of early-activated IgE regulatory CD8+ T cells. Immunology. 1993 Feb;78(2):226–236. [PMC free article] [PubMed] [Google Scholar]
- Erard F., Wild M. T., Garcia-Sanz J. A., Le Gros G. Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science. 1993 Jun 18;260(5115):1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- Erb K. J., Le Gros G. The role of Th2 type CD4+ T cells and Th2 type CD8+ T cells in asthma. Immunol Cell Biol. 1996 Apr;74(2):206–208. doi: 10.1038/icb.1996.29. [DOI] [PubMed] [Google Scholar]
- Germann T., Guckes S., Bongartz M., Dlugonska H., Schmitt E., Kolbe L., Kölsch E., Podlaski F. J., Gately M. K., Rüde E. Administration of IL-12 during ongoing immune responses fails to permanently suppress and can even enhance the synthesis of antigen-specific IgE. Int Immunol. 1995 Oct;7(10):1649–1657. doi: 10.1093/intimm/7.10.1649. [DOI] [PubMed] [Google Scholar]
- Groves P. L., Pech M. H., Troutt A. B., Kelso A. Limiting dilution analysis reveals the precursors of interleukin-4-producing CD4+ cells induced by protein immunization. Immunology. 1994 Sep;83(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Kelso A., Groves P. A single peripheral CD8+ T cell can give rise to progeny expressing type 1 and/or type 2 cytokine genes and can retain its multipotentiality through many cell divisions. Proc Natl Acad Sci U S A. 1997 Jul 22;94(15):8070–8075. doi: 10.1073/pnas.94.15.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Groves P., Troutt A. B., Pech M. H. Rapid establishment of a stable IL-4/IFN-gamma production profile in the antigen-specific CD4+ T cell response to protein immunization. Int Immunol. 1994 Oct;6(10):1515–1523. doi: 10.1093/intimm/6.10.1515. [DOI] [PubMed] [Google Scholar]
- Kelso A., Metcalf D. Clonal heterogeneity in colony stimulating factor production by murine T lymphocytes. J Cell Physiol. 1985 Apr;123(1):101–110. doi: 10.1002/jcp.1041230115. [DOI] [PubMed] [Google Scholar]
- Kelso A., Troutt A. B., Maraskovsky E., Gough N. M., Morris L., Pech M. H., Thomson J. A. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol Rev. 1991 Oct;123:85–114. doi: 10.1111/j.1600-065x.1991.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Kraal G., Schornagel K., Savelkoul H., Maruyama T. Activation of high endothelial venules in peripheral lymph nodes. The involvement of interferon-gamma. Int Immunol. 1994 Aug;6(8):1195–1201. doi: 10.1093/intimm/6.8.1195. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S. E., Warrier R. R., Carvajal D. M., Wu C. Y., Ferrante J., Stewart C., Sarmiento U., Faherty D. A., Gately M. K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996 May;4(5):471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Troutt A. B., Kelso A. Co-engagement of CD3 with LFA-1 or ICAM-1 adhesion molecules enhances the frequency of activation of single murine CD4+ and CD8+ T cells and induces synthesis of IL-3 and IFN-gamma but not IL-4 or IL-6. Int Immunol. 1992 Apr;4(4):475–485. doi: 10.1093/intimm/4.4.475. [DOI] [PubMed] [Google Scholar]
- McMenamin C., Holt P. G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993 Sep 1;178(3):889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C. C., Hewlett L. J., Prescott A. R., Shastri N., Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995 Dec;3(6):783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Oliveira S. C., Splitter G. A. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995 Sep;25(9):2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996 May 15;156(10):3721–3726. [PubMed] [Google Scholar]
- Rottenberg M. E., Sporrong L., Persson I., Wigzell H., Orn A. Cytokine gene expression during infection of mice lacking CD4 and/or CD8 with Trypanosoma cruzi. Scand J Immunol. 1995 Feb;41(2):164–170. doi: 10.1111/j.1365-3083.1995.tb03549.x. [DOI] [PubMed] [Google Scholar]
- Rus V., Svetic A., Nguyen P., Gause W. C., Via C. S. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J Immunol. 1995 Sep 1;155(5):2396–2406. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Sander B., Höidén I., Andersson U., Möller E., Abrams J. S. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. Cytokine detection by immunoassay and intracellular immunostaining. J Immunol Methods. 1993 Dec 3;166(2):201–214. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Seder R. A., Boulay J. L., Finkelman F., Barbier S., Ben-Sasson S. Z., Le Gros G., Paul W. E. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992 Mar 15;148(6):1652–1656. [PubMed] [Google Scholar]
- Sprent J. T and B memory cells. Cell. 1994 Jan 28;76(2):315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Karasuyama H., Garner A. M. Cytotoxic T lymphocytes against a soluble protein. Nature. 1987 Oct 1;329(6138):449–451. doi: 10.1038/329449a0. [DOI] [PubMed] [Google Scholar]
- Szabo S. J., Dighe A. S., Gubler U., Murphy K. M. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997 Mar 3;185(5):817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990 Jul 1;145(1):68–77. [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Noelle R. J., Roy M., Shepherd D. M., Aruffo A., Ledbetter J. A., Boersma W. J., Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993 Nov 1;178(5):1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner C. A., Güler M. L., Macatonia S. E., O'Garra A., Murphy K. M. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996 Feb 15;156(4):1442–1447. [PubMed] [Google Scholar]
- Westermann J., Persin S., Matyas J., van der Meide P., Pabst R. IFN-gamma influences the migration of thoracic duct B and T lymphocyte subsets in vivo. Random increase in disappearance from the blood and differential decrease in reappearance in the lymph. J Immunol. 1993 May 1;150(9):3843–3852. [PubMed] [Google Scholar]
- Xu H., DiIulio N. A., Fairchild R. L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996 Mar 1;183(3):1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yefenof E., Zehavi-Feferman R., Guy R. Control of primary and secondary antibody responses by cytotoxic T lymphocytes specific for a soluble antigen. Eur J Immunol. 1990 Aug;20(8):1849–1853. doi: 10.1002/eji.1830200833. [DOI] [PubMed] [Google Scholar]