Abstract
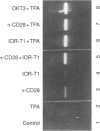
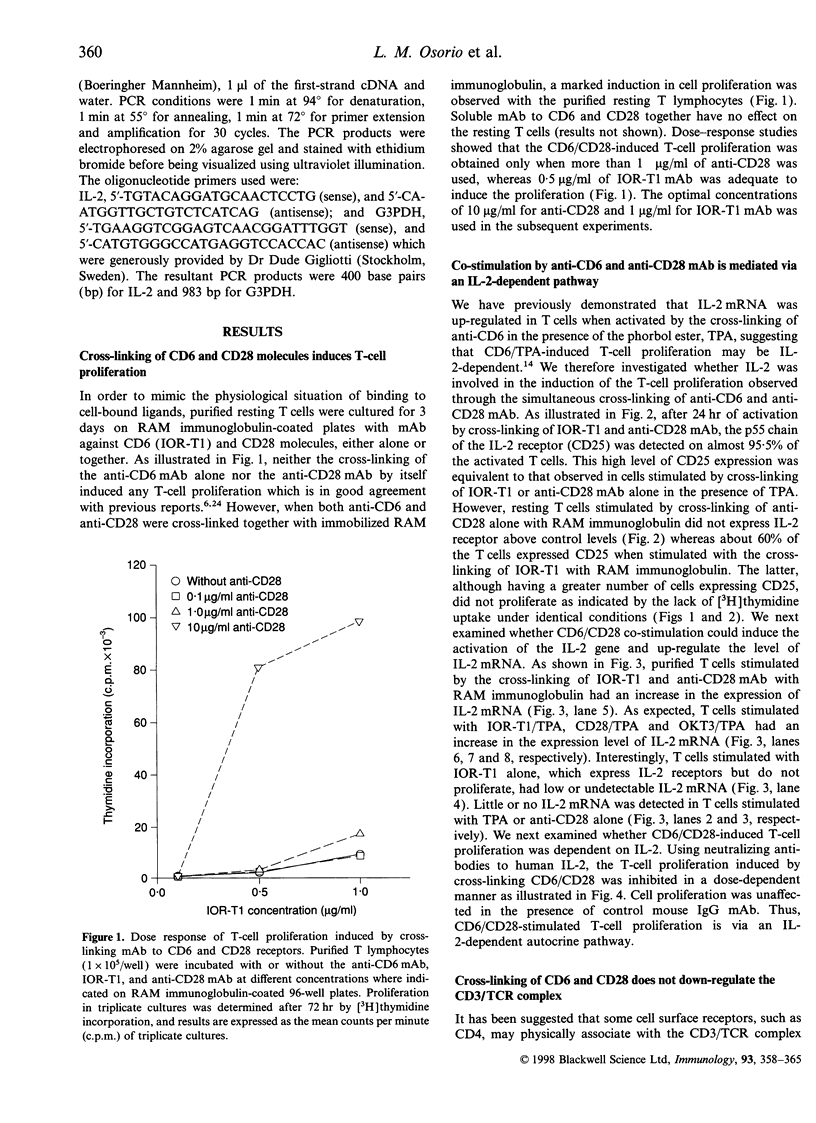
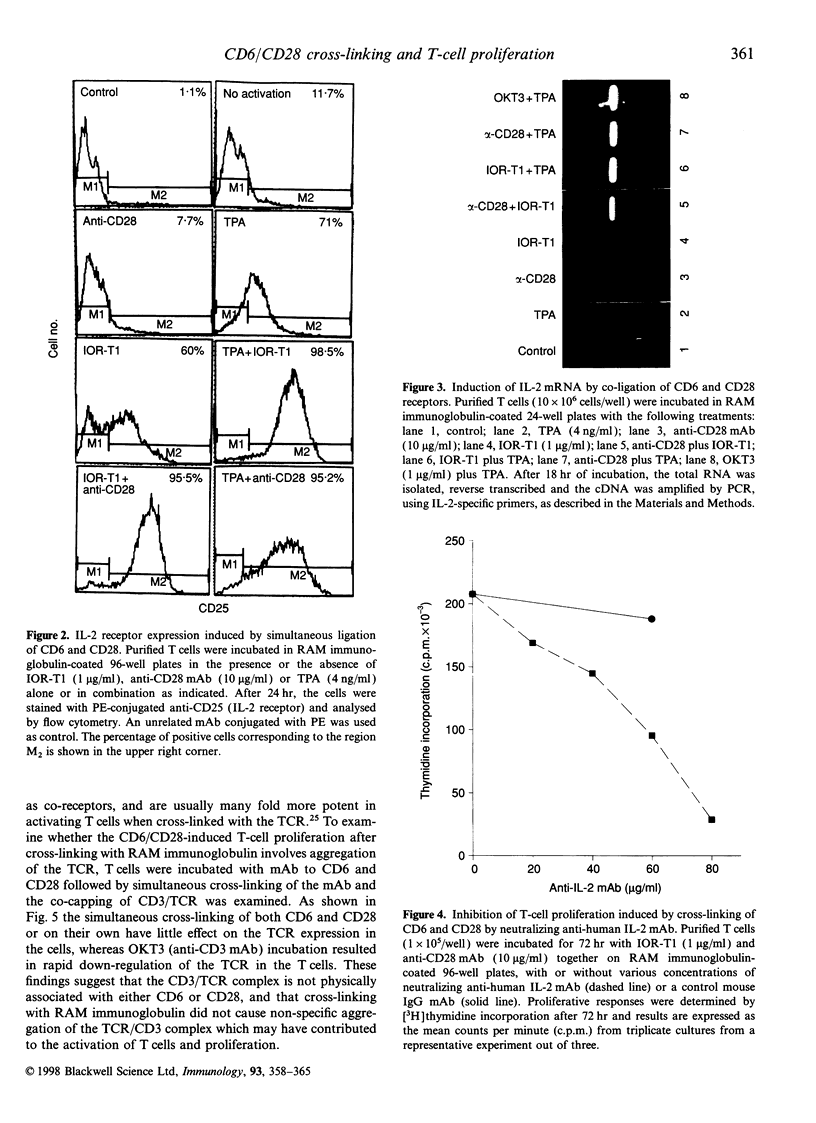
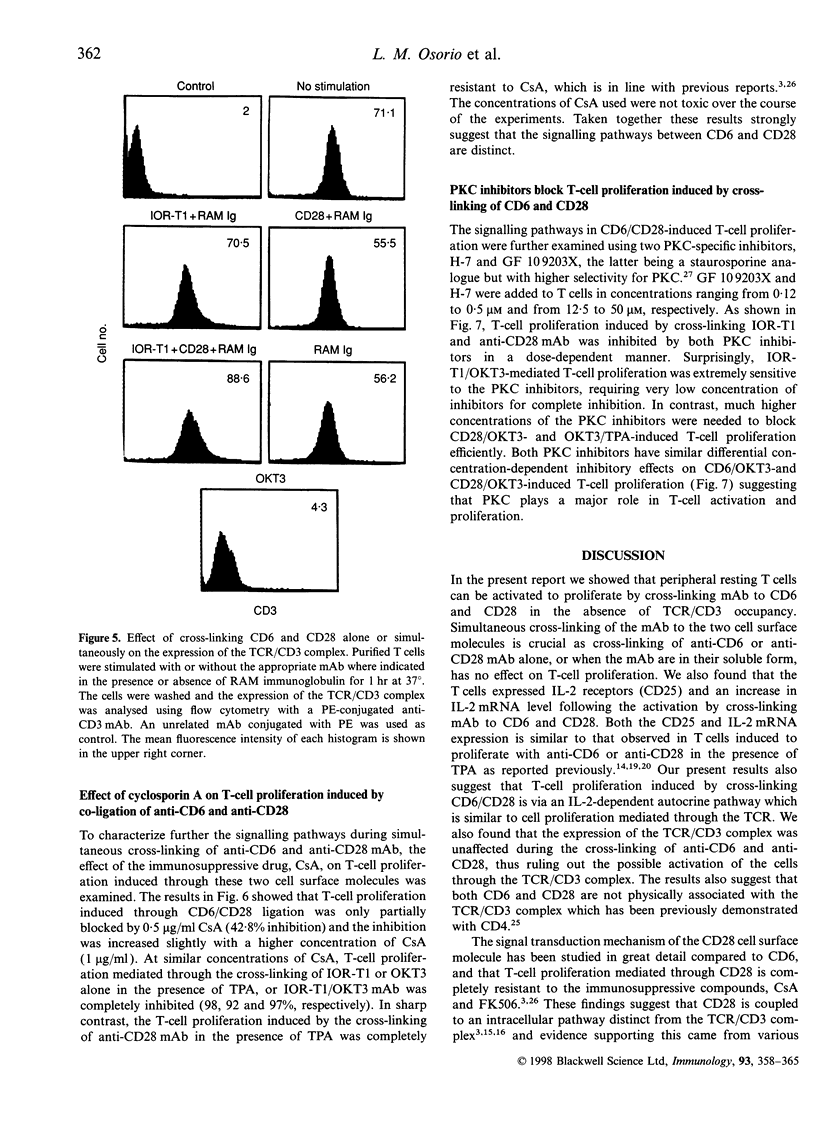
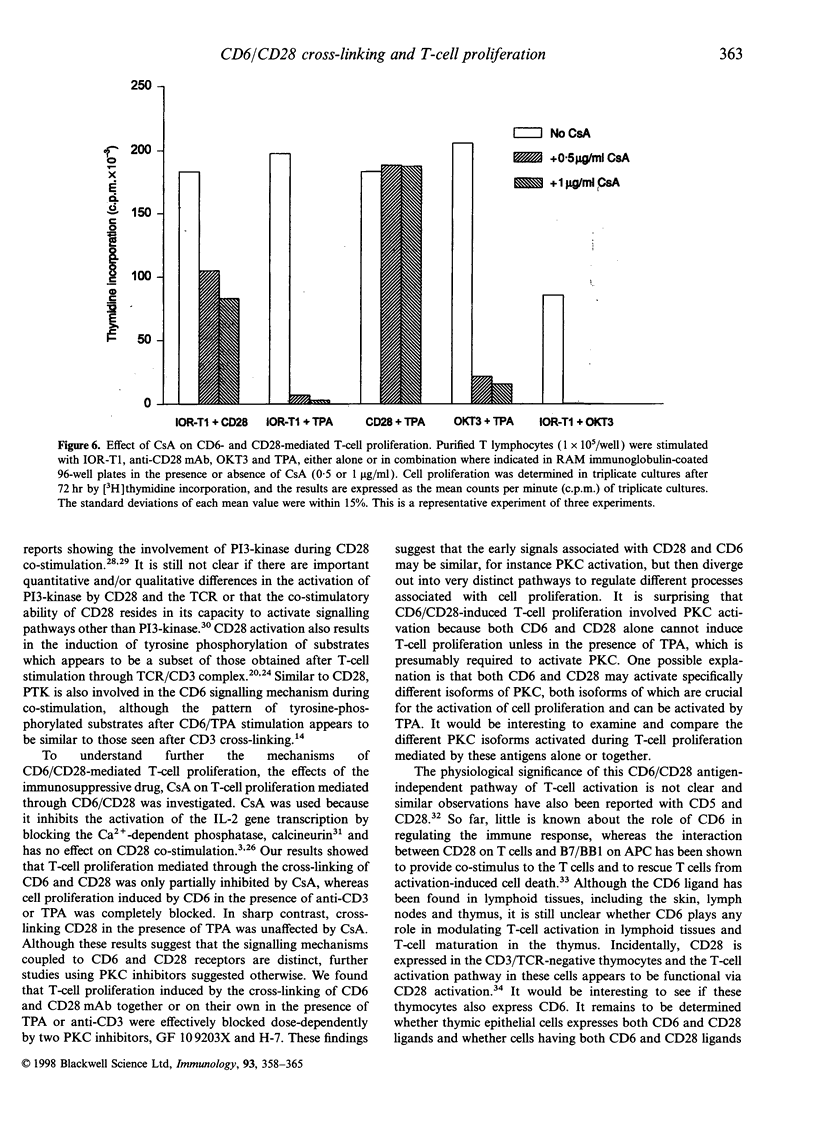
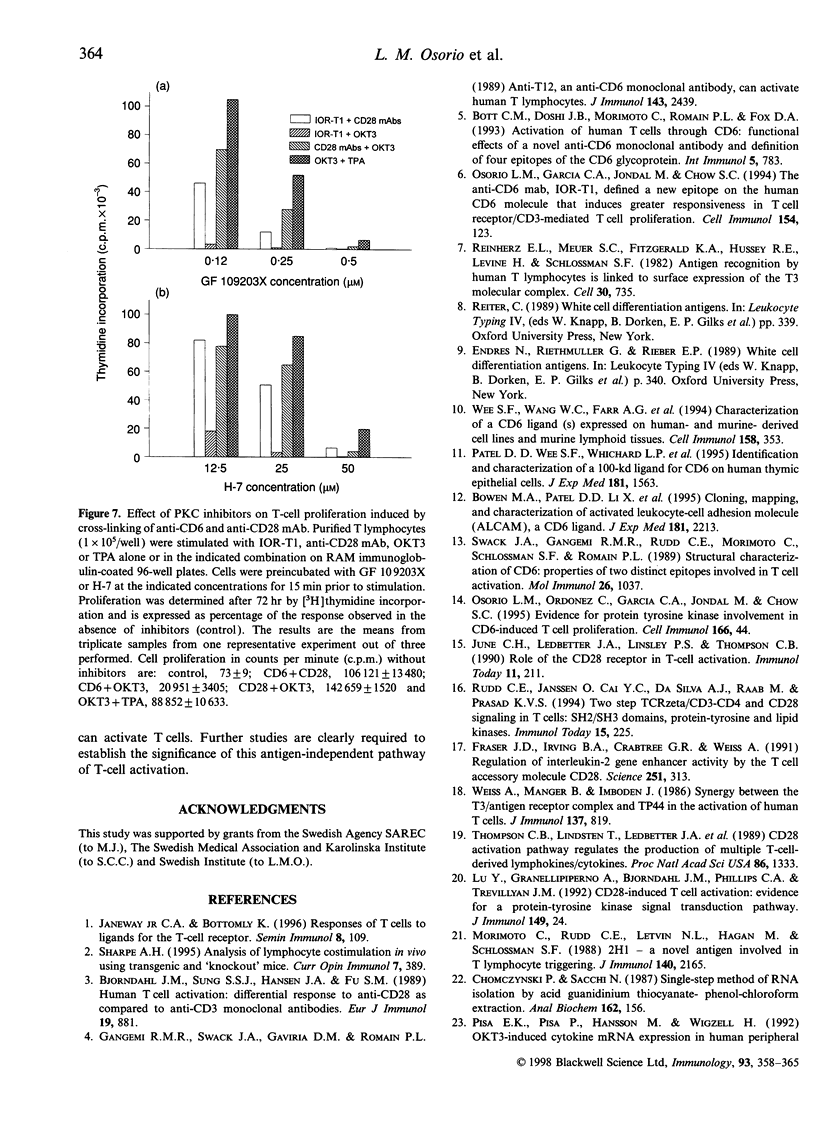
In the present study, we showed that simultaneous ligation of the monoclonal antibodies (mAb) against CD6 and CD28 induces T-cell proliferation in purified resting T lymphocytes in the absence of T-cell receptor (TCR) occupancy. No cell proliferation was observed when the mAb were cross-linked alone or used simultaneously in the soluble form. T-cell proliferation mediated through CD6/CD28 is accompanied by the up-regulation of interleukin-2 (IL-2) mRNA and expression of IL-2 receptors on the cell surface. In the presence of IL-2-neutralizing mAb the proliferative response of the T cell induced through CD6/CD28 was inhibited dose dependently. Cross-linking mAb to CD6 and CD28 alone or together did not down-regulate the CD3/TCR complex. T-cell proliferation mediated through CD6/CD28 was only partially blocked by the immunosuppressive drug, cyclosporin A (CsA), whereas anti-CD28-induced T-cell proliferation in the presence of the phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA), was unaffected. In sharp contrast T-cell proliferation mediated by anti-CD6 in the presence of TPA was efficiently blocked by CsA. In addition, two protein kinase C (PKC) inhibitors, GF 109203X and H-7 dose-dependently inhibited T-cell proliferation mediated through CD6/CD28, suggesting that PKC activation may be involved. Furthermore, there was a marked differential dose-dependent inhibitory effect of the PKC inhibitors on T-cell proliferation mediated by the co-ligation of anti-CD6 or anti-CD28 in the presence of anti-CD3, with the former being more sensitive to PKC inhibition. Taken collectively, our results suggest that T-cell activation can occur through an antigen-independent pathway by cross-linking the accessory molecules, CD6 and CD28, and that these two cell surface antigens may have distinct signalling pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorndahl J. M., Sung S. S., Hansen J. A., Fu S. M. Human T cell activation: differential response to anti-CD28 as compared to anti-CD3 monoclonal antibodies. Eur J Immunol. 1989 May;19(5):881–887. doi: 10.1002/eji.1830190515. [DOI] [PubMed] [Google Scholar]
- Bott C. M., Doshi J. B., Morimoto C., Romain P. L., Fox D. A. Activation of human T cells through CD6: functional effects of a novel anti-CD6 monoclonal antibody and definition of four epitopes of the CD6 glycoprotein. Int Immunol. 1993 Jul;5(7):783–792. doi: 10.1093/intimm/5.7.783. [DOI] [PubMed] [Google Scholar]
- Bowen M. A., Patel D. D., Li X., Modrell B., Malacko A. R., Wang W. C., Marquardt H., Neubauer M., Pesando J. M., Francke U. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995 Jun 1;181(6):2213–2220. doi: 10.1084/jem.181.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Courage C., Budworth J., Gescher A. Comparison of ability of protein kinase C inhibitors to arrest cell growth and to alter cellular protein kinase C localisation. Br J Cancer. 1995 Apr;71(4):697–704. doi: 10.1038/bjc.1995.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage C., Snowden R., Gescher A. Differential effects of staurosporine analogues on cell cycle, growth and viability in A549 cells. Br J Cancer. 1996 Oct;74(8):1199–1205. doi: 10.1038/bjc.1996.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani U., Shaw A., al-Ramadi B. K., Kubo R. T., Janeway C. A., Jr Physical association of CD4 with the T cell receptor. J Immunol. 1992 Feb 1;148(3):678–688. [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Gangemi R. M., Swack J. A., Gaviria D. M., Romain P. L. Anti-T12, an anti-CD6 monoclonal antibody, can activate human T lymphocytes. J Immunol. 1989 Oct 15;143(8):2439–2447. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Kunz J., Hall M. N. Cyclosporin A, FK506 and rapamycin: more than just immunosuppression. Trends Biochem Sci. 1993 Sep;18(9):334–338. doi: 10.1016/0968-0004(93)90069-y. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Imboden J. B., Schieven G. L., Grosmaire L. S., Rabinovitch P. S., Lindsten T., Thompson C. B., June C. H. CD28 ligation in T-cell activation: evidence for two signal transduction pathways. Blood. 1990 Apr 1;75(7):1531–1539. [PubMed] [Google Scholar]
- Morimoto C., Rudd C. E., Letvin N. L., Hagan M., Schlossman S. F. 2H1--a novel antigen involved in T lymphocyte triggering. J Immunol. 1988 Apr 1;140(7):2165–2170. [PubMed] [Google Scholar]
- Osorio L. M., Garcia C. A., Jondal M., Chow S. C. The anti-CD6 mAb, IOR-T1, defined a new epitope on the human CD6 molecule that induces greater responsiveness in T cell receptor/CD3-mediated T cell proliferation. Cell Immunol. 1994 Mar;154(1):123–133. doi: 10.1006/cimm.1994.1062. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Wee S. F., Whichard L. P., Bowen M. A., Pesando J. M., Aruffo A., Haynes B. F. Identification and characterization of a 100-kD ligand for CD6 on human thymic epithelial cells. J Exp Med. 1995 Apr 1;181(4):1563–1568. doi: 10.1084/jem.181.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Janssen O., Cai Y. C., da Silva A. J., Raab M., Prasad K. V. Two-step TCR zeta/CD3-CD4 and CD28 signaling in T cells: SH2/SH3 domains, protein-tyrosine and lipid kinases. Immunol Today. 1994 May;15(5):225–234. doi: 10.1016/0167-5699(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H. Analysis of lymphocyte costimulation in vivo using transgenic and 'knockout' mice. Curr Opin Immunol. 1995 Jun;7(3):389–395. doi: 10.1016/0952-7915(95)80115-4. [DOI] [PubMed] [Google Scholar]
- Swack J. A., Gangemi R. M., Rudd C. E., Morimoto C., Schlossman S. F., Romain P. L. Structural characterization of CD6: properties of two distinct epitopes involved in T cell activation. Mol Immunol. 1989 Nov;26(11):1037–1049. doi: 10.1016/0161-5890(89)90068-0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt K. E., Shi J., Gibson S., Segal L. G., Mills G. B., Imboden J. B. CD28 delivers costimulatory signals independently of its association with phosphatidylinositol 3-kinase. J Immunol. 1995 Nov 15;155(10):4702–4710. [PubMed] [Google Scholar]
- Verwilghen J., Vandenberghe P., Wallays G., de Boer M., Anthony N., Panayi G. S., Ceuppens J. L. Simultaneous ligation of CD5 and CD28 on resting T lymphocytes induces T cell activation in the absence of T cell receptor/CD3 occupancy. J Immunol. 1993 Feb 1;150(3):835–846. [PubMed] [Google Scholar]
- Ward S. G., June C. H., Olive D. PI 3-kinase: a pivotal pathway in T-cell activation? Immunol Today. 1996 Apr;17(4):187–197. doi: 10.1016/0167-5699(96)80618-9. [DOI] [PubMed] [Google Scholar]
- Wee S., Wang W. C., Farr A. G., Nelson A. J., Patel D. D., Haynes B. F., Linsley P. S., Aruffo A. Characterization of a CD6 ligand(s) expressed on human- and murine-derived cell lines and murine lymphoid tissues. Cell Immunol. 1994 Oct 15;158(2):353–364. doi: 10.1006/cimm.1994.1282. [DOI] [PubMed] [Google Scholar]
- Weiss A., Manger B., Imboden J. Synergy between the T3/antigen receptor complex and Tp44 in the activation of human T cells. J Immunol. 1986 Aug 1;137(3):819–825. [PubMed] [Google Scholar]
- Zocchi M. R., Poggi A. Activation of CD3/TCR negative human thymocytes via CD28 molecule. Cell Immunol. 1991 Aug;136(1):105–112. doi: 10.1016/0008-8749(91)90385-o. [DOI] [PubMed] [Google Scholar]