Abstract
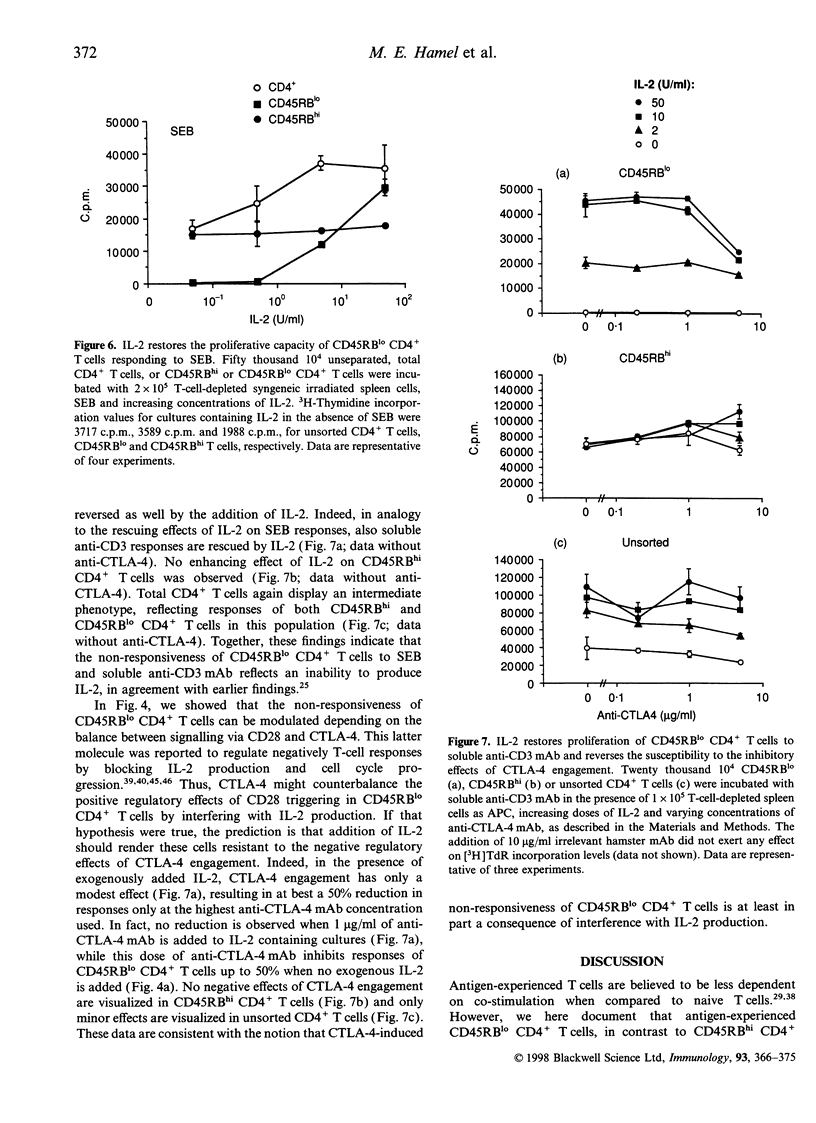
We recently reported that previously activated T cells, irrespective of the nature of the first stimulus they encountered, are unable to respond to Staphylococcal enterotoxin B (SEB), nor to soluble anti-CD3 monoclonal antibody (mAb) presented by splenic antigen-presenting cells (APC). Such previously activated T cells are, however, fully capable of responding to plate-bound anti-CD3 plus splenic APC. These data suggest differential integration of the T-cell receptor (TCR) and co-stimulatory signalling pathways in naive versus antigen-experienced T cells. Consistent with this hypothesis, anti-CD28 mAb restores the proliferative capacity of resting ex vivo CD45RBlo CD4+ T cells (representing previously activated T cells) to both soluble anti-CD3 mAb and SEB. Interestingly, mAb-mediated engagement of cytotoxic T-lymphocyte antigen-4 (CTLA-4) completely negates the rescue effects mediated by anti-CD28 mAb in CD45RBlo cells. Nevertheless, the non-responsiveness of CD45RBlo CD4+ T cells cannot be reversed by anti-CTLA-4 Fab fragments, indicating that it is not related to negative regulatory effects of CTLA-4 engagement itself. Interestingly, the addition of interleukin-2 (IL-2) restores the proliferative capacity of CD45RBlo CD4+ T cells to SEB and soluble anti-CD3 mAb. Moreover, when rescued by IL-2, the cells are less susceptible to the negative regulatory effects of CTLA-4 engagement. Together, these findings suggest that the non-responsiveness of CD45RBlo CD4+ T cells to certain stimuli may be related to inadequate TCR signalling, primarily affecting IL-2 production.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Birkeland M. L., Johnson P., Trowbridge I. S., Puré E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., Minn A. J., Noel P. J., June C. H., Accavitti M. A., Lindsten T., Thompson C. B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995 Jul;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Croft M., Swain S. L. T-cell memory: new perspectives. Immunol Today. 1993 May;14(5):197–199. doi: 10.1016/0167-5699(93)90161-D. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Duncan D. D., Yoshimoto K., Swain S. L. Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J Immunol. 1993 Apr 15;150(8 Pt 1):3119–3130. [PubMed] [Google Scholar]
- Chambers C. A., Allison J. P. Co-stimulation in T cell responses. Curr Opin Immunol. 1997 Jun;9(3):396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- Croft M., Bradley L. M., Swain S. L. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994 Mar 15;152(6):2675–2685. [PubMed] [Google Scholar]
- Croft M., Duncan D. D., Swain S. L. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992 Nov 1;176(5):1431–1437. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani U., Luqman M., Rojo J., Yagi J., Baron J. L., Woods A., Janeway C. A., Jr, Bottomly K. Molecular associations on the T cell surface correlate with immunological memory. Eur J Immunol. 1990 Oct;20(10):2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- Dianzani U., Redoglia V., Malavasi F., Bragardo M., Pileri A., Janeway C. A., Jr, Bottomly K. Isoform-specific associations of CD45 with accessory molecules in human T lymphocytes. Eur J Immunol. 1992 Feb;22(2):365–371. doi: 10.1002/eji.1830220212. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Engel P., Gribben J. G., Freeman G. J., Zhou L. J., Nozawa Y., Abe M., Nadler L. M., Wakasa H., Tedder T. F. The B7-2 (B70) costimulatory molecule expressed by monocytes and activated B lymphocytes is the CD86 differentiation antigen. Blood. 1994 Sep 1;84(5):1402–1407. [PubMed] [Google Scholar]
- Ericsson P. O., Orchansky P. L., Carlow D. A., Teh H. S. Differential activation of phospholipase C-gamma 1 and mitogen-activated protein kinase in naive and antigen-primed CD4 T cells by the peptide/MHC ligand. J Immunol. 1996 Mar 15;156(6):2045–2053. [PubMed] [Google Scholar]
- Farber D. L., Luqman M., Acuto O., Bottomly K. Control of memory CD4 T cell activation: MHC class II molecules on APCs and CD4 ligation inhibit memory but not naive CD4 T cells. Immunity. 1995 Mar;2(3):249–259. doi: 10.1016/1074-7613(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Hathcock K. S., Laszlo G., McKnight A. J., Kim J., Du L., Lombard D. B. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993 Nov 5;262(5135):907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Segil J. M., Lee G., Whitman J. F., Nadler L. M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989 Oct 15;143(8):2714–2722. [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravestein L. A., Nieland J. D., Kruisbeek A. M., Borst J. Novel mAbs reveal potent co-stimulatory activity of murine CD27. Int Immunol. 1995 Apr;7(4):551–557. doi: 10.1093/intimm/7.4.551. [DOI] [PubMed] [Google Scholar]
- Hamel M. E., Eynon E. E., Savelkoul H. F., van Oudenaren A., Kruisbeek A. M. Activation and re-activation potential of T cells responding to staphylococcal enterotoxin B. Int Immunol. 1995 Jul;7(7):1065–1077. doi: 10.1093/intimm/7.7.1065. [DOI] [PubMed] [Google Scholar]
- Hamel M. E., Leupers T. J., Kruisbeek A. M. Nonresponsiveness and susceptibility to CTLA-4 of antigen-exposed CD4 T cells are not regulated by the Bcl-2 family of apoptotic mediators, but can be restored by IL-2. Thymus. 1997;24(4):259–277. [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Hayden K. A., Tough D. F., Webb S. R. In vivo response of mature T cells to Mlsa antigens. Long-term progeny of dividing cells include cells with a naive phenotype. J Immunol. 1996 Jan 1;156(1):48–55. [PubMed] [Google Scholar]
- Hintzen R. Q., Lens S. M., Lammers K., Kuiper H., Beckmann M. P., van Lier R. A. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995 Mar 15;154(6):2612–2623. [PubMed] [Google Scholar]
- Holsti M. A., Raulet D. H. IL-6 and IL-1 synergize to stimulate IL-2 production and proliferation of peripheral T cells. J Immunol. 1989 Oct 15;143(8):2514–2519. [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M., Inaba M., Hathcock K. S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P. S., Ikehara S. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994 Nov 1;180(5):1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990 Oct 1;172(4):1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M. F., Allison J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995 Aug 1;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M. F., Allison J. P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996 Jun 1;183(6):2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Vitetta E. S. Memory T cells are anergic to the superantigen staphylococcal enterotoxin B. J Exp Med. 1992 Aug 1;176(2):575–579. doi: 10.1084/jem.176.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitenberg D., Novak T. J., Farber D., Smith B. R., Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996 Jan 1;183(1):249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Walunas T. L., Bluestone J. A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Bradshaw J., Greene J., Peach R., Bennett K. L., Mittler R. S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996 Jun;4(6):535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K., Eguchi K., Kawabe Y., Tsukada T., Ichinose Y., Nagataki S., Ochi A. Defective TCR-mediated signaling in anergic T cells. J Immunol. 1995 Dec 1;155(11):5083–5087. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr Opin Immunol. 1995 Jun;7(3):375–381. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Farber D., Leitenberg D., Hong S. C., Johnson P., Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994 May;1(2):109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Patarca R., Wei F. Y., Iregui M. V., Cantor H. Differential induction of interferon gamma gene expression after activation of CD4+ T cells by conventional antigen and Mls superantigen. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2736–2739. doi: 10.1073/pnas.88.7.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel J. T., Thomas M. L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989 Sep 22;58(6):1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Correa-Oliveira R., Mauze S., Coffman R. L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994 Feb 1;179(2):589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill H., Schwartz R. H. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987 Jun 1;138(11):3704–3712. [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Rellahan B. L., Jones L. A., Kruisbeek A. M., Fry A. M., Matis L. A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990 Oct 1;172(4):1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Taddie J. A. Role of tyrosine kinases in lymphocyte activation. Curr Opin Immunol. 1994 Jun;6(3):372–379. doi: 10.1016/0952-7915(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Stone J. D., Conroy L. A., Byth K. F., Hederer R. A., Howlett S., Takemoto Y., Holmes N., Alexander D. R. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-zeta, and ZAP-70. J Immunol. 1997 Jun 15;158(12):5773–5782. [PubMed] [Google Scholar]
- Swain S. L. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994 Oct;1(7):543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Thomas M. L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- Viola A., Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996 Jul 5;273(5271):104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- Walunas T. L., Bakker C. Y., Bluestone J. A. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996 Jun 1;183(6):2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Pingel J. T., Nelson J. O., Thomas M. L. CD8+ T-cell clones deficient in the expression of the CD45 protein tyrosine phosphatase have impaired responses to T-cell receptor stimuli. Mol Cell Biol. 1991 Sep;11(9):4415–4422. doi: 10.1128/mcb.11.9.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Woodland D. L., Blackman M. A. How do T-cell receptors, MHC molecules and superantigens get together? Immunol Today. 1993 May;14(5):208–212. doi: 10.1016/0167-5699(93)90164-G. [DOI] [PubMed] [Google Scholar]
- Zimmerman C., Brduscha-Riem K., Blaser C., Zinkernagel R. M., Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996 Apr 1;183(4):1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]